修回日期: 2012-10-10
接受日期: 2012-10-29
在线出版日期: 2012-11-08
目的: 研究化学合成的siRNA干扰巨噬细胞移动抑制因子(macrophage migration-inhibitory factor, MIF)后, 对大肠癌CT-26细胞侵袭的影响并探讨其可能的机制.
方法: 实验组用靶向MIF的siRNA处理CT-26细胞, 对照组用非特异性的siRNA处理CT-26细胞, 空白组不添加任何干扰剂. Transwell细胞侵袭试验检测细胞侵袭情况, ELISA检测培养上清中MIF蛋白的含量, 逆转录聚合酶链反应(RT-PCR)检测MIF、CD74、T淋巴瘤侵袭转移因子1(tiam1)、E-Cadherin mRNA的表达, Western blot检测细胞内MIF及CD74蛋白表达.
结果: 实验组经化学合成的MIF siRNA处理大肠癌CT-26细胞24 h后, 与对照组和空白组相比, 其侵袭受到明显抑制(P = 0.012); 实验组培养上清中MIF蛋白的含量与对照组和空白组比较有显著下降(P = 0.02); 实验组中MIF、CD74、tiam1的mRNA的表达较对照组和空白组相比显著下降(PMIF = 0.001; PCD74 = 0.001; Ptiam1 = 0.004); 实验组中E-Cadherin的表达较对照组和空白组升高(PE-Cadherin = 0.001); 实验组细胞内的MIF及CD74蛋白较对照组和空白组表达明显下降(PMIF = 0.006; PCD74 = 0.016).
结论: 化学合成的MIF siRNA抑制了CT-26细胞的侵袭, 其可能机制是靶向MIF的siRNA抑制了MIF的表达, 降低了CD74与tiam1的表达, 升高了E-Cadherin的表达.
引文著录: 徐慧鲜, 马伟钦, 杨荣娇, 王亚敏, 王丽京, 臧林泉, 何兴祥. 靶向MIF的siRNA对大肠癌细胞侵袭的影响. 世界华人消化杂志 2012; 20(31): 3000-3004
Revised: October 10, 2012
Accepted: October 29, 2012
Published online: November 8, 2012
AIM: To investigate the effect of small interfering RNA (siRNA)-mediated knockdown of the macrophage migration-inhibitory factor (MIF) gene on cell invasion in murine colorectal cancer cell line CT-26 and to explore possible mechanisms involved.
METHODS: CT-26 cells were divided into three groups: experimental group, control group, and blank group. The experimental group and control group were treated with a siRNA specific for the MIF gene (MIF siRNA) and a nonspecific siRNA, respectively, while the blank group was not treated with any agent. Transwell assay was used to determine cell invasion. ELISA was used to determine the level of MIF protein in cell supernatants, and the expression of MIF, CD74, tiam1 and E-cadherin mRNAs was detected by RT-PCR.
RESULTS: Twenty-four hours after treatment, cell invasion was significantly inhibited and the level of MIF protein in supernatants significantly declined in the experimental group compared to the control and blank groups (P = 0.012, 0.020). Compared to the control and blank groups, the expression of MIF, CD74 and tiam1 mRNAs decreased significantly and that of E-cadherin mRNA increased significantly (PE-Cadherin = 0.001) in the experimental group. In addition, the levels of MIF and CD74 proteins significantly declined in the experimental group compared to the control and the blank groups (PMIF = 0.006; PCD74 = 0.016).
CONCLUSION: MIF siRNA inhibits the invasion of CT-26 cells possibly by down-regulating the expression of MIF, CD74 and tiam1 and up-regulating the expression of E-cadherin.
- Citation: Xu HX, Ma WQ, Yang RJ, Wang YM, Wang LJ, Zang LQ, He XX. Small interfering RNA-mediated MIF knockdown reduces cell invasion in murine colorectal cancer cell line CT-26. Shijie Huaren Xiaohua Zazhi 2012; 20(31): 3000-3004
- URL: https://www.wjgnet.com/1009-3079/full/v20/i31/3000.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i31.3000
巨噬细胞移动抑制因子(macrophage migration-inhibitory factor, MIF)是一种具有多种生物学功能的细胞因子, 在很多肿瘤的发生、发展中起到了重要的作用, 并认为对肿瘤的增殖、侵袭有影响[1-3]. 近年来在前列腺癌的研究中发现MIF siRNA可以特异性的降解MIF mRNA, 改善肿瘤的预后[4], 我们前期的研究结果显示MIF促进了大肠癌细胞的侵袭[2,3]. 本实验研究靶向MIF的siRNA对大肠癌细胞侵袭的影响, 并分析其可能的作用机制.
大肠癌CT26细胞购自美国ATCC公司, Transwell购自美国corning公司; Matrigel基质胶购自美国BD公司; ELISA试剂盒购自Promega公司; RT-PCR购自日本东洋纺公司; 柱式TRIzol总RNA抽提试剂盒购自Promega公司; RIPA裂解液购自碧云天公司; CD74、MIF一抗及二抗购自美国Sata公司.
1.2.1 细胞培养与分组: 按1×105接种细胞, 24 h后待细胞生长稳定, 实验组、对照组分别加入 MIF siRNA与非特异性的siRNA, 空白组只加与前面两组相同的培养液, 置37 ℃含50 mL/L CO2的培养箱中培养24 h.
1.2.2 Transwell试验检测细胞侵袭: 4 ℃溶解Matrigel, 每孔40 μL加入预冷的Transwell小室的上室中, 将培养板置于37 ℃孵育1 h, 使Matrigel聚合成凝胶. 稀释细胞密度为1×105, 每孔Transwell上室内加入细胞悬液100 μL, 下室加入含10%PBS的1640培养液作为趋化因子, 放入37 ℃含50 mL/L CO2的培养箱中培养24 h后弃去上室液, 取出聚碳酸酯膜, 擦净膜上的Matrigel胶及上室未穿膜的细胞, 4%多聚甲醛固定30 min, 1%结晶紫染色, 使迁移到聚碳酸酯膜下层的细胞充分着色, 随机在上、下、左、右、中5个视野计数细胞穿膜数, 取平均值. 每组设3个复孔, 重复3次试验.
1.2.3 半定量RT-PCR: 细胞培养24 h后, 用柱式TRIzol总RNA抽提试剂盒抽提总RNA, 使用日本东洋纺一步法试剂盒检测MIF、CD74、T淋巴瘤侵袭转移因子1(tiam1)、E-Cadherin mRNA的表达. 引物和反应条件如表1, 所得产物用含7M尿素的16%聚丙烯酰胺凝胶电泳, 硝酸银染色, 所有实验重复3次. 用Quantity one软件分析数据.
| 引物 | 引物序列 | 扩增片段长度(bp) | 反应条件 | |
| GAPDH | 正义链 | ACC ACA GTC CAT GCC ATC AC | 460 | 94 ℃ 1 min后, 98 ℃ 10 s; 53 ℃ 2 s; 74 ℃ 30 s, 共30循环, 74 ℃延伸10 min |
| 反义链 | TCC ACC ACC CTG TTG CTG TA | |||
| MIF | 正义链 | CCA TGC CTA TGT TCA TCG TG | 250 | 94 ℃ 1 min后, 98 ℃ 10 s; 51℃ 2 s; 74 ℃ 30 s, 共30循环, 74 ℃延伸10 min |
| 反义链 | AGG CCA CAC AGC AGC TTA CT | |||
| CD74 | 正义链 | CCA CTG GAC ATG GAA GAC CT | 231 | 94 ℃ 1 min后, 98 ℃ 10 s; 52 ℃ 2 s; 74 ℃ 30 s, 共30循环, 74 ℃延伸10 min |
| 反义链 | GAC TTC ATT TGC CGT GTC CT | |||
| tiam1 | 正义链 | CTA CCG AAG CTT TGC AGG TC | 280 | 94 ℃ 1 min后, 98 ℃ 10 s; 51 ℃ 2 s; 74 ℃ 30 s, 共30循环, 74 ℃延伸10 min |
| 反义链 | TCC GTT TTG AGG AGC TGT CT | |||
| E-Cadherin | 正义链 | CAA GGA CAG CCT TCT TTT CG | 165 | 94 ℃ 1 min后, 98 ℃ 10 s; 51 ℃ 2 s; 74 ℃ 30 s, 共30循环, 74 ℃延伸10 min |
| 反义链 | TGG ACT TCA GCG TCA CTT TG |
1.2.4 ELISA检测细胞培养液中MIF蛋白的含量: 实验组、对照组及空白组分别加入等量的MIF siRNA, 非特异性siRNA及培养基, 培养24 h, 收集实验组、对照组及空白组中细胞培养液, 1 200 r/min离心5 min, 去除细胞碎片, 按照ELISA检测试剂盒说明进行测定.
1.2.5 Western blot检测细胞内蛋白的表达: 用RIPA裂解液按说明书抽提3组中总蛋白并上样, SDS-PAGE分离后电转移至硝酸纤维素膜上, 用含3%BSA的封闭液封闭2 h, 加入1:1 000稀释的兔抗鼠MIF、CD74单克隆抗体, 4 ℃孵育过夜. 加入辣根过氧化物酶偶联的羊抗兔二抗(1:750, TBST稀释), 平稳摇动, 室温2 h, 加入显影液, 于暗室内显影, 重复试验3 次. 用Quantity one软件分析数据.
统计学处理 所有数据运用SPSS17.0统计软件分析, 采用F检验和秩和检验, 检验水准α = 0.05.
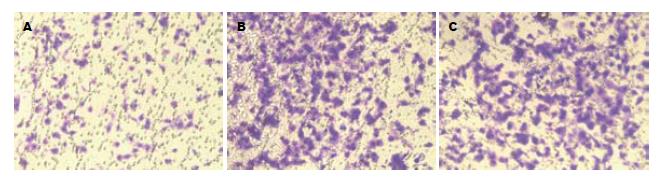
实验组细胞穿膜数较对照组和空白组明显减少(72.75±20.65 vs 276.75±8.47; 72.75±20.65 vs 297.25±15.00, P = 0.012), 而对照组与空白组无明显差异(276.75±8.47 vs 297.25±15.00, P = 0.083, 图1).
MIF、CD74、tiam1在实验组中的表达明显降低, E-Cadherin mRNA在实验组中的表达明显升高, 空白组和阴性对照组无明显改变(图2, 表2).
实验组较对照和空白组明显减少(6.12±0.57 vs 10.21±0.12, 6.12±0.57 vs 10.22±0.14, P = 0.02), 而对照组与空白组无统计学意义(10.22±0.12 vs 10.22±0.14, P = 0.86).
肿瘤细胞的生长发育受许多因素的调控, 其中侵袭在肿瘤的进程中起到重要作用. 在胃癌、大肠癌、黑色素癌、卵巢癌、前列腺癌等研究中发现MIF呈高表达[4-8]. MIF通过LPA依赖Rho途径促进细胞侵袭[9], 也可以通过ERK1/2 MAPK途径促进肿瘤细胞的侵袭[10]. 我们的前期研究发现在大肠癌肝转移模型中, 基质金属蛋白酶9(matrix metallopeptidase 9, MMP-9)的表达与血清MIF呈正相关[1]; MIF促进MMP-9表达, 诱导血管内皮生长因子(vascular endothelial growth factor, VEGF)和IL-8的表达, 以此促进癌细胞向周围组织和血管侵袭[2,3]. 本研究发现靶向MIF的siRNA处理大肠癌CT-26细胞后, MIF的mRNA与蛋白的表达均明显下降, 细胞侵袭能力下降, 进一步说明MIF的表达可以促进大肠癌细胞的侵袭.
CD74属于MHC的Ⅱ型跨膜蛋白, 是MIF的受体. CD74在与MIF结合可以促进NF-κB活性, 导致IL-8分泌, 以此促进肿瘤的侵袭[11], 也可以通过PI3K/AKT和MEK/ERK依赖途径促进肿瘤细胞VEGF的表达, 增加肿瘤细胞内血管的形成[12]. tiam1可以特异性的结合到Rac1上, 促进Rac1活性, 激活GTPase酶, 以此调节细胞信号途径, 参与肿瘤细胞的迁移和侵袭[13,14]. 而E-Cadherin是上皮细胞的主要分子, 正常情况下介导细胞与细胞间的粘附, 但是当E-Cadherin表达缺失时, 细胞之间的粘附作用下降, 致使癌细胞转移[15,16]. 为进一步探讨MIF对大肠癌细胞侵袭的影响, 我们研究其受体CD74及与肿瘤侵袭有关的细胞因子tiam1及E-Cadherin, 结果发现当MIF siRNA抑制MIF的表达后, 其受体CD74亦随之下降, tiam1的表达也下降, 而E-Cadherin的表达上升, 具体的机制有待进一步研究. Veillat等[17]研究发现, 当用ISO-1抑制MIF的表达, 或者CD74 siRNA敲低CD74表达后, 上皮细胞中ERK和p38MAPKs途径受到抑制, VEGF、IL-8和MCP-1表达下调, 细胞侵袭能力下降, 提示MIF与CD74在肿瘤细胞的侵袭中起到重要作用. 但是为什么siRNA干扰MIF的表达后, CD74的表达亦下降, 两者之间的相互关系有待进一步研究. 而Singleton等[18]研究发现CD44可以上调肝细胞生长因子介导的tiam1, 从而上调血管的生成, 而CD44与CD74结合可以通过NF-κB途径促进肿瘤细胞的侵袭[19]. Roger等[20]研究发现当野生型p53突变时, P53的表达下降, E-Cadherin的表达下降, 结肠癌细胞间的侵袭能力上调. 由此我们推测MIF siRNA影响tiam1及E-Cadherin的表达可能是通过NF-κB途径及P53突变来实现的, 具体机制需进一步研究.
总之, 本实验表明靶向MIF的siRNA可以抑制大肠癌细胞的侵袭, 可能的机制是靶向MIF的siRNA敲低MIF表达后, 导致、CD74、tiam1表达下降, E-Cadherin表达上升.
大肠癌占胃肠道肿瘤发病率的第3位, 每年呈上升趋势. 研究发现巨噬细胞移动抑制因子(MIF)参与许多肿瘤的发生、发展, 而本课题组前期的研究也证实MIF参与大肠癌的侵袭, 为此, 本问研究靶向MIF的siRNA对大肠癌细胞侵袭的影响, 希望找到抑制大肠癌的方法, 以达到治疗大肠癌的目的.
陈卫昌, 教授, 苏州大学附属第一医院消化内科
近年来在前列腺癌的研究中发现MIF siRNA可以特异性的降解MIF mRNA, 改善肿瘤的预后.
本研究发现靶向MIF的siRNA处理大肠癌CT-26细胞后, MIF的mRNA与蛋白的表达均明显下降, 细胞侵袭能力下降, 进一步说明MIF的表达可以促进大肠癌细胞的侵袭.
本研究方法较为成熟, 结果可靠, 有一定价值.
编辑: 李军亮 电编:鲁亚静
| 2. | 李 晓宇, 何 兴祥. 巨噬细胞移动抑制因子对大肠癌细胞侵袭的影响. 现代临床医学生物工程学杂志. 2006;12:402-404. |
| 4. | Meyer-Siegler KL, Iczkowski KA, Leng L, Bucala R, Vera PL. Inhibition of macrophage migration inhibitory factor or its receptor (CD74) attenuates growth and invasion of DU-145 prostate cancer cells. J Immunol. 2006;177:8730-8739. [PubMed] |
| 5. | He XX, Yang J, Ding YW, Liu W, Shen QY, Xia HH. Increased epithelial and serum expression of macrophage migration inhibitory factor (MIF) in gastric cancer: potential role of MIF in gastric carcinogenesis. Gut. 2006;55:797-802. [PubMed] [DOI] |
| 6. | He XX, Chen K, Yang J, Li XY, Gan HY, Liu CY, Coleman TR, Al-Abed Y. Macrophage migration inhibitory factor promotes colorectal cancer. Mol Med. 2009;15:1-10. [PubMed] [DOI] |
| 7. | Swant JD, Rendon BE, Symons M, Mitchell RA. Rho GTPase-dependent signaling is required for macrophage migration inhibitory factor-mediated expression of cyclin D1. J Biol Chem. 2005;280:23066-23072. [PubMed] [DOI] |
| 8. | Hagemann T, Robinson SC, Thompson RG, Charles K, Kulbe H, Balkwill FR. Ovarian cancer cell-derived migration inhibitory factor enhances tumor growth, progression, and angiogenesis. Mol Cancer Ther. 2007;6:1993-2002. [PubMed] [DOI] |
| 9. | Sun B, Nishihira J, Yoshiki T, Kondo M, Sato Y, Sasaki F, Todo S. Macrophage migration inhibitory factor promotes tumor invasion and metastasis via the Rho-dependent pathway. Clin Cancer Res. 2005;11:1050-1058. [PubMed] |
| 10. | Piette C, Deprez M, Roger T, Noël A, Foidart JM, Munaut C. The dexamethasone-induced inhibition of proliferation, migration, and invasion in glioma cell lines is antagonized by macrophage migration inhibitory factor (MIF) and can be enhanced by specific MIF inhibitors. J Biol Chem. 2009;284:32483-32492. [PubMed] [DOI] |
| 11. | Binsky I, Haran M, Starlets D, Gore Y, Lantner F, Harpaz N, Leng L, Goldenberg DM, Shvidel L, Berrebi A. IL-8 secreted in a macrophage migration-inhibitory factor- and CD74-dependent manner regulates B cell chronic lymphocytic leukemia survival. Proc Natl Acad Sci U S A. 2007;104:13408-13413. [PubMed] [DOI] |
| 12. | Liu YH, Lin CY, Lin WC, Tang SW, Lai MK, Lin JY. Up-regulation of vascular endothelial growth factor-D expression in clear cell renal cell carcinoma by CD74: a critical role in cancer cell tumorigenesis. J Immunol. 2008;181:6584-6594. [PubMed] |
| 13. | Lee SH, Kunz J, Lin SH, Yu-Lee LY. 16-kDa prolactin inhibits endothelial cell migration by down-regulating the Ras-Tiam1-Rac1-Pak1 signaling pathway. Cancer Res. 2007;67:11045-11053. [PubMed] [DOI] |
| 14. | Malliri A, Rygiel TP, van der Kammen RA, Song JY, Engers R, Hurlstone AF, Clevers H, Collard JG. The rac activator Tiam1 is a Wnt-responsive gene that modifies intestinal tumor development. J Biol Chem. 2006;281:543-548. [PubMed] [DOI] |
| 15. | Shin Y, Kim IJ, Kang HC, Park JH, Park HW, Jang SG, Lee MR, Jeong SY, Chang HJ, Ku JL. A functional polymorphism (-347 G--& gt; GA) in the E-cadherin gene is associated with colorectal cancer. Carcinogenesis. 2004;25:2173-2176. [PubMed] [DOI] |
| 16. | Andl CD, Fargnoli BB, Okawa T, Bowser M, Takaoka M, Nakagawa H, Klein-Szanto A, Hua X, Herlyn M, Rustgi AK. Coordinated functions of E-cadherin and transforming growth factor beta receptor II in vitro and in vivo. Cancer Res. 2006;66:9878-9885. [PubMed] [DOI] |
| 17. | Veillat V, Carli C, Metz CN, Al-Abed Y, Naccache PH, Akoum A. Macrophage migration inhibitory factor elicits an angiogenic phenotype in human ectopic endometrial cells and triggers the production of major angiogenic factors via CD44, CD74, and MAPK signaling pathways. J Clin Endocrinol Metab. 2010;95:E403-E412. [PubMed] [DOI] |
| 18. | Singleton PA, Salgia R, Moreno-Vinasco L, Moitra J, Sammani S, Mirzapoiazova T, Garcia JG. CD44 regulates hepatocyte growth factor-mediated vascular integrity. Role of c-Met, Tiam1/Rac1, dynamin 2, and cortactin. J Biol Chem. 2007;282:30643-30657. [PubMed] [DOI] |
| 19. | Gore Y, Starlets D, Maharshak N, Becker-Herman S, Kaneyuki U, Leng L, Bucala R, Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J Biol Chem. 2008;283:2784-2792. [PubMed] [DOI] |
| 20. | Roger L, Jullien L, Gire V, Roux P. Gain of oncogenic function of p53 mutants regulates E-cadherin expression uncoupled from cell invasion in colon cancer cells. J Cell Sci. 2010;123:1295-1305. [PubMed] [DOI] |