修回日期: 2012-07-02
接受日期: 2012-07-20
在线出版日期: 2012-08-08
目的: 分析聚乙二醇干扰素α-2a(peginterferonα-2a, Peg-IFNα-2a)治疗HBeAg阳性慢性乙型肝炎应答不佳者联合阿德福韦酯(adefovir dipivoxil, ADV)治疗48 wk的疗效、安全性, 并评价48 wk疗效预测指标.
方法: 140例患者初始均接受Peg-IFNα-2a每周1次皮下注射单药治疗, 根据24 wk时HBV DNA水平进行分组. A组90例患者治疗24 wk时HBV DNA≥2.0×103 IU/mL且HBV DNA下降≥2 log10 IU/mL, 其中A1组45例患者加用ADV治疗至48 wk, A2组45例患者继续Peg-IFNα-2a单药治疗至48 wk; B组50例患者治疗24 wk时HBV DNA<2.0×103 IU/mL, 继续Peg-IFNα-2a治疗至48 wk. 比较各组基线及治疗中HBV DNA、HBsAg、HBeAg及ALT水平.
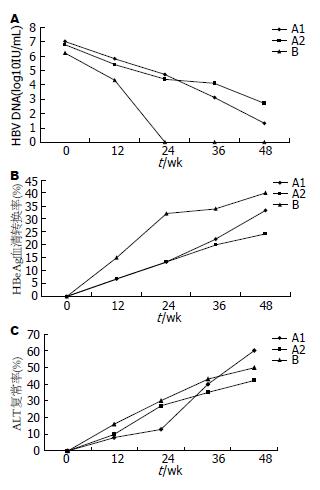
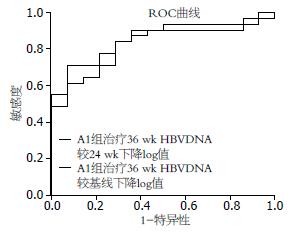
结果: 治疗36、48 wk时HBV DNA转阴率B组>A1组>A2组(P<0.01). 治疗36 wk时HBV DNA下降值B组>A1组>A2组(P<0.01). 48 wk时HBV DNA下降值A1组>A2组(P<0.01), A1、B组之间差异无统计学意义. 治疗24-48 wk期间, A1组HBeAg血清转换率升高幅度>A2组和B组(P<0.01). 治疗48 wk时HBsAg下降A1组4例、A2组1例、B组2例. 治疗36、48 wk时A1与A2、B组ALT复常率差异无统计学意义. A1组治疗48 wk时HBV DNA阴转与治疗36 wk HBV DNA下降值有关. 治疗36 wk时HBV DNA较基线下降值对48 wk时HBV DNA阴转的阳性预测值为90.5%, 较24 wk时下降值对48 wk时HBV DNA阴转的阳性预测值为95.7%.
结论: Peg-IFNα-2a治疗HBeAg阳性慢性乙型肝炎应答不佳者联合ADV治疗提高病毒应答、HBeAg血清转换率, 其中治疗36 wk时HBV DNA下降值可预测48 wk疗效.
引文著录: 丁洋, 吴发玲, 盛秋菊, 赵连荣, 夏婷婷, 王静艳, 石理兰, 王淑兰, 单红, 安萍, 段红岩, 窦晓光. 聚乙二醇干扰素α-2a联合阿德福韦酯对HBeAg阳性慢性乙型肝炎的治疗. 世界华人消化杂志 2012; 20(22): 2036-2042
Revised: July 2, 2012
Accepted: July 20, 2012
Published online: August 8, 2012
AIM: To analyze the efficacy and safety of adefovir dipivoxil (ADV) as an add-on therapy in patients with HBeAg-positive chronic hepatitis B (CHB) who have a suboptimal response to peginterferonα-2a (Peg-IFNα-2a) treatment for 48 weeks, and to evaluate the predictors of response to the combination therapy.
METHODS: Ninety HBeAg-positive CHB patients who had been being treated with Peg-IFNα-2a for 24 weeks and had HBV DNA ≥ 2.0 × 103 IU/mL and HBV DNA decrease ≥ 2 log10 IU/mL were randomly assigned either to add on ADV (group A1, 45 patients) or to continue Peg-IFNα-2a monotherapy (group A2, 45 patients).Other 55 patients with HBV DNA < 2.0 × 103 IU/mL were assigned to continue Peg-IFNα-2a monotherapy. The levels of HBV DNA, HBsAg, HBeAg and ALT at baseline and during treatment were compared among the three groups.
RESULTS: The rates of undetectable serum HBV DNA at weeks 36 and 48 were highest in group B, followed by group A1 and group A2 (P < 0.01). HBV DNA level at week 36 declined more significantly in group B than in group A1, and in group A1 than in group A2 (both P < 0.01). At week 48, HBV DNA level declined more significantly in groups A1 than in group A2 (P < 0.01), while there was no significant difference between group A1 and B. Rates of HBeAg loss and HBeAg seroconversion in groups A1, A2 and B were not statistically different at weeks 36 and 48. Amplitude of HBeAg seroconversion rate from week 24 to week 48 in group A1 was higher than those in group A2 and group B (both P < 0.01). There were 4, 1 and 2 cases of HBsAg reduction in group A1, group A2 and group B at week 48, respectively. Rates of ALT normalization in group A1, group A2 and group B were not statistically different at weeks 36 and 48. All the patients finished 48-week therapy without severe adverse effects. Rate of undetectable serum HBV DNA in group A1 was only associated with the amplitude of HBV DNA level at week 36. HBV DNA reduction levels at week 36 from baseline or week 24 could predict undetectable serum HBV DNA at week 48 with a PPV of 90.5% and 95.7%, respectively.
CONCLUSION: ADV as an add-on therapy in patients with HBeAg-positive CHB who have a suboptimal response to Peg-IFNα-2a treatment for 48 weeks could improve HBeAg seroconversion rate and undetectable HBV DNA rate with tolerable adverse effects. HBV DNA reduction levels at week 36 from baseline or week 24 could predict the viral response to the combination therapy at week 48.
- Citation: Ding Y, Wu FL, Sheng QJ, Zhao LR, Xia TT, Wang JY, Shi LL, Wang SL, Shan H, An P, Duan HY, Dou XG. Combination therapy with peginterferonα-2a and adefovir dipivoxil for HBeAg-positive chronic hepatitis B: A prospective multicenter cohort study. Shijie Huaren Xiaohua Zazhi 2012; 20(22): 2036-2042
- URL: https://www.wjgnet.com/1009-3079/full/v20/i22/2036.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i22.2036
慢性乙型肝炎(chronic hepatitis B, CHB)是由乙型肝炎病毒(hepatitis B virus, HBV)在体内持续高水平复制、机体发生免疫应答引起的慢性进展性疾病. 治疗策略是最大限度地长期抑制病毒复制或通过增强机体免疫反应, 使机体自发清除病毒, 延缓疾病进展, 减少肝硬化、肝癌等的发生, 方法包括抗病毒、免疫调节、抗氧化、抗纤维化和对症治疗, 其中抗病毒治疗是关键[1-3]. 目前国内外公认的有效抗病毒药物主要包括干扰素(interferon, IFN)类和核苷(酸)类似物[Nucleos(t)ide, NUC]类, 2者各有优缺点, 当一种治疗方法效果不明显甚至无效时改用或联合应用另一种方法可能仍然有效. 对于初始应用IFN治疗的CHB患者, 经一段时间治疗后应答不佳者联合NUC理论上不会影响后续治疗的疗效, 而且应用IFN所致基础免疫状态发生改变可能会提高机体对NUC的应答[4], 但实际上临床疗效如何目前尚无定论. 本临床观察通过对聚乙二醇干扰素α-2a(peginterferonα-2a, Peg-IFNα-2a)治疗HBeAg阳性CHB应答不佳者联合阿德福韦酯(adefovir dipivoxil, ADV)或继续Peg-IFNα-2a单药治疗, 比较治疗中的各指标差异, 评价48 wk疗效, 为更多患者选择合适的联合治疗方案提供参考.
采集2010-02/2012-02在中国医科大学附属盛京医院、辽宁省人民医院、沈阳市第六人民医院、大连市第六人民医院和盘锦辽河油田第二医院传染科就医并完成Peg-IFNα-2a治疗48 wk的180例HBeAg阳性CHB患者抗病毒治疗前和治疗中空腹静脉血血清, 排除治疗24 wk时HBV DNA≥2.0×103 IU/mL且HBV DNA下降<2 log10 IU/mL的患者40例, 入选其余140例. 患者治疗前均符合下列入组条件: 年龄>18岁、HBeAg阳性CHB、2×正常上限≤ALT≤10×正常上限、总胆红素<2×正常上限、HBV DNA≥2.0×104 IU/mL, 近6 mo无IFN或NUC用药史, 无IFNα和ADV治疗禁忌证, 无甲型肝炎病毒(hepatitis A virus, HAV)、丙型肝炎病毒(hepatitis C virus, HCV)、人类免疫缺陷病毒(human immunodeficiency virus, HIV)重叠感染.
1.2.1 治疗: 入组140例患者初始均接受Peg-IFNα-2a 135 μg(体质量≤65 kg)或180 μg(体质量>65 kg)每周1次皮下注射单药治疗, 根据24 wk时HBV DNA水平进行分组. A组90例患者治疗24 wk时HBV DNA≥2.0×103 IU/mL且HBV DNA下降≥2 log10 IU/mL, 其中A1组45例患者加用ADV 10 mg, 每日1次口服治疗至48 wk, A2组45例患者继续Peg-IFNα-2a单药治疗至48 wk; B组50例患者治疗24 wk时HBV DNA<2.0×103 IU/mL, 继续Peg-IFNα-2a治疗至48 wk. 比较各组基线及治疗中HBV DNA、HBsAg、HBeAg及ALT水平.
1.2.2 ALT检测: 采用美国Beckman全自动生化仪及其配套试剂检测, 检测标本为空腹静脉血血清.
1.2.3 HBV DNA检测: 采用Real Time PCR法, 试剂由深圳匹基生物工程有限公司生产, 敏感度为103 IU/mL, 检测标本为空腹静脉血血清.
1.2.4 HBsAg、HBeAg检测: 采用Achitect(Abbott)微粒子化学发光免疫分析法、两步法原理, HBsAg assay定量检测敏感度为0.05 IU/mL, HBeAg assay定量检测敏感度为1.0 s/co, 检测标本为空腹静脉血血清.
统计学处理 采用SPSS18.0统计软件进行统计分析, 两组间均数的比较采用t检验; 多组间均数比较采用单因素方差分析(ANOVA)和组间两两比较; 组间计数资料的比较采用χ2检验. 以α = 0.05作为检验水准. 疗效预测采用ROC曲线, AUC为曲线下面积, PPV为阳性预测值, NPV为阴性预测值.
患者治疗前基线材料见表1.
A、B两组患者平均年龄、性别分布及基线HBeAg水平差异无统计学意义, A组基线ALT水平低于B组(P<0.05), 基线HBV DNA水平高于B组(P<0.01, 表1); 3组患者治疗24 wk分组时平均年龄、性别分布及ALT水平差异无统计学意义, A1、A2组HBV DNA、HBeAg水平高于B组(P<0.01), 但A1、A2组之间差异无统计学意义(表2).
治疗36、48 wk时HBV DNA转阴率B组>A1组>A2组, 差异有统计学意义(P<0.01). 治疗36 wk HBV DNA下降值B组>A1组>A2组, 差异有统计学意义(P<0.01). 48 wk HBV DNA下降值A1>A2, 差异有统计学意义(P<0.01); 但A1与B组之间差异无统计学意义(表3). B组发生HBV DNA阴转时间较A1组早, A1组较A2组早(图1A).
| 应答 | 治疗36 wk | 治疗48 wk | ||||||||||
| A1组(n = 45) | A2组(n = 45) | B组(n = 50) | A1组(n = 45) | A2组(n = 45) | B组(n = 50) | |||||||
| 病毒学应答 | ||||||||||||
| HBV DNA转阴 | n(%) | 12(26.7) | 2(4.4)b | 50(100)b | 30(66.7) | 16(35.6)b | 50(100)b | |||||
| HBV DNA下降 | (log10 IU/mL) | 4.0±1.9 | 2.9±0.9b | 6.2±1.2b | 5.7±2.0 | 4.2±2.0b | 6.2±1.2 | |||||
| 血清学应答 | ||||||||||||
| HBeAg转阴 | n(%) | 13(28.9) | 12(26.7) | 21(42) | 19(42.2) | 13(28.9) | 31(62) | |||||
| HBeAg血清转换 | n(%) | 10(22.2) | 9(20) | 17(34) | 15(33.3) | 11(24.4) | 20(40) | |||||
| HBsAg下降 | n(%) | 4(8.9) | 1(2.2) | 2(4) | ||||||||
| 生化学应答 | ||||||||||||
| ALT复常 | n(%) | 15(33.3) | 16(35.6) | 21(42) | 26(57.8) | 19(42.2) | 25(50) | |||||
治疗36、48 wk时A1组与A2组、B组HBeAg转阴率、血清转换率差异无统计学意义(表3). 治疗24-48 wk间, A1组HBeAg血清转换率升高幅度>A2组、B组(P<0.01). 治疗48 wk, HBsAg下降: A1组4例、A2组1例、B组2例, 差异无统计学意义(图1B).
除40例患者因24 wk时HBV DNA≥2.0×103 IU/mL, 且HBV DNA下降<2 log10 IU/mL中途退出本临床试验外, 其余140例患者均完成了48 wk治疗. 大多数患者应用Peg-IFNα-2a早期出现发热、头痛、全身肌肉酸痛等流感样症状, 一般应用3-4针后症状好转或消失. 大多数患者有白细胞、中性粒细胞下降, 一般不需要特殊处理, 少数患者加用粒细胞集落刺激因子, 且停药后逐渐恢复到基线水平. 未发现ADV导致的明显不良反应.
分析A1组患者情况发现, 治疗48 wk时HBV DNA阴转与基线、治疗24 wk时ALT、HBeAg、HBV DNA水平无关, 与治疗36 wk HBV DNA下降值有关. 采用ROC曲线分析发现, 治疗36 wk HBV DNA较基线下降3.6 log10 IU/mL对48 wk HBV DNA阴转预测的PPV 90.5%、NPV 54.2%, AUC 0.822; 而治疗36 wk HBV DNA较24 wk下降0.8 log10 IU/mL对48 wk HBV DNA阴转预测的PPV 95.7%、NPV 63.6%, AUC 0.876(图2).
Peg-IFNα-2a有抗病毒和调节免疫双重作用, 且疗程相对固定, 被推荐作为CHB治疗的一线用药[5]. 但难治性基因型如C型、D型HBV对Peg-IFNα-2a敏感性较差[6], 治疗中应答不佳或治疗后病毒反弹时有发生. 研究发现, 病毒反弹与应答不佳有关, 早期病毒应答者能获得更高的持久应答率[7]. 目前对于难治性CHB, 专家建议根据优化治疗原则选择联合治疗方案[8], 但由于个体差异大, 联合抗病毒药物种类、剂量、时机、治疗终点不一, 目前尚无统一方案. 曾有报道初始Peg-IFNα-2a联合拉米夫定治疗难治性基因D型CHB能提高联合应答率(即病毒学应答+生化学应答)[9], 然而全球性大样本试验证实联合治疗48 wk的病毒应答率、HBeAg血清转换率并不优于Peg-IFNα-2a单药治疗[10,11]. Peg-IFNα-2a联合替比夫定增加周围神经病变的发生风险[12], 不推荐使用. ADV虽起效慢, 却是体内唯一能降低肝内cccDNA的NUC, 标准量治疗病毒变异率低, 耐药发生较晚[13]. 研究显示Peg-IFNα-2a联合ADV治疗CHB能提高病毒学应答率[14-16], 延长疗程可逐渐降低肝内cccDNA[17].
HBV DNA阴转是CHB治疗的基本目标, 治疗中HBV DNA变化水平是评价疗效的基本指标[18]. Piccolo等[19]研究发现, Peg-IFNα-2a联合ADV治疗HBeAg阴性患者24 wk HBV DNA阴转率可达73.3%. 本研究发现, Peg-IFNα-2a治疗HBeAg阳性CHB 24 wk应答不佳者联合ADV治疗至36、48 wk时HBV DNA下降值、阴转率均较继续Peg-IFNα-2a单药治疗显著提高, 且发生阴转的时间早于单药组, 说明联合治疗可迅速、显著抑制病毒复制, 提高病毒学应答. 分析可能原因是IFN改变机体免疫状态, 调动机体免疫功能, 联合ADV后机体免疫程度与病毒下降速度相匹配, 发生疗效叠加. 观察HBV DNA变化曲线并进行疗效相关性分析发现, 48 wk病毒转阴与36 wk(即联合治疗12 wk)HBV DNA下降值显著相关, 与基线、24 wk(联合治疗初)ALT、HBeAg、HBV DNA水平无关. 36 wk HBV DNA下降值对48 wk病毒应答有预测价值, 其中较24 wk下降0.8 log10 IU/mL比较基线下降3.6 log10 IU/mL更有意义. 我们由此推测, HBV DNA下降值对Peg-IFNα-2a治疗HBeAg阳性CHB 24 wk应答不佳者联合ADV治疗的远期疗效有一定预测意义, 具体价值还有待进一步研究.
既往研究发现, 病毒低载量, ALT升高, 肝细胞炎症活动度大, 机体进入免疫清除期, 此时行抗病毒治疗能获得更好疗效[20]. 本研究发现, Peg-IFNα-2a治疗HBeAg阳性CHB早期能否发生完全病毒学应答与基线ALT、HBV DNA水平有关, 基线ALT水平高, 病毒低载量, 治疗中HBV DNA下降显著, 疗效佳.
既往研究发现, HBeAg水平与患者远期愈后、生存率、肝脏组织病理改变有关[21], 是评价HBeAg阳性患者抗病毒疗效的关键指标, Peg-IFNα联合ADV治疗CHB能提高HBeAg血清转换率、阴转率[14,22,23]. 国内专家基于持续缓解CHB进展的考虑, 建议以HBeAg血清转换作为IFN治疗目标[7]. 本研究发现, 24 wk时病毒应答佳者24 wk时的HBeAg血清转换率、阴转率均较应答不佳者高, 说明HBV DNA早期下降与HBeAg血清转换相关, 这与Janssen等[24]的研究结果相一致, 考虑可能原因为HBV DNA早期下降者对IFN的作用敏感性强, 一方面IFN达到血药浓度后抑制病毒复制能力强, 使HBV DNA快速下降; 另一方面免疫调节作用强, 使HBeAg迅速发生血清转换. 而应答不佳者加用ADV治疗至36、48 wk时HBeAg血清转换率、阴转率均较前明显升高, 但较单药治疗者未见明显升高, 考虑与样本量少、联合治疗时间短有关, 建议扩大样本量、延长疗程并定期随访以进一步研究.
HBsAg转阴是公认的理想治疗目标, 往往发生率低, 在IFN停药后随访24 wk时为3%, 3年时增加至8%[25]. 而治疗中HBsAg定量变化也是较好的疗效预测指标, 尤其对HBeAg阴性CHB患者[26]. 国外学者发现24 wk血清HBsAg≤1 500 IU/mL, 继续治疗至48 wk并停药24 wk观察HBeAg血清转换率升高. 本研究发现, 治疗48 wk HBsAg<250 IU/mL, 联合组4(45)例、单药组3(105)例, 对于上述患者我们建议延长疗程以提高HBsAg转阴率.
本研究中除40例因24 wk HBV DNA≥2.0×103 IU/mL且HBV DNA下降<2 log10 IU/mL中途退出临床试验外, 其余140例患者均完成了48 wk治疗, 未发生因严重不良反应停药失访或等事件. 治疗中出现的不适症状或血清学异常与既往IFN单药治疗出现的一样[10], 且对症治疗或停药后逐渐恢复到基线水平, 说明该治疗方案安全可行.
总之, Peg-IFNα-2a治疗CHB应答不佳者联合ADV治疗可提高病毒应答率、HBeAg血清转换率, 治疗36 wk HBV DNA下降值可预测48 wk疗效, 该方案安全、有效, 可供临床医师参考.
衷心感谢中国医科大学附属盛京医院、辽宁省人民医院、沈阳市第六人民医院、大连市第六人民医院和盘锦辽河油田第二医院感染科、检验科全体医师、护士在本研究过程中给予的大力支持, 感谢中国医科大学附属盛京医院感染科冯国和教授、李智伟教授在论文书写过程中提供的中肯意见!
Peg-IFNα-2a是慢性乙型肝炎(CHB)抗病毒治疗的一线用药, 具有抗病毒和调节免疫的双重作用, 但治疗中应答不佳的情况时有发生. 对于这类患者, 专家建议联合核苷(酸)类似物优化治疗.
赵秀英, 副教授, 首都医科大学附属北京佑安医院; 周霞秋, 教授, 上海瑞金医院感染科
目前国内外学者分别对联合治疗开始时机、药物种类、剂量、治疗终点等方面进行深入研究, 以期为患者提供合理的治疗策略.
Bart等研究发现Peg-IFNα-2a联合ADV治疗CHB能提高病毒学应答率, Wursthorn等研究发现延长疗程可逐渐降低肝内cccDNA水平. Takkenberg等研究发现2者联合治疗能提高HBeAg血清转换率、阴转率.
Peg-IFNα-2a治疗CHB应答不佳者联合ADV治疗可提高病毒应答率、HBeAg血清转换率, 治疗36 wk HBV DNA下降值可预测远期疗效, 该方案安全、有效, 可供临床医师参考.
应答不佳: 治疗24 wk血清HBV DNA≥2.0×103 IU/mL且较基线下降≥2 log10 IU/mL.
本文设计较科学, 数据分析合理, 结果较为客观, 对临床有一定指导意义和实用价值.
编辑: 张姗姗 电编:闫晋利
| 1. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [PubMed] [DOI] |
| 2. | Lai CL, Ratziu V, Yuen MF, Poynard T. Viral hepatitis B. Lancet. 2003;362:2089-2094. [PubMed] [DOI] |
| 4. | Hui CK, Leung N, Yuen ST, Zhang HY, Leung KW, Lu L, Cheung SK, Wong WM, Lau GK. Natural history and disease progression in Chinese chronic hepatitis B patients in immune-tolerant phase. Hepatology. 2007;46:395-401. [PubMed] [DOI] |
| 6. | Craxì A, Di Bona D, Cammà C. Interferon-alpha for HBeAg-positive chronic hepatitis B. J Hepatol. 2003;39 Suppl 1:S99-S105. [PubMed] [DOI] |
| 7. | ter Borg MJ, van Zonneveld M, Zeuzem S, Senturk H, Akarca US, Simon C, Hansen BE, Haagmans BL, de Man RA, Schalm SW. Patterns of viral decline during PEG-interferon alpha-2b therapy in HBeAg-positive chronic hepatitis B: relation to treatment response. Hepatology. 2006;44:721-727. [PubMed] [DOI] |
| 9. | Bonino F, Marcellin P, Lau GK, Hadziyannis S, Jin R, Piratvisuth T, Germanidis G, Yurdaydin C, Diago M, Gurel S. Predicting response to peginterferon alpha-2a, lamivudine and the two combined for HBeAg-negative chronic hepatitis B. Gut. 2007;56:699-705. [PubMed] [DOI] |
| 10. | Lau GK, Piratvisuth T, Luo KX, Marcellin P, Thongsawat S, Cooksley G, Gane E, Fried MW, Chow WC, Paik SW. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med. 2005;352:2682-2695. [PubMed] [DOI] |
| 11. | Marcellin P, Lau GK, Bonino F, Farci P, Hadziyannis S, Jin R, Lu ZM, Piratvisuth T, Germanidis G, Yurdaydin C. Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. N Engl J Med. 2004;351:1206-1217. [PubMed] [DOI] |
| 12. | Risk of peripheral neuropathy in patients treated with telbivudine (SEBIVO) and interferon-For Health Professionals. Available from: http://www.hc-sc.gc.ca/dhp-mps/medeff/advisories-avis/public/_2008/sebivo_pc-cp-eng.php. |
| 13. | Werle-Lapostolle B, Bowden S, Locarnini S, Wursthorn K, Petersen J, Lau G, Trepo C, Marcellin P, Goodman Z, Delaney WE. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750-1758. [PubMed] |
| 14. | Takkenberg B, Terpstra V, Zaaijer H, Weegink C, Dijkgraaf M, Jansen P, Beld M, Reesink H. Intrahepatic response markers in chronic hepatitis B patients treated with peginterferon alpha-2a and adefovir. J Gastroenterol Hepatol. 2011;26:1527-1535. [PubMed] [DOI] |
| 15. | 陈 禄彪, 舒 欣, 揭 育胜, 杨 小安, 张 卡, 李 刚, 徐 启恒. 聚乙二醇干扰素α-2a加用阿德福韦酯治疗HBeAg阳性慢性乙肝的短期疗效观察. 中华实验和临床病毒学杂志. 2010;24:39-41. |
| 16. | Ingiliz P, Valantin MA, Thibault V, Duvivier C, Dominguez S, Katlama C, Poynard T, Benhamou Y. Efficacy and safety of adefovir dipivoxil plus pegylated interferon-alpha2a for the treatment of lamivudine-resistant hepatitis B virus infection in HIV-infected patients. Antivir Ther. 2008;13:895-900. [PubMed] |
| 17. | Wursthorn K, Lutgehetmann M, Dandri M, Volz T, Buggisch P, Zollner B, Longerich T, Schirmacher P, Metzler F, Zankel M. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44:675-684. [PubMed] [DOI] |
| 18. | Hansen BE, Buster EH, Steyerberg EW, Lesaffre E, Janssen HL. Prediction of the response to peg-interferon-alfa in patients with HBeAg positive chronic hepatitis B using decline of HBV DNA during treatment. J Med Virol. 2010;82:1135-1142. [PubMed] [DOI] |
| 19. | Piccolo P, Lenci I, Demelia L, Bandiera F, Piras MR, Antonucci G, Nosotti L, Mari T, De Santis A, Ponti ML. A randomized controlled trial of pegylated interferon-alpha2a plus adefovir dipivoxil for hepatitis B e antigen-negative chronic hepatitis B. Antivir Ther. 2009;14:1165-1174. [PubMed] [DOI] |
| 20. | Erhardt A, Blondin D, Hauck K, Sagir A, Kohnle T, Heintges T, Häussinger D. Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut. 2005;54:1009-1013. [PubMed] [DOI] |
| 21. | Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, Alberti A, Realdi G. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology. 1986;6:167-172. [PubMed] [DOI] |
| 22. | Chen EQ, Tang H. Hepatitis B e antigen as a predictor for hepatitis B e antigen-positive chronic hepatitis B patients with peginterferon alfa-2a therapy. Hepatology. 2009;50:1677; author reply 1677-1679. [PubMed] |
| 23. | Chan HL, Wong VW, Tse AM, Tse CH, Chim AM, Chan HY, Wong GL, Sung JJ. Serum hepatitis B surface antigen quantitation can reflect hepatitis B virus in the liver and predict treatment response. Clin Gastroenterol Hepatol. 2007;5:1462-1468. [PubMed] [DOI] |
| 24. | Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-129. [PubMed] [DOI] |
| 25. | Marcellin P, Bonino F, Lau GK, Farci P, Yurdaydin C, Piratvisuth T, Jin R, Gurel S, Lu ZM, Wu J. Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology. 2009;136:2169-2179.e1-e4. [PubMed] |
| 26. | Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, Paik SW, Liaw YF, Button P. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47:428-434. [PubMed] [DOI] |