修回日期: 2012-06-07
接受日期: 2012-06-30
在线出版日期: 2012-07-28
目的: 定量分析肝病患者血可溶性细胞间粘附分子-1(sICAM-1)浓度和外周血单核细胞磷脂酰肌醇蛋白多糖-3(GPC-3)mRNA, 探讨对肝癌(HCC)诊断与预后的价值.
方法: 收集住院肝病患者外周血, 分离单核细胞, 制备总RNA, 经逆转录合成GPC-3 cDNA, 以荧光定量PCR扩增; 并以酶联免疫吸附法(ELISA)定量分析血ICAM-1水平.
结果: 肝病发展过程中血ICAM-1表达呈梯度增加, HCC患者血ICAM-1表达显著高于肝硬化(t = 3.184, P = 0.002)和慢性肝炎患者(t = 3.962, P<0.001), 与伴门脉癌栓(t = 2.941, P = 0.005)及肝外转移(t = 3.282, P = 0.002)明显相关, 与患者年龄、性别、HBsAg阳性与否、AFP浓度及肿瘤大小间未见明显相关. GPC-3 mRNA阳性仅见肝癌患者(70.9%); 肝硬化、慢性肝炎患者及正常对照组中未检出(χ2 = 26.773, P<0.001). GPC-3 mRNA阳性表达与HBsAg阳性(χ2 = 14.601, P<0.001)、肝癌TNM分期(χ2 = 17.732, P<0.001)、伴门脉癌栓及肝外转移(χ2 = 22.271, P<0.001)显著相关, 与瘤体直径、数目、AFP浓度及分化程度未见明显相关; 两者可互补诊断, 提高诊断肝癌阳性率.
结论: sICAM-1和GPC-3 mRNA检测是肝癌诊断和转移监测的良好标志物, 且对AFP阴性肝癌具有互补诊断价值.
引文著录: 姚敏, 杨君伶, 蔚丹丹, 严晓娣, 陈洁, 姚登福. sICAM-1和GPC-3 mRNA对肝癌诊断与转移监测的临床价值. 世界华人消化杂志 2012; 20(21): 1927-1932
Revised: June 7, 2012
Accepted: June 30, 2012
Published online: July 28, 2012
AIM: To quantitatively detect the levels of circulating soluble intercellular adhesion molecule-1 (sICAM-1) and glypican-3 (GPC-3) mRNA expression in peripheral blood mononuclear cells (PBMCs) in patients with liver diseases for exploring their significance in the diagnosis and prognostic evaluation for hepatocellular carcinoma (HCC).
METHODS: Total RNA was extracted from circulating PBMCs collected from patients with liver diseases. GPC-3 cDNA was synthetized by reverse transcription and amplified by fluorescent quantitative PCR. The levels of circulating ICAM-1 were quantitatively detected by enzyme-linked immunosorbent assay.
RESULTS: The levels of sICAM-1 were increasing during the development of liver diseases, significantly higher in HCC patients than in those with liver cirrhosis (t = 3.184, P = 0.002) or chronic hepatitis (t = 3.962, P < 0.001). sICAM-1 levels were significantly associated with periportal cancerous embolus (t = 2.941, P = 0.005) and extrahepatic metastasis (t = 3.282, P = 0.002), but not with patients' age, sex, AFP level, HBsAg positivity, or tumor size. The positive expression of GPC-3 mRNA in PBMCs was found only in HCC patients (70.9%, 39 of 55), and not in patients with liver cirrhosis, chronic hepatitis, metastatic liver cancer, non-liver tumors, or normal controls (χ2 = 26.773, P < 0.001). The expression of GPC-3mRNA in PBMCs was associated with HBV infection (χ2 = 14.601, P < 0.001), TNM stage (χ2 = 17.732, P < 0.001), periportal cancerous embolus, and extrahepatic metastasis (χ2 = 22.271, P < 0.001), and not with tumor size, tumor number, AFP level or differentiation degree. Combined detection of GPC-3 mRNA and serum AFP level could improve the detection rate of HCC.
CONCLUSION: Detection of circulating sICAM-1 and GPC-3 mRNA in PBMCs could be used for the diagnosis and metastasis monitoring of HCC, especially in AFP-negative patients with HCC.
- Citation: Yao M, Yang JL, Wei DD, Yan XD, Chen J, Yao DF. Clinical significance of circulating sICAM-1 and GPC-3 mRNA expression in the diagnosis and prognostic evaluation of hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2012; 20(21): 1927-1932
- URL: https://www.wjgnet.com/1009-3079/full/v20/i21/1927.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i21.1927
肝细胞癌(hepatocellular carcinoma, HCC)是一种血供丰富、传统疗法易耐受、预后极差的高度恶性实体瘤[1-3]. 虽然HCC诊治进展较快, 但他的转移复发仍是临床上HCC防治的难题. HCC侵袭和转移是肝癌治疗失败和患者致死的主要原因[4,5], 肝癌细胞脱落、侵入基质、进入脉管系统、瘤栓形成、在继发组织器官定位生长及侵袭的多步骤复杂生物学过程中, 多种机制参与转移调控[6,7], 如癌基因表达上调、抑癌基因失活、免疫基因失调、癌细胞间黏附作用丧失、新生血管形成、蛋白水解酶合成、细胞迁移能力增强、癌细胞和基底膜黏附等, 都是促进HCC转移复发的重要因素[8]. 肝癌的预后取决于早期诊断和是否转移. 本文收集肝病患者外周血标本, 定量检测了磷脂酰基醇蛋白多糖-3(glypican-3, GPC-3)mRNA和可溶性细胞间粘附分子(soluble intercellular adhesion molecule-1, sICAM-1)浓度, 分析其临床病理学特征, 探讨对肝癌诊断和转移监测中的临床价值.
收取南通大学附属医院2010-2011年HCC、肝硬化、肝炎正常人新鲜抗凝血5 mL, 包括肝癌患者55例(男46例, 女9例), 平均年龄57.5岁, 经临床、AFP、影像学(B超、CT或MRI检查)和/或病理学确诊; 肝硬化患者20例(男10例, 女10例), 平均年龄55.4岁; 慢性肝炎患者20例(男14例, 女6例), 平均年龄47.5岁; 健康对照20例(男10例, 女10例), 平均年龄53.1岁. 所有病例均有完整病理诊断、住院记录和随访资料, 并按全国肝癌防治协作组制定的标准核实诊断[9].
1.2.1 单核细胞分离与总RNA制备: 采用淋巴细胞分离液(Cedarlane, 加拿大)分离外周血单核细胞. 抗凝血于2 h内, 以淋巴细胞分离液及密度梯度离心法分离有核细胞, 经生理盐水洗涤2次后进行有核细胞计数. 细胞或肝组织50 mg置于无RNAase匀浆器中, 加入TRIzol(MRC, 美国)1.0 mL匀浆2 min; 制备总RNA, 最后加入TE缓冲液100 μL. 经紫外分光扫描鉴定已纯化的RNA未受到污染或降解, 并以紫外分光光度计定量总RNA浓度, 置于-85 ℃保存备用.
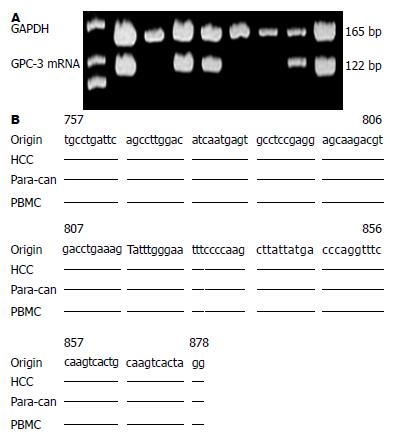
1.2.2 cDNA合成及引物设计: 取总RNA 1.0 μg及cDNA合成试剂盒(Fermentas, 立陶宛), 经随机引物和逆转录酶合成GPC-3 cDNA. 参照文献GPC-3序列, 设计引物并由上海英骏公司(Invitrogen, 美国)合成. GPC-3上游引物为5'-TGCCTGATTCAGCCTTG GAC-3'(nt 757-776, GPC-3P1),下游引物5'-CCTAGTGACTTGCAGTGACTTG-3'(nt 857-878, GPC-3P2), 大小122 bp; GAPDH内参引物: 上游5'-CACTGGC GTCTTCACCACCAT-3'(nt 396-416),下游5'-GTGCAGGAGGCATTGCTGAT-3'(nt 541-560), 扩增产物大小165 bp.
1.2.3 GPC-3 mRNA扩增: cDNA合成后, 进行巢式PCR. 第一阶段PCR反应体系(25 μL): 1 μL cDNA(0.1 μg/μL); 外部引物P1和P2各0.5 μL(20 μmol/L); Premix Taq 12.5 μL, 加双蒸水至2 μL. 扩增条件: 94 ℃预变性5 min; 94 ℃变性25 s, 55 ℃退火30 s, 72 ℃延伸90 s, 30个循环; 72 ℃延伸10 min, 4 ℃保存. 第二阶段PCR反应体系(25 μL): 第一阶段PCR产物(稀释100倍)1 μL; 内部引物P3和P4各0.5 μL(20 mmol/L); PreMix Taq 12.5 μL, 加双蒸水至25 μL. 扩增条件: 94 ℃预变性5 min; 94 ℃变性25 s, 55 ℃退火30 s, 72 ℃延伸90 s, 30个循环; 72 ℃延伸10 min, 4 ℃保存.
1.2.4 GPC-3 mRNA定量分析: 以染料法定量PCR反应体系: SYBR Premix Ex TaqTM 12.5 μL, PCR正向引物(10 μmol/L)和反向引物(10 μmol/L)各0.5 μL, GPC-3 cDNA 2 μL, 双蒸水9.5 μL. 反应条件: 95 ℃ 2 min; 95 ℃ 12 s, 60 ℃ 40 s, 扩增40个循环.
1.2.5 GPC-3 mRNA相对表达率计算: 以GPC-3和GAPDH cDNA的Ct值进行相对定量: △Ct = Ct(目的基因)-Ct(内参基因), 相对表达量 = 2-△Ct; △△Ct = △Ct(实验组)-△Ct(对照组平均值), 相对表达率 = 2-△△Ct.
1.2.6 DNA测序分析: 取含目的DNA凝胶, 以MontageTM DNA装置提取DNA(4 ℃离心, 9 000 r/min离心10 min), 以DYEnamic ET Dye末端循环测序试剂盒制备测序标本. 反应体系: 待测DNA 0.2-2.0 μg, 2 μmol/L引物(上游引物或下游引物)2.5 μL, 测序混合试剂8.0 μL, 加双蒸水至20 μL. 扩增条件: 95 ℃预变性30 s; 95 ℃变性20 s, 50 ℃退火15 s, 60 ℃延伸1 min, 30个循环, 15 ℃保存备用. 产物经醋酸铵、乙醇沉淀, 即可在MegaBACE DNA测序仪上检测DNA序列, 以MegaBACE系统软件(Version 3.0)分析DNA序列, 并与Gene Bank中基因源序列进行比对.
1.2.7 sICAM-1测定及结果计算: 肝病患者按临床诊断资料分为肝癌、肝硬化及正常对照组. 在酶标包被板上待测样品孔中先加已用样品稀释液稀释的样品100 μL, 酶标板加上盖, 37 ℃反应90 min. 甩去酶标板内液体, 用吸水纸拍干, 将准备好的生物素抗人sICAM-1抗体工作液100 μL加入酶标板(空白显色孔除外), 37 ℃反应60 min. 洗涤液洗涤3次, 每次浸泡1 min左右, 每孔加入ABC工作液100 μL(空白显色孔除外), 37 ℃温育30 min. 洗涤液洗涤3次, 每次浸泡1-2 min, 每孔加入显色液90 μL, 37 ℃避光反应17-22 min. 取出酶标板, 每孔加终止液100 μL终止反应, 此时蓝色立转黄色. 在酶标仪上, 以450 nm波长检测其吸光度(A)值. 检测前2 h内配制标准品, 浓度分别为: 10.000、5.000、2.500、1.250、0.625、0.313、0.156及0.000 ng/L, 标准品孔中加入稀释好的标准品100 μL. 参照以上方法同时检测, 将检测的A值和对应的标准浓度绘制标准曲线, 计算样品中sICAM-1的浓度.
统计学处理 计量资料以mean±SD表示, 以t检验分析; 计数资料以χ2检验分析, 以P<0.05表示有显著性差异.
所采集的外周血标本, 各组患者的年龄、HBV感染及AFP浓度等临床资料见表1. 肝癌和肝硬化患者按Child-pugh A、B和C分类, 分别为30、19、6例和11、6、3例. 慢性肝病患者中69例HBsAg阳性(72.6%), 肝癌患者中25例血AFP浓度≥400 μg/L(45.5%).
| 分组 | n | 男/女 | 年龄(岁) | HBsAg(+/-) | AFP(µg/L) | ||
| ≤20 | 21-399 | ≥400 | |||||
| 肝癌 | 55 | 46/9 | 41-81 | 39/16 | 12 | 18 | 25 |
| 肝硬化 | 20 | 10/10 | 20-82 | 14/6 | 16 | 4 | 0 |
| 慢性肝炎 | 20 | 14/6 | 18-80 | 16/4 | 17 | 3 | 0 |
| 正常对照 | 20 | 10/10 | 40-69 | 0/30 | 20 | 0 | 0 |
肝病患者血GPC-3 mRNA和GAPDH基因片段的扩增图谱见图1A. 取PCR产物测序结果显示, 肝癌及癌周组织、外周血PBMC中GPC-3 mRNA扩增片段的序列(图1B)均与Gene Bank中GPC-3基因相应的源序列完全一致, 证实所扩增基因即为GPC-3基因片段.
55例肝细胞癌者血中39例阳性(70.9%, 表2), 在肝硬化、慢性肝炎及健康对照组中未检出阳性例(P<0.001). GPC-3 mRNA在26例不伴肝外转移组阳性率38.46%, GPC-3 mRNA相对表达量为0.0036±0.0013; 29例肝癌伴肝外转移组阳性率为100%, GPC-3 mRNA相对表达量为0.0277±0.0003, 两组相对表达量平均相差7倍以上(t = 3.190, P = 0.003). 健康对照组sICAM-1表达水平低于慢性肝炎(t = 7.331, P<0.001)、肝硬化(t = 8.673, P<0.001)和肝癌(t = 11.476, P<0.001).
| 分组 | n | GPC-3 mRNA(%) | χ2值 | P值 | sICAM-1(µg/L) | t值 | P值 |
| 肝细胞癌 | 55 | 70.9 | - | - | 140.0 ± 31.3 | - | - |
| 肝硬化 | 20 | 0 | 26.773 | <0.001 | 114.8 ± 27.3 | 3.184 | 0.002 |
| 慢性肝炎 | 20 | 0 | 26.773 | <0.001 | 107.0 ± 28.0 | 3.962 | <0.001 |
| 正常对照 | 20 | 0 | 26.773 | <0.001 | 57.5 ± 11.3 | 11.476 | <0.001 |
肝癌患者GPC-3 mRNA检出率与TNM分期(χ2 = 17.732, P<0.001), Ⅲ-Ⅳ肝癌(91.2%)显著高于Ⅰ-Ⅱ肝癌(38.1%); 与HBV相关(χ2 = 14.601, P<0.001), HBsAg阳性肝癌(87.2%)显著高于HBsAg阴性肝癌(31.3%); 肝癌患者伴有门脉癌栓者全数异常(100%)显著高于不伴有门脉癌栓者(20%)及有无肝外转移(χ2 = 22.271, P<0.001)密切相关, 而与性别、年龄、分化程度、AFP浓度和肿瘤大小间未见明显相关(表3), 肝癌患者sICAM-1浓度与肝癌TNM分期(t = 2.750, P = 0.008)、门脉癌栓(t = 2.941, P = 0.005)及肝外转移(t = 3.282, P = 0.002)显著相关.
| 分组 | n | GPC-3 mRNA(n) | sICAM-1(µg/L) | |
| 阳性 | 阴性 | |||
| TNM分期 | ||||
| I-II | 21 | 8 | 13 | 125.6±32.4 |
| III-IV | 34 | 31b | 3 | 148.3±28.0b |
| 分化程度 | ||||
| 高 | 16 | 6 | 10 | 120.3±20.3 |
| 中 | 25 | 15 | 10 | 155.0±38.5 |
| 低 | 14 | 8 | 6 | 134.1±30.1 |
| 肿瘤大小 | ||||
| ≥5 cm | 30 | 21 | 9 | 132.2±31.5 |
| <5 cm | 25 | 18 | 7 | 140.9±29.8 |
| AFP(µg/L) | ||||
| ≥400 | 25 | 18 | 7 | 137.6±29.1 |
| <400 | 30 | 21 | 9 | 142.5±34.0 |
| HBsAg | ||||
| 阳性 | 39 | 34b | 5 | 140.7±30.6 |
| 阴性 | 16 | 5 | 11 | 136.0±37.1 |
| 门脉癌栓 | ||||
| 有 | 35 | 35b | 0 | 153.4±28.7b |
| 无 | 20 | 4 | 16 | 128.5±32.7 |
| 肝内外转移 | ||||
| 有 | 29 | 29b | 0 | 157.1±27.2b |
| 无 | 26 | 10 | 16 | 131.7±30.2 |
肝癌患者sICAM-1、GPC-3 mRNA和AFP异常表达, 对肝癌的诊断价值见表4. 如以sICAM-1>115 μg/L和AFP>50 μg/L为界, 对肝癌的阳性率为74.5%和63.6%, 但在良性肝病有30%左右的假阳性; 外周血GPC-3 mRNA仅见于肝癌患者(70.9%). 从表4可见3种标志物对肝癌诊断率均在70%左右, 如将sICAM-1>115 μg/L、GPC-3 mRNA阳性和AFP>50 μg/L联合诊断肝癌, 其综合评价见表5. 若以GPC-3 mRNA和AFP联合, 阳性率为87.2%(48/55); 若以sICAM-1、GPC-3 mRNA和AFP联检, 阳性率为92.7%(51/55). GPC-3 mRNA、sICAM-1和与AFP分析, 对肝癌具有互补诊断价值.
| sICAM-1(>115 µg/L) | GPC-3mRNA(阳性) | AFP(>50 µg/L) | 三者联合 | |
| 灵敏度 | 74.5 | 70.9 | 63.6 | 92.7 |
| 特异性 | 72.5 | 100 | 87.5 | 67.5 |
| 真阳性 | 76.7 | 100 | 87.5 | 79.6 |
| 真阴性 | 67.4 | 71.4 | 63.6 | 87.0 |
| 准确度 | 73.6 | 83.1 | 73.6 | 82.1 |
AFP诊断肝癌虽被广泛应用, 但单AFP难以满足临床需要, 且敏感性和特异性均难令人满意[10,11]. 随着基因组学、蛋白组学、转录组学等多种"组学"技术的发展, 越来越多与肝细胞恶性转化相关的分子如肝癌特异性蛋白被发现, 可改进肝癌诊断特异性; 肝癌相关基因转录异常, 可监测肝癌微小转移[12,13]. 早期诊断与术后转移复发监测, 仍是肝癌防治研究的热点[14]. 研究发现GPC-3和ICAM-1过表达与肝癌发生发展关系密切[15,16]. 所以, 定量检测肝病患者血GPC-3 mRNA和sICAM-1浓度, 可探讨其临床病理学特征和在肝癌诊断和转移监测中的临床价值.
GPC-3基因位于人染色体X26.10, 由8个外显子组成, 启动子区含多个转录因子结合位点, 核苷酸为2 130 bp, 编码580个氨基酸. 在HCC发生、发展过程中, GPC-3基因被再次激活. GPC-3表达位于胞质和胞膜, 癌旁定位于胞质[17]. 肝癌组织GPC-3表达强度高于癌旁和远癌组织[18]. 肝癌模型发现肝细胞GPC-3过表达与肝细胞恶性转化相关, 是肝细胞恶性改变的早期事件[5,19]. GPC-3过表达与基质金属蛋白酶(matrix metalloproteinases, MMP)显著相关, 抑制GPC-3可下调MMP的表达; GPC-3可致胞核/胞质β-catenin显著增高, 进而与MMP及增殖信号结合, 促进HCC进展[20]. HCC患者中GPC-3 mRNA阳性率为70.9%, 而在肝硬化、肝炎及正常对照组均未检出, 提示GPC-3 mRNA是肝癌特异诊断的有用分子标志物.
分子结构为单链糖蛋白的粘附分子由723-748个氨基酸构成, 胞浆区高度保守, 肝病患者sICAM-1水平与肝细胞坏死严重程度和肝功能受损程度相关. 正常肝细胞缺乏sICAM-1表达, 仅在窦状隙内皮细胞和散在的炎症单核细胞上有ICAM-1微弱表达, 肝病时表达增加. sICAM-1除介导粘附外, 可从癌细胞表面脱落进入循环系统, 可助癌细胞逃逸CTL和NK细胞监视, 促进转移[21]. 肝癌患者sICAM-1水平能反映其病情进展及预后[22]. sICAM-1、GPC-3 mRNA及AFP联合阳性率达92.7%, 对AFP阴性肝癌具有互补诊断价值.
血行转移是肝癌的主要转移途径[6,11], GPC-3 mRNA检出率与TNM分期、门脉癌栓、肝外转移有关. Ⅲ-Ⅳ肝癌显著高于Ⅰ-Ⅱ肝癌; HBsAg阳性肝癌显著高于HBsAg阴性肝癌; 肝癌患者伴有门脉癌栓者全数异常显著高于不伴有门脉癌栓者及肝外转移密切相关; sICAM-1浓度与肝癌TNM分期、门脉癌栓及肝外转移显著相关, sICAM-1和GPC-3 mRNA是监测肝癌转移或术后复发的可靠分子标志[23]. 肝癌早诊早治和术后复发监测, 可能是提高患者存活率的唯一途径. 随着对肝细胞恶性转化关键信号通路的机制阐明, 多种具诊断特异性标志物的应用[24,25], 将更有利于早期发现AFP阴性或低浓度肝癌, 为以分子标志为基础发病机制转化医学和分子靶向治疗研究奠定基础[25-27].
肝细胞癌(HCC)为血供丰富、传统疗法易耐受、术后易复发、预后极差的高度恶性实体瘤. 肝癌虽然诊治进展研究较快, 但其转移复发仍是临床上HCC防治的难题, 术后转移复发监测仍是肝癌防治研究的热点.
唐南洪, 教授, 福建医科大学附属协和医院省肝胆外科研究所
随着基因组学、蛋白组学、转录组学等多种"组学"技术的发展, 与肝细胞恶性转化相关的分子如肝癌特异性蛋白被发现, 肝癌相关基因转录水平的检测可用于肝癌微小转移的临床诊断.
Yao等以肝细胞发生恶性转化的大鼠为模型, 动态观察了磷脂酰基醇蛋白多糖-3(GPC-3) mRNA和GPC-3的表达与改变, 发现GPC-3具有癌胚性, 与肝细胞的恶性转化显著相关, 是肝癌早期诊断的特异性生物标志物.
血行转移是肝癌的主要转移途径, GPC-3 mRNA检出率和sICAM-1异常与肝癌的TNM分期、伴门脉癌栓和肝外转移有关, 两者检测是监测肝癌转移或术后复发的可靠分子标志.
GPC-3基因: 位于人染色体X26.10, 由8个外显子组成, 启动子区含多个转录因子结合位点, 核苷酸为2 130 bp, 编码580个氨基酸, 在HCC发生发展过程中, GPC-3基因被再次激活, 导致癌胚型的GPC-3过表达.
本研究旨在探讨sICAM-1和GPC-3 mRNA在肝癌诊断中的临床价值, 具有一定的科研意义.
编辑: 张姗姗 电编:鲁亚静
| 1. | Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48:2047-2063. [PubMed] [DOI] |
| 2. | Qian J, Yao D, Dong Z, Wu W, Qiu L, Yao N, Li S, Bian Y, Wang Z, Shi G. Characteristics of hepatic igf-ii expression and monitored levels of circulating igf-ii mRNA in metastasis of hepatocellular carcinoma. Am J Clin Pathol. 2010;134:799-806. [PubMed] [DOI] |
| 3. | Zhou JY, Zhang L, Li L, Gu GY, Zhou YH, Chen JH. High hepatitis B virus load is associated with hepatocellular carcinomas development in Chinese chronic hepatitis B patients: a case control study. Virol J. 2012;9:16. [PubMed] [DOI] |
| 4. | Qiao SS, Cui ZQ, Gong L, Han H, Chen PC, Guo LM, Yu X, Wei YH, Ha SA, Kim JW. Simultaneous measurements of serum AFP, GPC-3 and HCCR for diagnosing hepatocellular carcinoma. Hepatogastroenterology. 2011;58:1718-1724. [PubMed] [DOI] |
| 5. | Yao M, Yao DF, Bian YZ, Zhang CG, Qiu LW, Wu W, Sai WL, Yang JL, Zhang HJ. Oncofetal antigen glypican-3 as a promising early diagnostic marker for hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2011;10:289-294. [PubMed] [DOI] |
| 7. | Helaly GF, Abou Shamaa LA. Influence of hepatitis C virus infection on circulating levels of sICAM-1 and VEGF in patients with hepatitis C and hepatocellular carcinoma (HCC) and their role in enhancing detection of HCC. Egypt J Immunol. 2006;13:27-38. [PubMed] |
| 8. | Moriyama M, Matsumura H, Shioda J, Aoki H, Nakamura H, Arakawa Y, Nirei K, Yamagami H, Kaneko M, Tanaka N. Measurement of human intercellular adhesion molecule 1 in the blood is useful for predicting the occurrence of hepatocellular carcinomas from chronic hepatitis C and liver cirrhosis. Intervirology. 2006;49:327-338. [PubMed] [DOI] |
| 10. | Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497-513. [PubMed] [DOI] |
| 12. | Jung CW, Song TJ, Lee KO, Choi SB, Kim WB, Suh SO, Kim YC, Choi SY. Characterization of hepatocellular carcinoma cell lines based on cell adhesion molecules. Int J Mol Med. 2012;29:1158-1164. |
| 14. | Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene. 2010;29:4989-5005. [PubMed] [DOI] |
| 15. | Wang Y, Shen Z, Zhu Z, Han R, Huai M. Clinical values of AFP, GPC3 mRNA in peripheral blood for prediction of hepatocellular carcinoma recurrence following OLT: AFP, GPC3 mRNA for prediction of HCC. Hepat Mon. 2011;11:195-199. [PubMed] |
| 16. | Ho M. Advances in liver cancer antibody therapies: a focus on glypican-3 and mesothelin. BioDrugs. 2011;25:275-284. [PubMed] [DOI] |
| 17. | Yang D, Yang J, Lu F, Li C, Yang J, Liang J. A new membrane re-anchored protein originating from GPC3 against hepatoma cells HepG2. Mol Med Rep. 2011;4:1067-1073. [PubMed] [DOI] |
| 18. | Ozkan H, Erdal H, Koçak E, Tutkak H, Karaeren Z, Yakut M, Köklü S. Diagnostic and prognostic role of serum glypican 3 in patients with hepatocellular carcinoma. J Clin Lab Anal. 2011;25:350-353. [PubMed] [DOI] |
| 19. | Sun CK, Chua MS, He J, So SK. Suppression of glypican 3 inhibits growth of hepatocellular carcinoma cells through up-regulation of TGF-β2. Neoplasia. 2011;13:735-747. [PubMed] [DOI] |
| 20. | Nobuoka D, Motomura Y, Shirakawa H, Yoshikawa T, Kuronuma T, Takahashi M, Nakachi K, Ishii H, Furuse J, Gotohda N. Radiofrequency ablation for hepatocellular carcinoma induces glypican-3 peptide-specific cytotoxic T lymphocytes. Int J Oncol. 2012;40:63-70. [PubMed] |
| 21. | Raskopf E, Gerceker S, Vogt A, Standop J, Sauerbruch T, Schmitz V. Plasminogen fragment K1-3 inhibits expression of adhesion molecules and experimental HCC recurrence in the liver. Int J Colorectal Dis. 2009;24:837-844. [PubMed] [DOI] |
| 22. | O'Beirne J, Farzaneh F, Harrison PM. Generation of functional CD8+ T cells by human dendritic cells expressing glypican-3 epitopes. J Exp Clin Cancer Res. 2010;29:48. [PubMed] [DOI] |
| 23. | Yao DF, Dong ZZ, Yao M. Specific molecular markers in hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2007;6:241-247. [PubMed] |
| 24. | Chan SL, Yeo W. Targeted therapy of hepatocellular carcinoma: present and future. J Gastroenterol Hepatol. 2012;27:862-872. [PubMed] [DOI] |
| 25. | Kudo M. Molecular targeted therapy for hepatocellular carcinoma: bench to bedside. Dig Dis. 2011;29:273-277. [PubMed] [DOI] |
| 26. | Tazi el M, Essadi I, M'rabti H, Touyar A, Errihani PH. Systemic treatment and targeted therapy in patients with advanced hepatocellular carcinoma. N Am J Med Sci. 2011;3:167-175. [PubMed] |