修回日期: 2012-02-18
接受日期: 2012-03-06
在线出版日期: 2012-04-18
目的: 比较血液标志物及胰腺外炎症CT评分(extrapancreatic inflammation on CT score, EPIC)对急性胰腺炎(acute pancreatitis, AP)严重性的早期预测价值.
方法: 对2010-09/2011-09住院的96例AP患者首个24 h内的临床、实验室及CT资料进行分析. 临床上重症急性胰腺炎(severe acute pancreatitis, SAP)的标准为: 死亡或持续器官衰竭及/或入住ICU, 及/或手术治疗. 对重症急性胰腺炎组及轻症急性胰腺炎(mild acute pancreatitis, MAP)组患者血液标志物及胰腺外炎症CT评分进行t检验, 血液标志物及EPIC预测AP严重性的相关性检验及预测AP严重性的ROC分析, 并计算预测敏感性、阳性预测值及准确度.
结果: MAP76例, SAP20例. 重症患者的血液标志物及胰腺外炎症CT评分均明显较轻症患者的大[白细胞: (15.16±5.06)×109/L vs (11.05±1.76)×109/L, 中性粒细胞与淋巴细胞比值: 18.95±12.13 vs 6.63±3.44, 高敏C-反应蛋白: 58.35 mg/L±20.47 mg/L vs 28.59 mg/L±12.92 mg/L, D-二聚体: 1596.95 μg/L±1409.05 μg/L vs 412.52 μg/L±316.66 μg/L, 胰腺外炎症CT评分: 3.30±0.86 vs 1.50±0.96, P = 0.000]. 白细胞、中性粒细胞与淋巴细胞比值、高敏C-反应蛋白、D-二聚体及胰腺外炎症CT评分与AP严重性的Spearman相关系数(rs)分别为0.419、0.571、0.568、0.434及0.61(P = 0.000). 白细胞、中性粒细胞与淋巴细胞比值、高敏C-反应蛋白、D-二聚体及胰腺外炎症CT评分对AP严重性预测的曲线下面积分别为0.798(0.670-0.925)、0.906(0.830-0.981)、0.904(0.838-0.970)、0.808(0.638-0.938)以及0.917(0.851-0.983); 预测敏感性分别为70.00%、85.00%、85.00%、75.00%及85.00%; 阳性预测值分别为58.33%、73.91%、51.52%、48.39%及72.00%; 预测准确度分别为83.33%、90.63%、80.21%、78.13%及90.63%.
结论: 白细胞及D-二聚体对AP严重性的预测价值中等, 中性粒细胞与淋巴细胞比值、高敏C-反应蛋白及胰腺外炎症CT评分的预测价值较高, 其中中性粒细胞与淋巴细胞比值和胰腺外炎症CT评分预测的准确度最高, 胰腺外炎症CT评分与AP严重性的相关系数最大, 其预测AP严重性的受试者曲线下面积最大.
引文著录: 余贤恩. 血液标志物与胰腺外炎症CT评分对急性胰腺炎严重性早期预测的比较. 世界华人消化杂志 2012; 20(11): 969-974
Revised: February 18, 2012
Accepted: March 6, 2012
Published online: April 18, 2012
AIM: To compare the value of blood markers and extrapancreatic inflammation on CT score (EPIC) in early prediction of the severity of acute pancreatitis (AP).
METHODS: The clinical, laboratory and CT data obtained on admission (within 24 h of hospitalization) for 96 patients with AP who were hospitalized from September 2010 to September 2011 were analyzed. Severe AP (SAP) was defined as the presence of one or more of the following signs: mortality, persistent organ failure and/or admission ICU, and/or operation. Blood markers and EPIC were compared between SAP group and mild AP (MAP) using the t test. The correlation between blood markers, EPIC and severity of AP was analyzed. The value of blood markers and EPIC in predicting the severity of AP was assessed using receiver operation curve analysis. The sensitivity, positive predictive value and accuracy were also studied.
RESULTS: There were 20 patients with SAP and 76 patients with MAP. The levels of blood markers and EPIC in the SAP group were significantly higher than those in the MAP group [white blood cell (WBC): (15.16 ± 5.06) × 109/L vs (11.05 ± 1.76) × 109/L, neutrophil-lymphocyte ratio (NLR): 18.95 ± 12.13 vs 6.63 ± 3.44, high-sensitivity C-reactive protein (hs-CRP): 58.35 mg/L ± 20.47 mg/L vs 28.59 mg/L ± 12.92 mg/L, D-dimer (DD): 1596.95 μg/L ± 1409.05 μg/L vs 412.52 μg/L ± 316.66 μg/L, EPIC: 3.30 ± 0.86 vs 1.50 ± 0.96, all P = 0.000]. The Spearman correlation coefficients (rs) between severity of AP and WBC, NLR, hs-CRP, DD and EPIC were 0.419, 0.571, 0.568, 0.434 and 0.613, respectively (all P = 0.000). The area under the curve (AUC) of WBC, NLR, hs-CRP, DD and EPIC in predicting the severity of AP were 0.798 (0.670-0.925), 0.906 (0.830-0.981), 0.904 (0.838-0.970), 0.808 (0.638-0.938) and 0.917 (0.851-0.983), respectively. The predictive sensitivities were 70.00%, 85.00%, 85.00%, 75.00% and 85.00%; the positive predictive values were 58.33%, 73.91%, 51.52%, 48.39% and 72.00%; and the accuracies were 83.33%, 90.63%, 80.21%, 78.13% and 90.63%, respectively.
CONCLUSION: WBC and DD have a moderate value in predicting the severity of AP, while NLR, hs-CRP, and EPIC have a much higher value.
- Citation: Yu XE. Comparative evaluation of blood markers and extrapancreatic inflammatim on CT score in the early prediction of the severity of acute panereatitis. Shijie Huaren Xiaohua Zazhi 2012; 20(11): 969-974
- URL: https://www.wjgnet.com/1009-3079/full/v20/i11/969.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v20.i11.969
急性胰腺炎(acute pancreatitis, AP)是消化系疾病中第3位常见疾病, 是由胆系疾病、酒精、高脂血症、肥胖等引起的全身炎症性疾病. 大多数AP是轻症并多为自限性, 而约1/5患者会发展为重症急性胰腺(severe acute pancreatitis, SAP). SAP严重影响患者康复, 其病死率达20%-30%, 所以早期预测AP严重性以及给予相应处理显得很重要[1], 而采取哪些便捷方法能尽早识别及预测SAP是对AP患者进行科学分类、合理治疗、减少病死率及促进康复的关键. 多种单个因素如血糖、C-反应蛋白(C-reactive protein, CRP)、白蛋白、红细胞压积等均能评估AP严重性[2]. Azab等[3]报道, 中性粒细胞(neutrophil, N)与淋巴细胞(lymphocyte, L)比值较白细胞(white blood cell, WBC)总数更能预测AP患者入住ICU及住院天数. Prongprasobchai等[4]报道, 在患者入院36 h, CRP≥150 mg/L对AP严重性预测的敏感性为86%, 特异性为87%, 阳性预测值为75%. Ke等[5]报道, D-二聚体(D-Dimer, DD)是预测AP严重性的一个易操作、价廉的指标. Ke等[5]研究发现, 患者入院24 h内CT评分对AP严重性预测准确度与临床多因素评分系统的预测结果相类似. 本研究拟比较WBC、N与L比值、CRP、DD和胰腺外炎症CT评分(extrapancreatic inflammation on CT score, EPLC)对AP严重性预测情况并探索各种因素预测的敏感性、特异性、阳性拟然比、阳性预测值及准确度.
对2010-09/2011-09住院的96例AP患者资料进行回顾性分析. 纳入病例符合中华医学会胰腺病学组2003年制定《中国急性胰腺炎诊疗指南(草案)》的标准, 临床上诊断SAP的条件为: 死亡或持续器官衰竭及/或入住ICU, 及/或手术治疗[6]. 患者年龄22-81岁, 男77例, 女19例. 轻症急性胰腺炎(mild acute pancreatitis, MAP)76例, SAP 20例, 其中肾衰12例, 呼衰8例, 手术6例, 死亡4例. 排除感染病例.
患者入院24 h内采取外周静脉血2 mL, 采用ABX DX120全自动血球仪及ABX配套试剂进行血常规检查, 采用颗粒增强免疫透射比浊法[试剂盒由德赛诊断系统(上海)有限公司提供]进行高敏CRP(higher sensitivity CRP, hs-CRP)检查及采用Destiny Max血凝仪(光学比浊法)进行DD检查. 患者入院24 h内接受64排螺旋CT进行腹部扫描检查及EPIC评分[7].
统计学处理 SAP组及MAP组患者的血WBC总数、N与L比值、hs-CRP浓度、DD浓度及EPIC评分结果以mean±SD表示, 使用SSPS17.0对数据进行t检验、相关性检验及ROC分析, 以P<0.05为差异具有统计学意义. 并分别计算各标志物及EPIC对SAP预测的敏感性、特异性、阳性拟然比、阳性预测值及准确度.
96例AP患者中, SAP组患者的WBC总数、N与L比值、hs-CRP浓度、DD浓度及EPIC数值均较MAP组患者的相应数值高, 经t检验, 均具有明显差异(P = 0.000, 表1).
| 分组 | n | WBC (×109/L) | N与L比值 | hs-CRP(mg/L) | DD(μg/L) | EPIC |
| SAP组 | 20 | 15.16±5.06 | 18.95±12.13 | 58.35±20.47 | 1596.95±1409.05 | 3.30±0.86 |
| MAP组 | 76 | 11.05±1.76 | 6.63±3.44 | 28.59±12.92 | 412.52±316.66 | 1.50±0.96 |
| P值 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
2.2 AP患者血液标志物及EPIC预测AP严重性的相关性检验
AP患者WBC总数、N与L比值、hs-CRP浓度、DD浓度和EPIC数据经非参数检验(Npar test)结果为不服从正态分布资料, 采用Spearman相关性检验显示WBC总数、N与L比值、hs-CRP浓度、DD浓度和EPIC数值与AP严重性的相关系数(rs)分别为0.419、0.571、0.568、0.434及0.613(P值均为0.000), 即这些指标均与AP严重性明显相关. 而且WBC总数与N与L比值的rs为0.391(P = 0.000), 即N与L比值大小与WBC总数明显相关.
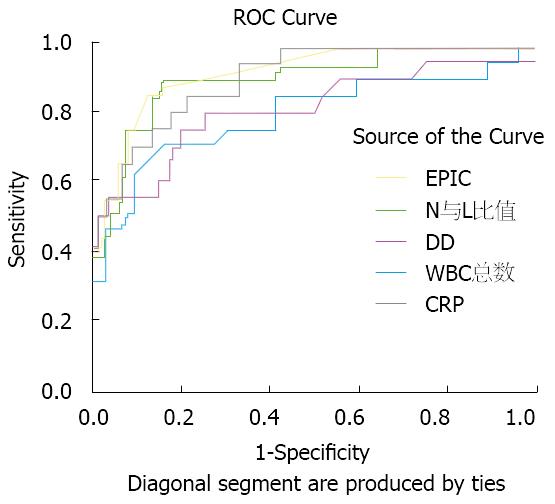
AP患者的WBC总数、N与L比值、hs-CRP浓度、DD浓度及EPIC数值预测AP严重性的ROC曲线下面积(area under the cure, AUC)分别为0.798(95%CI: 0.670-0.925)、0.906(95%CI: 0.830-0.981)、0.904(0.838-0.970)、0.808(0.638-0.938)、0.917(0.851-0.983). 其中以EPIC的AUC最大, 即EPIC的预测有效性最大. AP患者的WBC总数、N与L比值、hs-CRP浓度、DD浓度及EPIC数值预测AP严重性的ROC曲线见图1.
在WBC总数、N与L比值、hs-CRP浓度、DD浓度及EPIC预测SAP的敏感性、特异性、阳性拟然比、阳性预测值及准确度的比较中, 以N与L比值及EPIC预测的特异性及准确性较高, 均超过90%(表2).
| 实验室指标 | AUC | 预测分界值 | 敏感性(%) | 特异性(%) | 阳性拟然比 | 阳性预测值(%) | 准确度(%) |
| WBC总数 | 0.798 | 12.55×109/L | 70.00 | 86.84 | 5.32 | 58.33 | 83.33 |
| N与L比值 | 0.906 | 7.85 | 85.00 | 92.11 | 10.77 | 73.91 | 90.63 |
| hs-CRP | 0.904 | 37.85 mg/L | 85.00 | 78.95 | 4.04 | 51.52 | 80.21 |
| DD | 0.808 | 616.00 μg/L | 75.00 | 78.95 | 3.56 | 48.39 | 78.13 |
| EPIC | 0.917 | 2.5 | 85.00 | 90.79 | 9.77 | 72.00 | 90.63 |
AP是以胰腺病变并全身性的炎症性疾病, 其发病机制是胰腺酶原激活、炎症介质释放、细胞因子产生, 导致多个系统、器官、组织及细胞受到影响, 可引起20% AP患者发生SAP, 而使病死率升高达20%-30%[2]. 对AP严重性进行早期识别, 以便于早期有效治疗, 改善SAP预后[1]. 多年来对AP严重性的预测方法进行了广泛研究, 分别建立了Ranson评分、APACHEⅡ评分、Glosgow评分等, 尽管这些评分标准都具有较好的预测价值, 但这些评分系统的评分项目较多, 操作不易. 所以对AP严重性的简捷预测方法一直处于探索之中, 临床上多种单一因素已用于对AP严重性的评估[2], 并取得了较好的结果.
WBC是AP的重要炎症细胞, Ramudo等[8]研究中发现外周血循环WBC在大鼠实验性AP中具有重要作用. WBC中的N具有趋化、粘附、吞噬和杀菌等作用. AP时细胞因子生成增多, 使N表达抗凋亡蛋白A1、Bcl-x1等, 延迟N凋亡, 而且caspase-2也抑制N凋亡[9], SAP患者的核转录因子κB(NK-κB)也延迟N凋亡[10]. N数量增加后, 在趋化因子作用下聚集并粘附于胰腺腺泡细胞, 就会释放髓过氧化物酶、吞噬素、溶菌酶、β葡萄糖苷酸酶、碱性磷酸酶等, 并激活胰蛋白酶原[11], 导致胰腺自身消化并进一步损伤及加重炎症反应. Azab等[3]报道, N与L比值较WBC总数更能预测AP患者入住ICU及住院天数等情况. Dambrauskas等[12]对发作72 h内的108例AP患者进行研究发现, SAP具有典型的全身炎症反应及促细胞因子(IL-6、IL-8及巨噬细胞迁移抑制因子等)过度表达, 而且重症及坏死性AP的外周血淋巴细胞枯竭. 所以, SAP患者的外周血L明显减少, 那么就可能导致N与L的比值增大. 我院研究显示, SAP组较MAP组的WBC总数明显多[(15.16±5.060×109/L vs (11.05±1.76)×109/L, P = 0.000], 而且Spearman相关性检验显示WBC总数与AP严重性的rs为0.419(P = 0.000), 说明WBC总数与AP严重性明显相关. 研究还显示WBC总数与N与L比值的rs为0.391(P = 0.000), 即N与L比值大小与WBC总数明显相关. 而N与L比值与AP严重性的rs为0.571(P = 0.000), 说明两者明显相关, 且SAP组的N与L比值较MAP组的高(18.95±12.13 vs 6.63±3.43, P = 0.000), 说明WBC总数越大, N与L比值越大, AP就越严重, 这与文献报道相类似. 我院研究显示预测SAP的N与L比值为7.85, 更与文献报道的N与L比值≥7.6时患者入住ICU及住院时间延长的情况相一致. 经ROC分析, WBC预测SAP的AUC为0.798(95%CI: 0.670-0.925), 而N与L比值预测SAP的AUC为0.906(95%CI: 0.830-0.981), 两者预测AP严重性的敏感性、特异性、阳性拟然比、阳性预测值、准确度分别为70.00%、86.84%、5.32、58.33%、83.33%和85.00%、92.11%、10.77、73.91%、90.63%. 显示N与L比值较WBC总数更准确预测AP严重性.
CRP是由多种细胞因子等炎症分子剌激肝脏细胞合成的五聚体蛋白, 是炎症反应的急时象性蛋白, 是非特异炎症的标志物, 也用于AP的评估[13]. 尽管Andersson等[14]研究报道, CRP预测SAP的敏感性较组织因子(tissue factor, TF)的预测值低(AUC为0.653 vs 0.775), 但是Dambrauskas等[15]报道认为CRP仍然是预测SAP最有用的生化因子, 其具有准确、价廉及便捷的特点. 我院研究显示, hs-CRP与AP严重性的相关系数rs为0.568(P = 0.000), 说明CRP浓度越高, AP越严重, 明显相关. 研究还显示hs-CRP预测SAP的AUC为0.904(0.838-0.970), 表明其具有较高的预测价值. 我院研究显示CRP预测SAP的AUC为0.904, 而Andersson等[14]研究CRP预测SAP的AUC为0.653, 这种差异可能与我院是检测hs-CRP而非普通CRP有一定的关系. Gürleyik等[16]报道, 在患者入院48 h CRP≥150 mg/L对AP严重性预测的敏感性为85%, 特异性为74%, 阳性预测值为50%, 阴性预测值为94%, 准确度76%. 2010年Pongrasobchai等[4]报道, 在患者入院36 h, CRP≥150 mg/L对AP严重性预测敏感性86%、特异性87%、阳性预测值75%、阴性预测值为93%. Kim等[17]对119例住院的AP患者研究表明, CRP切割值为83 mg/L时, 患者持续疼痛的阳性拟然比为3.84, CRP可用于评估AP的严重性. 我院研究显示, 患者入院24 h内, hs-CRP预测分界值为37.85 mg/L时对SAP预测的敏感性、特异性、阳性拟然比、阳性预测值、准确度分别为85.00%、78.95%、4.04、51.52%及80.21%, 与文献报道相类似. 而且, Wang等[18]报道, 在AP患者入院24 h内CRP>170 mg/L及白蛋白<30 g/L增加SAP住院患者病死率, 即高浓度的CRP还预示高病死率.
AP时由于液体渗出等改变, 导致器官组织的血液循环障碍及体内纤溶功能改变或高凝状态. 而DD是继发纤溶时交联纤维蛋白特异性的降解产物, 其在炎症反应及器官衰竭时大量增加, 所以DD是炎症反应的标志物之一. Ke等[5]报道, DD对预测AP严重性是一个易操作、价廉的指标, 在SAP患者, DD浓度为正常值高限的6倍[19], DD是判断SAP合并器官衰竭的敏感指标[20]. 我院研究显示, DD与AP严重性相关系数rs为0.434(P = 0.000), 也表明两者明显相关, 而且入院24 h内, DD预测AP严重性的AUC为0.808(0.638-0.938), DD在预测分界值为616 μg/L时其预测SAP的敏感性、特异性、阳性拟然比、阳性预测值及准确度分别为75.00%、78.95%、3.56、48.39%及78.13%, 属于中度预测水平.
CT在AP诊断、监测及严重性评估方面具有关键性的作用[13], CT平扫、增强CT成像或动态CT检查在能准确诊断胰腺及胰周炎症, 并用于AP病情评估及预后判断[21-24]. Gürleyik等[16]报道, CT严重指数(CT severity index, CTSI)对AP严重性预测的敏感性85%, 特异性98%, 阳性预测值92%, 阴性预测值为95%, 准确性为95%, 优于APACHE-Ⅱ对SAP的预测值. Papachristou等[25]报道, BIAP、Ranson评分、APACHE-Ⅱ评分及CTSI预测SAP的AUC分别为0.81、0.94、0.78及0.84. 最近Bollen等[6]报道, 入院第1天CT检查对AP严重性的预测准确性与临床多系统评分的预测价值相类似, 在Balthazar CT评分、CTSI、改良CT严重指数(modified CTSI, MCTSI)、胰腺体积指数、胰腺外评分、EPIC、肠系膜水肿及腹腔积液评分等CT评分预测系统中, Balthazar CT评分及CTSI具有最高的准确性, 但无统计学差异. Bollen等[26]也报道, CTSI及MCTSI与AP严重性明显相关(P<0.0001), 两者诊断SAP的准确性优于APACHE-Ⅱ(AUC为0.87). Heiss等[27]报道, CT检查发现胰腺坏死以及肾后间隙和结肠后积液都与SAP病死率有关, 这些CT影像可以预测SAP的预后. Imamura等[28]报道, AP患者腹部CT检查后的肾脏外周分级(1-3)可用来评估AP严重性, 肾脏外周分级评分与Balthazar评分、CTSI、Ranson评分及APACHE-Ⅱ评分对SAP及死亡率预测的AUC价值相当. 也说明CT对胰腺外炎症的判定结果对于SAP的预测具有很高的价值. De Waele等[7]研究报道, EPIC评分优于Balthazar CT评分及CTSI, 当EPIC评分≥4时, 对AP严重性预测的敏感性100%, 特异性为70.8%. 我院研究显示EPIC与AP严重性的相关系数rs为0.613(P = 0.000), 两者明显相关, EPIC预测SAP的AUC为0.917(0.851-0.983), 具有很高的价值. 在EPIC>2.5时, 其预测SAP敏感性为85%、特异性为90.79%、阳性拟然比为9.77, 阳性预测值为72.00%, 准确度90.63%, 与文献报道类似, 即EPIC对AP严重性有很高的预测价值.
我院研究显示, 在WBC、N与L比值、hs-CRP、DD及EPIC对AP严重性预测价值比较中, 各指标与AP严重性的相关系数rs分别为0.419、0.571、0.568、0.434及0.613, 以EPIC的相关系数最大, 而各指标预测AP严重性的AUC分别为0.798、0.906、0.904、0.808及0.917, N与L比值、hs-CRP及EPIC预测的AUC值均超过0.9, 说明这3个指标的预测价值较高, 而以EPIC的预测价值最高. N与L比值及EPIC预测SAP的准确度最高, 均为90.63%.
总之, 我院研究表明, SAP患者的血液标志物及EPIC数值均明显较MAP患者的相应指标大, WBC及DD对AP严重性预测价值中等, N与L比值、hs-CRP及EPIC对AP严重性预测价值较高, 其中N与L比值和EPIC预测的准确度最高, EPIC与AP严重性的相关性最大, 其预测的AUC最高. 所以, 可以单独或联合使用这些指标对AP严重性进行早期预测, 以便及时有效治疗AP并改善其预后.
急性胰腺炎(acute pancreatitis, AP)是消化系统第3位常见疾病, 其中约20%发展为重症急性胰腺炎(SAP), 而后者的病死率高达20%-30%, 严重影响人们健康. 所以早期识别及早期预测重症病例对于积极而恰当的治疗及改善其预后具有重要意义.
黄颖秋, 教授, 本溪钢铁(集团)总医院消化内科
目前对预测AP严重性的研究主要采用血液或尿液单个指标、临床多因素评分系统、CT评分系统、磁共振检查以及超声内镜检查等, 而采用哪个或哪些简便、准确、有效的早期预测方法是研究的热点之一.
本研究表明重症与轻症AP患者的血液标志物及胰腺外炎症CT评分(EPIC)有显著差异, EPIC与AP严重性的相关系数最大, 而EPIC预测AP严重性的受试者曲线下面积也最大, 中性粒细胞与淋巴细胞比值及EPIC对AP严重性预测准确性最高, 这对早期预测AP严重性具有重要的临床意义.
该文采用中性粒细胞与淋巴细胞比值和胰腺外炎症CT评分预测急性胰腺炎的严重程度, 对临床有一定参考价值.
编辑: 张姗姗 电编: 鲁亚静
| 1. | Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World J Gastroenterol. 2009;15:5260-5265. [PubMed] [DOI] |
| 2. | Brisinda G, Vanella S, Crocco A, Mazzari A, Tomaiuolo P, Santullo F, Grossi U, Crucitti A. Severe acute pancreatitis: advances and insights in assessment of severity and management. Eur J Gastroenterol Hepatol. 2011;23:541-551. [PubMed] [DOI] |
| 3. | Azab B, Jaglall N, Atallah JP, Lamet A, Raja-Surya V, Farah B, Lesser M, Widmann WD. Neutrophil-lymphocyte ratio as a predictor of adverse outcomes of acute pancreatitis. Pancreatology. 2011;11:445-452. [PubMed] [DOI] |
| 4. | Pongprasobchai S, Jianjaroonwong V, Charatcharoenwitthaya P, Komoltri C, Tanwandee T, Leelakusolvong S, Pausawasdi N, Srikureja W, Chainuvati S, Prachayakul V. Erythrocyte sedimentation rate and C-reactive protein for the prediction of severity of acute pancreatitis. Pancreas. 2010;39:1226-1230. [PubMed] [DOI] |
| 5. | Ke L, Ni HB, Tong ZH, Li WQ, Li N, Li JS. D-dimer as a marker of severity in patients with severe acute pancreatitis. J Hepatobiliary Pancreat Sci. 2012;19:259-265. [PubMed] |
| 6. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. A comparative evaluation of radiologic and clinical scoring systems in the early prediction of severity in acute pancreatitis. Am J Gastroenterol. 2012;107:612-619. [PubMed] |
| 7. | De Waele JJ, Delrue L, Hoste EA, De Vos M, Duyck P, Colardyn FA. Extrapancreatic inflammation on abdominal computed tomography as an early predictor of disease severity in acute pancreatitis: evaluation of a new scoring system. Pancreas. 2007;34:185-190. [PubMed] [DOI] |
| 8. | Ramudo L, Yubero S, Manso MA, Recio JS, Weruaga E, De Dios I. Effect of dexamethasone on peripheral blood leukocyte immune response in bile-pancreatic duct obstruction-induced acute pancreatitis. Steroids. 2010;75:362-367. [PubMed] [DOI] |
| 9. | Nakamura Y, Do JH, Yuan J, Odinokova IV, Mareninova O, Gukovskaya AS, Pandol SJ. Inflammatory cells regulate p53 and caspases in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2010;298:G92-G100. [PubMed] [DOI] |
| 10. | Yubero S, Ramudo L, Manso MA, De Dios I. Mechanisms of dexamethasone-mediated chemokine down-regulation in mild and severe acute pancreatitis. Biochim Biophys Acta. 2009;1792:1205-1211. [PubMed] [DOI] |
| 11. | Abdulla A, Awla D, Thorlacius H, Regnér S. Role of neutrophils in the activation of trypsinogen in severe acute pancreatitis. J Leukoc Biol. 2011;90:975-982. [PubMed] [DOI] |
| 12. | Dambrauskas Z, Giese N, Gulbinas A, Giese T, Berberat PO, Pundzius J, Barauskas G, Friess H. Different profiles of cytokine expression during mild and severe acute pancreatitis. World J Gastroenterol. 2010;16:1845-1853. [PubMed] [DOI] |
| 13. | Pezzilli R, Zerbi A, Di Carlo V, Bassi C, Delle Fave GF. Practical guidelines for acute pancreatitis. Pancreatology. 2010;10:523-535. [PubMed] [DOI] |
| 14. | Andersson E, Axelsson J, Eckerwall G, Ansari D, Andersson R. Tissue factor in predicted severe acute pancreatitis. World J Gastroenterol. 2010;16:6128-6134. [PubMed] [DOI] |
| 15. | Dambrauskas Z, Gulbinas A, Pundzius J, Barauskas G. Value of the different prognostic systems and biological markers for predicting severity and progression of acute pancreatitis. Scand J Gastroenterol. 2010;45:959-970. [PubMed] [DOI] |
| 16. | Gürleyik G, Emir S, Kiliçoglu G, Arman A, Saglam A. Computed tomography severity index, APACHE II score, and serum CRP concentration for predicting the severity of acute pancreatitis. JOP. 2005;6:562-567. [PubMed] |
| 17. | Kim YS, Lee BS, Kim SH, Seong JK, Jeong HY, Lee HY. Is there correlation between pancreatic enzyme and radiological severity in acute pancreatitis? World J Gastroenterol. 2008;14:2401-2405. [PubMed] [DOI] |
| 18. | Wang X, Cui Z, Li H, Saleen AF, Zhang D, Miao B, Cui Y, Zhao E, Li Z, Cui N. Nosocomial mortality and early prediction of patients with severe acute pancreatitis. J Gastroenterol Hepatol. 2010;25:1386-1393. [PubMed] [DOI] |
| 19. | Kong H, Ding Z, Zhu XC, Gao XY, Wu J, Qian W, Wang CY, Hou XH. d-Dimer change in human acute pancreatitis as determined by serumal triglyceride. Pancreas. 2011;40:1103-1106. [PubMed] [DOI] |
| 20. | Radenkovic D, Bajec D, Ivancevic N, Milic N, Bumbasirevic V, Jeremic V, Djukic V, Stefanovic B, Stefanovic B, Milosevic-Zbutega G. D-dimer in acute pancreatitis: a new approach for an early assessment of organ failure. Pancreas. 2009;38:655-660. [PubMed] [DOI] |
| 21. | Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World J Gastroenterol. 2007;13:5043-5051. [PubMed] [DOI] |
| 22. | Ocampo C, Zandalazini H, Kohan G, Silva W, Szelagowsky C, Oría A. Computed tomographic prognostic factors for predicting local complications in patients with pancreatic necrosis. Pancreas. 2009;38:137-142. [PubMed] [DOI] |
| 23. | Takeda K, Yokoe M, Takada T, Kataoka K, Yoshida M, Gabata T, Hirota M, Mayumi T, Kadoya M, Yamanouchi E. Assessment of severity of acute pancreatitis according to new prognostic factors and CT grading. J Hepatobiliary Pancreat Sci. 2010;17:37-44. [PubMed] [DOI] |
| 24. | Bharwani N, Patel S, Prabhudesai S, Fotheringham T, Power N. Acute pancreatitis: the role of imaging in diagnosis and management. Clin Radiol. 2011;66:164-175. [PubMed] [DOI] |
| 25. | Papachristou GI, Muddana V, Yadav D, O'Connell M, Sanders MK, Slivka A, Whitcomb DC. Comparison of BISAP, Ranson's, APACHE-II, and CTSI scores in predicting organ failure, complications, and mortality in acute pancreatitis. Am J Gastroenterol. 2010;105:435-441; quiz 442. [PubMed] [DOI] |
| 26. | Bollen TL, Singh VK, Maurer R, Repas K, van Es HW, Banks PA, Mortele KJ. Comparative evaluation of the modified CT severity index and CT severity index in assessing severity of acute pancreatitis. AJR Am J Roentgenol. 2011;197:386-392. [PubMed] [DOI] |
| 27. | Heiss P, Bruennler T, Salzberger B, Lang S, Langgartner J, Feuerbach S, Schoelmerich J, Hamer OW. Severe acute pancreatitis requiring drainage therapy: findings on computed tomography as predictor of patient outcome. Pancreatology. 2010;10:726-733. [PubMed] [DOI] |