修回日期: 2011-08-10
接受日期: 2011-08-17
在线出版日期: 2011-08-18
目的: 建立一种新颖的高通量二维细胞识别、定位技术, 为肝癌干细胞的鉴定、异质性分化细胞分类等提供技术平台.
方法: 收集原发性肝细胞癌患者手术标本1例, 每个标本分别石蜡包埋, 以1 μm的厚度连续切5片. 每张切片取第一片做常规HE染色, 病理诊断. 其余4张切片分别以肝癌组织中目前已报道的8个可能的肝癌干细胞标志物进行了免疫荧光标记, 利用试剂Hoechst33342(蓝色)标记细胞核定位, 异硫氰酸荧光素(fluorescein isothiocyanate, FITC), 四甲基异硫氰酸罗丹明(tetramethyl rhodamine isothiocyanate rhodamine)双荧光标记待测抗体, 荧光显微镜检测荧光位置及强度.
结果: 以4个切片中均能识别的细胞核做定位, 确定每个切片1个1×100显微镜视野中有效细胞的个数, 作出4个切片合并的细胞图谱, 建立了一种新颖的二维细胞识别技术. 检测得到有效细胞为2 772个. 检测的8个抗体均在肝癌细胞中表达, 并且不同肝癌细胞中肝癌干细胞标志物的表达情况不同, 同一肝癌细胞可同时表达多个肝癌干细胞的分子标志, 且可见8个抗体均阳性的肝癌细胞. 其中 2 772个有效细胞中有2 453个细胞为阴性, 所占比例为884.9‰.
结论: 完成了高通量二维细胞识别、定位技术方法的建立, 该方法可在一个肝癌干细胞上检测两种以上的肝癌干细胞分子标志的表达情况.
引文著录: 朱玉珍, 符达, 刘黎黎, 马雨水, 沈锡中, 陈锡美. 肝细胞癌中一种高通量二维细胞识别技术方法的建立. 世界华人消化杂志 2011; 19(23): 2455-2460
Revised: August 10, 2011
Accepted: August 17, 2011
Published online: August 18, 2011
AIM: To establish a novel high-throughput two-dimensional screening technique for cellular recognition and localization in hepatocellular carcinoma (HCC).
METHODS: HCC specimen was collected from a patient who underwent radical resection. The paraffin-embedded specimen was serially sectioned at a thickness of 1 μm. Five serial sections were used for staining: one for H&E staining and the other four for immunofluorescence staining for detecting eight reported liver cancer stem cell (LCSC) markers. Fluorescein isothiocyanate (FITC) and tetramethyl rhodamine isothiocyanate (rhodamine) were used for fluorescent imaging for double staining. The sections were counterstained with Hoechst33342 to demonstrate the nuclei for cellular localization. Fluorescence microscopy was used to detect the fluorescence intensity and localization.
RESULTS: We identified the valid number of cells in a visual field (1 × 100) of microscopy and delineated the merged cell map. The results showed that 8 LCSCs biomarkers could be detected in 2 772 valid cells. The expression levels of biomarkers were different in these cells and a single valid cell could express 0-8 biomarkers. No biomarkers could be detected in 2 453 cells (88.5%).
CONCLUSION: A high-throughput two-dimensional screening technique for cellular recognition and localization has been successfully developed and can be used to detect the expression of two or more LCSC markers in one liver cancer cell.
- Citation: Zhu YZ, Fu D, Liu LL, Ma YS, Shen XZ, Chen XM. A high-throughput two-dimensional screening technique for cellular recognition and localization in hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2011; 19(23): 2455-2460
- URL: https://www.wjgnet.com/1009-3079/full/v19/i23/2455.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i23.2455
随着对肿瘤干细胞研究的深入, 肿瘤干细胞理论已经逐渐受到广泛认可. 肿瘤干细胞分子标志物在乳腺癌、前列腺癌、结肠癌、神经胶质瘤、白血病、肺癌等肿瘤中已确立[1-6], 但是就肝癌干细胞的分子标志物而言, 目前的研究证据尚不能完全证实肝癌干细胞(liver cancer stem cell, LCSC)的存在. 肝癌中已证实有一小群具有干细胞样潜能的肿瘤细胞的存在, 并发现了一些人肝癌干细胞可能相关的生物标记, 包括侧群细胞[7-9]、CD133[10-14]、CD133+/CD44+[15]、CD133+/CD45-[16]、CD90[17]、CD90+/CD44+[18]、OV6[19]、AFP+/EpCAM+[20-23]、ALDH[24], 但尚无一致结论. 上述肝癌干细胞可能的标志物及相关标志物组合有8个, 但不同实验室的结果不能相互印证, 免疫组织化学、免疫荧光技术一次做多能标识两个抗体于同一细胞, 在同一细胞标记8个相关抗体目前技术仍无法达到. 因此, 我们利用组织免疫荧光、荧光显微镜检测技术建立一种新的高通量的二维细胞识别技术.
Hoechst33342染料(Sigma公司, 工作浓度1∶1 000), 异硫氰酸荧光素(fluorescein isothiocyanate, FITC), 四甲基异硫氰酸罗丹明(tetramethyl rhodamine isothiocyanate rhodamine), 80i正置荧光显微镜荧光显微镜(Nikon公司), 抗体ABCG2, AFP, CD133, c-Kit, CD34, CD44(PTG公司).
1.2.1 肝癌组织固定, 包埋与切片: 收集上海复旦大学附属中山医院原发性肝细胞癌患者手术标本1例, 每个标本经40 g/L的中性甲醛固定, 分别石蜡包埋, 以1 μm的厚度连续切5片. 每张切片取第一片做常规HE染色, 病理诊断. 其余4张切片分别以肝癌组织中目前已报道的8个可能的肝癌干细胞标志物进行了免疫荧光双标记.
1.2.2 标本脱蜡及水化: 将组织切片于恒温烘箱孵育半小时, 在二甲苯中浸3次, 每次10 min. 脱蜡后切片依次浸入1 000 mL/L、950 mL/L、700 mL/L梯度乙醇中脱二甲苯, 每次2 min. 至蒸馏水中浸5 min, 0.01 mol/L的PBS洗3次, 每次5 min.
1.2.3 抗原修复及封闭: 取出切片, 放置于3%过氧化氢溶液中, 室温孵育15 min, 0.01 mol/L的PBS振洗2次, 每次5 min. 取出标本, 放入95 ℃预热的抗原修复液(柠檬酸抗原修复液, pH6.0), 中低火煮沸15 min, 冷却至室温, 0.01 mol/L的PBS振洗3次, 每次3 min. 取出切片, 擦干切片组织周围的液体, 放置于湿盒中, 每份标本加入10%正常山羊血清, 每张片子约50 μL, 室温孵育30 min.
1.2.4 加入抗体及封片: 将切片的液体甩干, 滴加对应的一抗于组织上. 切片加入一抗后, 置于湿盒内, 4 ℃过夜. 取出后37 ℃复温60 min, 0.01 mol/L的PBS振洗3次, 每次5 min; Hoechst 33342(1∶100)染核. 将切片的液体甩干, 滴加对应的二抗于组织上, 二抗为1∶1混合FITC标记的羊抗兔IgG和罗丹明标记羊抗小鼠IgG, 37 ℃, 40 min. 0.01 mol/L的PBS振洗3次, 每次5 min, 10%缓冲甘油封片. 设空白对照(无一抗, 加PBS)和抗体特异性对照(只加一抗不加二抗或只加二抗不加一抗), 所有切片同时染色. 抗体名称及组合如下: EPCAM/AFP, CD90/CD44, ABCG2/CD133, c-Kit/CD34.
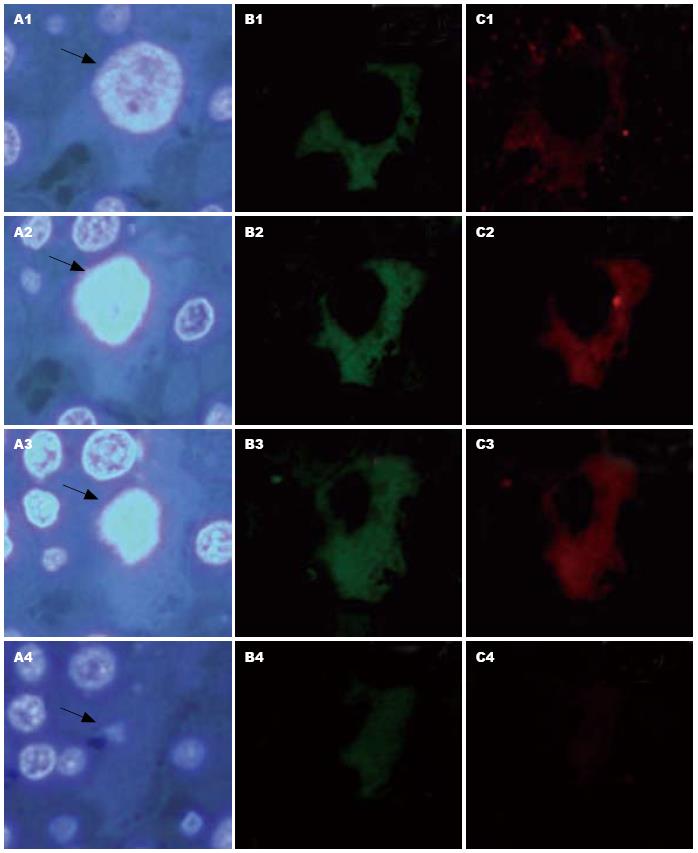
1.2.5 荧光显微镜检测荧光位置及强度: 细胞定位及计数: 以4个连续切片中均能识别的细胞核做定位, 记为有效细胞, 确定每个切片1个1×100显微镜视野中有效细胞的个数. 于中山医院中心实验室Nikon 80i正置荧光显微镜下4张连续切片分别观察, 于1个1×100的显微镜视野中每张切片均选择相同的视野, 拍照、保存. 用A3纸打印出图片, 4张纸都用3 mm的长度或宽度分别列出坐标, 横向为: X1, X2, X3...X99; 竖向为Y1, Y2, Y3...Y140(A3纸长宽分别为297 mm和420 mm), 4张切片中的相同坐标部位均有细胞的存在才能记为一个有效细胞. 在原图片进行荧光半定量计数, 按荧光强弱分为+++, ++, +, 阴性.
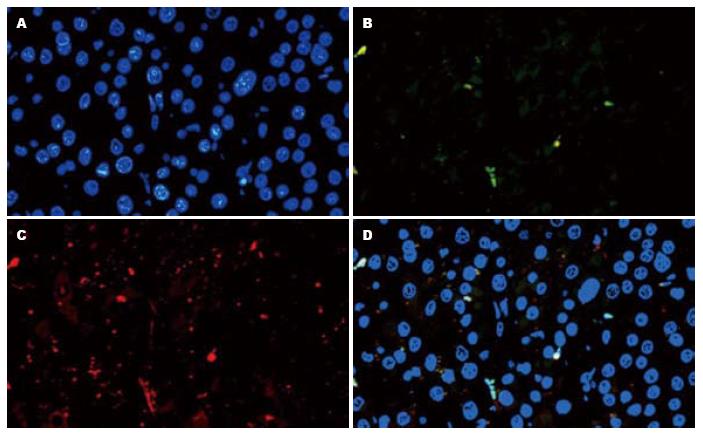
肝癌组织经组织免疫荧光后, 于×400的荧光显微镜下观察拍照. 图1为肝癌组织切片的EpCAM和AFP检测示意图. 肝癌组织切片利用试剂Hoechst33342(蓝色)标记细胞核定位, FITC(绿色)和Rhodamine(红色)分别标记EpCAM和AFP二抗, 荧光显微镜检测蓝, 绿, 红的成像再利用Nikon 80i正置荧光显微镜自带软件做出相关合成图.
4张切片中的相同坐标部位均有细胞的存在才能记为一个有效细胞, 共计算有效细胞为2 772个. 检测的8个抗体均在肝癌细胞中表达, 并且不同肝癌细胞中肝癌干细胞标志物的表达情况不同, 同一肝癌细胞可同时表达多个肝癌干细胞的分子标志(表1), 且可见8个抗体均阳性的肝癌细胞(图2). 其中, 2 772个有效细胞中有2 453个细胞为阴性, 所占比例为884.9‰.
| HCSCs标志物数目 | 阳性细胞数目 | 所占比例(‰) |
| 8 | 11 | 3.9 |
| 7 | 2 | 0.7 |
| 6 | 28 | 10.1 |
| 5 | 6 | 2.2 |
| 4 | 45 | 16.2 |
| 3 | 23 | 8.3 |
| 2 | 146 | 52.7 |
| 1 | 58 | 20.9 |
| 0 | 2 453 | 884.9 |
肝癌干细胞是指肝癌组织中一小群具有自我更新与分化潜能的肝癌细胞, 因其可能与肝癌发生和转移直接相关而备受关注. Ma等[14]的研究表明, CD133+ HCC细胞具有干细胞/祖细胞的特点. 也有一些实验显示C133+细胞CD133-细胞具有相同的致瘤能力[4]. Yamashita等[20]发现EpCAM+AFP+细胞亚群具有肝癌干细胞特征, 且在不同的细胞系中, 肝癌干细胞的标志物也有差别. AFP+的细胞系HuH1、HuH7、Hep3B同时也表达EpCAM、CD133, 但没有CD90阳性表达细胞; AFP+的细胞系SK-Hep-1、HLE、HLF有CD90+的细胞亚群, 但没有EpCAM+和或CD133+的亚群. 卵圆细胞标记OV6+的肝癌细胞具有更高的体内成瘤能力和化疗耐受性[19]. Zhu等[15]在对肝癌细胞系HepG2、Hep3B、Huh7、SMMC-7721、MHCC-LM3、MHCC-97L的研究中发现从CD133+细胞群中分离出的CD133+CD44+细胞具有干细胞的特性. Yang等[18]从肝癌细胞株和HCC患者肝癌组织中研究发现CD90+CD44+细胞具有肿瘤干细胞特性, 且较CD90+CD44-具有较强的侵袭能力与转移能力. 上述各个实验结果都证明各自的具有特殊分子标记的细胞具有LCSC的特征, 但是对于不同细胞系的研究分子标记有差异, 且相关的分子组合也只有2个.
在既往文献对肿瘤干细胞分子标记的报道中, 多采用流式[25-28]或磁珠分选[29-33]对癌细胞系中的具有癌干细胞分子标记的细胞进行分选, 此分选价格较昂贵且对1个细胞1次最多也只能分选具有2个的分子标记; 采用免疫组织化学或免疫荧光法对人肿瘤组织检测CSCs的存在时, 双抗也只能检测出1个细胞同时存在的两种分子标记[34,35]. 目前, 与LCSC相关的标志物有8种, 采用流式或磁珠分选、免疫组化技术只能用于两个相关的分子标记相互关系的研究. 本实验用8个已报道的肝癌干细胞标志物对肝癌患者肿瘤组织进行了检测、立体定位和分类, 结果发现在同一肝癌组织中, 多种肝癌干细胞标志物可在一个肝癌细胞上同时表达, 少数肝癌细胞可同时表达8个肝癌干细胞标记, 各个肝癌干细胞分子标记存在交叉表达的情况.
总之, 高通量二维细胞识别技术方法的建立, 可同时对目前8个肝癌干细胞相关的分子标记之间的相关性进行研究; 可用于推测肝癌干细胞在肝癌细胞中的比例与复发的相关性; 用于比较肝癌干细胞标志物在肝癌及癌旁组织的表达差异, 根据其与预后的关联, 探讨最佳的肝癌干细胞标志物组合及验证其在复发中的作用, 并检验肿瘤干细胞的特性, 为肝癌干细胞的分离和临床应用提供理论依据和实践基础.
肿瘤干细胞的发现是最近肿瘤研究中的一个重要方向. 肿瘤干细胞方面的的深入研究, 对于理解肿瘤的发展、转移、预后和治疗等有重要的意义.
代智, 副研究员, 复旦大学附属中山医院肝癌研究所
已有文献报道肝癌干细胞的分离和鉴定. 但目前还没有公认而统一的肝癌干细胞标志物. 不同肝癌干细胞亚群之间的差异特征和相关分子机制尚未见报道.
Yang等分离鉴定了CD90+肝癌干细胞. 证实CD90+CD44+的肝癌细胞具有较强的侵袭和转移能力, 差异基因组学筛选出一系列与侵袭转移相关的差异基因.
利用建立的一种新颖的高通量二维细胞识别技术. 可以对8个分子标记进行肝癌干细胞的分选或免疫双标.
高通量二维细胞识别技术方法的建立, 可用于推测肝癌干细胞在肝癌细胞中的比例与复发的相关性, 将为肝癌干细胞的分离和临床应用提供理论依据和实践基础.
本文有一定的创新性, 研究结果具有很好的临床价值.
编辑 何基才 电编 何基才
| 1. | Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8:755-768. [PubMed] [DOI] |
| 2. | Xu Y, He K, Goldkorn A. Telomerase targeted therapy in cancer and cancer stem cells. Clin Adv Hematol Oncol. 2011;9:442-455. [PubMed] |
| 3. | Medema JP, Vermeulen L. Microenvironmental regulation of stem cells in intestinal homeostasis and cancer. Nature. 2011;474:318-326. [PubMed] [DOI] |
| 4. | Lu X, Kang Y. Cell fusion hypothesis of the cancer stem cell. Adv Exp Med Biol. 2011;714:129-140. [PubMed] [DOI] |
| 5. | Davies EJ, Marsh V, Clarke AR. Origin and maintenance of the intestinal cancer stem cell. Mol Carcinog. 2011;50:254-263. [PubMed] [DOI] |
| 7. | Chiba T, Kita K, Zheng YW, Yokosuka O, Saisho H, Iwama A, Nakauchi H, Taniguchi H. Side population purified from hepatocellular carcinoma cells harbors cancer stem cell-like properties. Hepatology. 2006;44:240-251. [PubMed] [DOI] |
| 8. | Shi GM, Xu Y, Fan J, Zhou J, Yang XR, Qiu SJ, Liao Y, Wu WZ, Ji Y, Ke AW. Identification of side population cells in human hepatocellular carcinoma cell lines with stepwise metastatic potentials. J Cancer Res Clin Oncol. 2008;134:1155-1163. [PubMed] [DOI] |
| 9. | Zen Y, Fujii T, Yoshikawa S, Takamura H, Tani T, Ohta T, Nakanuma Y. Histological and culture studies with respect to ABCG2 expression support the existence of a cancer cell hierarchy in human hepatocellular carcinoma. Am J Pathol. 2007;170:1750-1762. [PubMed] [DOI] |
| 10. | Ma S, Chan KW, Hu L, Lee TK, Wo JY, Ng IO, Zheng BJ, Guan XY. Identification and characterization of tumorigenic liver cancer stem/progenitor cells. Gastroenterology. 2007;132:2542-2556. [PubMed] [DOI] |
| 11. | Yin S, Li J, Hu C, Chen X, Yao M, Yan M, Jiang G, Ge C, Xie H, Wan D. CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. Int J Cancer. 2007;120:1444-1450. [PubMed] [DOI] |
| 12. | Suetsugu A, Nagaki M, Aoki H, Motohashi T, Kunisada T, Moriwaki H. Characterization of CD133+ hepatocellular carcinoma cells as cancer stem/progenitor cells. Biochem Biophys Res Commun. 2006;351:820-824. [PubMed] [DOI] |
| 13. | Rountree CB, Senadheera S, Mato JM, Crooks GM, Lu SC. Expansion of liver cancer stem cells during aging in methionine adenosyltransferase 1A-deficient mice. Hepatology. 2008;47:1288-1297. [PubMed] [DOI] |
| 14. | Ma S, Lee TK, Zheng BJ, Chan KW, Guan XY. CD133+ HCC cancer stem cells confer chemoresistance by preferential expression of the Akt/PKB survival pathway. Oncogene. 2008;27:1749-1758. [PubMed] [DOI] |
| 15. | Zhu Z, Hao X, Yan M, Yao M, Ge C, Gu J, Li J. Cancer stem/progenitor cells are highly enriched in CD133+CD44+ population in hepatocellular carcinoma. Int J Cancer. 2010;126:2067-2078. [PubMed] |
| 16. | Rountree CB, Ding W, He L, Stiles B. Expansion of CD133-expressing liver cancer stem cells in liver-specific phosphatase and tensin homolog deleted on chromosome 10-deleted mice. Stem Cells. 2009;27:290-299. [PubMed] [DOI] |
| 17. | Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, Li ML, Tam KH, Lam CT, Poon RT. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47:919-928. [PubMed] [DOI] |
| 18. | Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P, Chu PW, Lam CT, Poon RT, Fan ST. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell. 2008;13:153-166. [PubMed] [DOI] |
| 19. | Yang W, Yan HX, Chen L, Liu Q, He YQ, Yu LX, Zhang SH, Huang DD, Tang L, Kong XN. Wnt/beta-catenin signaling contributes to activation of normal and tumorigenic liver progenitor cells. Cancer Res. 2008;68:4287-4295. [PubMed] [DOI] |
| 20. | Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, Jia H, Ye Q, Qin LX, Wauthier E. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012-1024. [PubMed] [DOI] |
| 21. | Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, Budhu A, Zanetti KA, Chen Y, Qin LX. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451-1461. [PubMed] [DOI] |
| 22. | Ji J, Yamashita T, Budhu A, Forgues M, Jia HL, Li C, Deng C, Wauthier E, Reid LM, Ye QH. Identification of microRNA-181 by genome-wide screening as a critical player in EpCAM-positive hepatic cancer stem cells. Hepatology. 2009;50:472-480. [PubMed] [DOI] |
| 24. | Ma S, Chan KW, Lee TK, Tang KH, Wo JY, Zheng BJ, Guan XY. Aldehyde dehydrogenase discriminates the CD133 liver cancer stem cell populations. Mol Cancer Res. 2008;6:1146-1153. [PubMed] [DOI] |
| 25. | Shmelkov SV, Butler JM, Hooper AT, Hormigo A, Kushner J, Milde T, St Clair R, Baljevic M, White I, Jin DK. CD133 expression is not restricted to stem cells, and both CD133 and CD133- metastatic colon cancer cells initiate tumors. J Clin Invest. 2008;118:2111-2120. [DOI] |
| 26. | Dotto GP. Calcineurin signaling as a negative determinant of keratinocyte cancer stem cell potential and carcinogenesis. Cancer Res. 2011;71:2029-2033. [PubMed] [DOI] |
| 27. | Dou J, Wen P, Hu W, Li Y, Wu Y, Liu C, Zhao F, Hu K, Wang J, Jiang C. Identifying tumor stem-like cells in mouse melanoma cell lines by analyzing the characteristics of side population cells. Cell Biol Int. 2009;33:807-815. [PubMed] [DOI] |
| 28. | Lu YR, Yuan Y, Wang XJ, Wei LL, Chen YN, Cong C, Li SF, Long D, Tan WD, Mao YQ. The growth inhibitory effect of mesenchymal stem cells on tumor cells in vitro and in vivo. Cancer Biol Ther. 2008;7:245-251. [PubMed] [DOI] |
| 29. | Fan H, Guo H, Zhang IY, Liu B, Luan L, Xu S, Hou X, Liu W, Zhang R, Wang X. The different HMGA1 expression of total population of glioblastoma cell line U251 and glioma stem cells isolated from U251. Brain Res. 2011;1384:9-14. [PubMed] [DOI] |
| 30. | Zhong Y, Guan K, Guo S, Zhou C, Wang D, Ma W, Zhang Y, Li C, Zhang S. Spheres derived from the human SK-RC-42 renal cell carcinoma cell line are enriched in cancer stem cells. Cancer Lett. 2010;299:150-160. [PubMed] [DOI] |
| 31. | Joo KM, Nam DH. Prospective identification of cancer stem cells with the surface antigen CD133. Methods Mol Biol. 2009;568:57-71. [PubMed] [DOI] |
| 32. | Shervington A, Lu C, Patel R, Shervington L. Telomerase downregulation in cancer brain stem cell. Mol Cell Biochem. 2009;331:153-159. [PubMed] [DOI] |
| 33. | Cammareri P, Lombardo Y, Francipane MG, Bonventre S, Todaro M, Stassi G. Isolation and culture of colon cancer stem cells. Methods Cell Biol. 2008;86:311-324. [PubMed] [DOI] |
| 34. | van der Horst G, van den Hoogen C, Buijs JT, Cheung H, Bloys H, Pelger RC, Lorenzon G, Heckmann B, Feyen J, Pujuguet P. Targeting of α(v)-integrins in stem/progenitor cells and supportive microenvironment impairs bone metastasis in human prostate cancer. Neoplasia. 2011;13:516-525. [PubMed] [DOI] |
| 35. | Kleist B, Xu L, Li G, Kersten C. Expression of the adult intestinal stem cell marker Lgr5 in the metastatic cascade of colorectal cancer. Int J Clin Exp Pathol. 2011;4:327-335. [PubMed] |