修回日期: 2011-03-29
接受日期: 2011-04-11
在线出版日期: 2011-04-28
目的: 探讨吴茱萸碱(EVO)联合CDK1的特异抑制剂RO3306对鼠结肠癌细胞CT26的生长抑制、诱导凋亡是否有协同增效作用.
方法: 采用MTT法求出EVO对CT26作用24 h的IC50及诱导CT26细胞发生不可逆凋亡的时间点, 比较EVO和RO3306同时用药与序贯用药(EVO先作用24 h, 再加入RO3306共同作用6 h)对CT26细胞的抑制作用. 采用金正均q值法检验其联合作用是否有协同性(q为实际药效与理论药效比值, q>1.15为协同性). 同时用药实验与序贯用药实验的分组情况均为对照组、2 mg/L EVO组, 4 mg/L EVO组, 15 mg/L RO3306组(加药时间同相应联合组), 2 mg/L EVO+15 mg/L RO3306联合组, 4 mg/L EVO+15 mg/L RO3306联合组. 采用克隆集落形成法检测药物单独和联合作用下对CT26细胞的抑制率. 采用流式细胞术检测药物作用对CT26凋亡率的影响.
结果: MTT法结果显示EVO对结肠癌细胞CT26具有显著抑制作用, 其抑制作用有明显的浓度依赖性, 作用24 h的IC50为10.8 mg/L; EVO诱导CT26细胞进入不可逆凋亡的时间点在24 h左右. MTT检测EVO和RO3306序贯用药的各组抑制率依次分别是22.0%±4.4%、30.4%±3.2%、12.3%±4.8%、48.0%±3.2%、62.2%±2.2%(序贯用药联合组q = 1.52, 1.60>1.15, 同时加药联合组q = 0.68, 0.72). 克隆集落形成法显示相应各组的抑制率依次分别是9.7%±5.8%、38.9%±3.8%, 10.8%±3.7%, 29.8%±10.7%, 68.3%±12.7%(q>1.15). 流式细胞术显示各组细胞凋亡率依次分别是5.5%±1.1%, 18.3%±1.9%, 25.6%±1.5%, 9.2%±1.1%, 39.1%±9.8%, 54.6%±1.2%(q>1.15).
结论: EVO能抑制鼠结肠癌细胞CT26的生长, 其作用呈剂量依赖关系, EVO诱导CT26发生不可逆凋亡的时间点约在24 h左右; EVO联合CDK1抑制剂RO3306并序贯用药对CT26的抑制作用具有协同增效效应, 同时加药联合作用未显示协同性.
引文著录: 崔娟, 吴映雅, 谭宇蕙, 张广献, 杜标炎, 常金荣. 吴茱萸碱联合CDK1抑制剂RO3306对鼠结肠癌CT26的协同杀伤作用. 世界华人消化杂志 2011; 19(12): 1244-1250
Revised: March 29, 2011
Accepted: April 11, 2011
Published online: April 28, 2011
AIM: To explore whether there is a synergistic effect between evodiamine (EVO) and RO3306, a specific cyclin-dependent kinase 1 (CDK1) inhibitor, on the proliferation and apoptosis of murine colon cancer CT26 cells.
METHODS: The inhibitory effect of EVO on the proliferation of CT26 cells was determined by MTT assay to calculate IC50 at 24 h and the time required for the induction of irreversible apoptosis. The inhibitory effect of combination treatment with EVO and RO3306 either in a simultaneous or sequential way (pretreatment with EVO for 24 h followed by addition of RO3306 for another 6 h) on cell proliferation was also detected. CT26 cells were divided into six groups: control group, 2 mg/L EVO group, 4 mg/L EVO group, 15 mg/L RO3306 group, 2 mg/L EVO + 15 mg/L RO3306 group, and 4 mg/L EVO + 15 mg/L RO3306 group. Colony-forming assay and flow cytometry (FCM) assay were used to detect the effect of these treatments on cell proliferation and apoptosis. q-value analysis was used to estimate the synergistic effect of evodiamine and RO3306. A q value of ≥1.15 indicates synergism.
RESULTS: Treatment with EVO alone for 24 h had a significant inhibitory effect on CT26 cell proliferation, and IC50 was around 10.8 mg/L. The time required for the induction of irreversible apoptosis was 24 h. Combination treatment with EVO and RO3306 in a sequential way resulted in the rates of reduced proliferation of 22.0 ± 4.4%, 30.4 ± 3.2%, 12.3 ± 4.8%, 48.0 ± 3.2%, and 62.2±2.2% in each treatment group. The q values of the two sequential treatment groups were 1.52 and 1.60, while those of simultaneous treatment groups were 0.68 and 0.72, respectively. Colony-forming assay showed the reduced rates of colony formation were 9.7 ± 5.8%, 38.9 ± 3.8%, 10.8 ± 3.7%, 29.8 ± 10.7%, and 68.3 ± 12.7% in each treatment group. The q values of the two sequential treatment groups were 1.41 and 1.47. FCM assay showed that the apoptotic rates were 5.5±1.1%, 18.3 ± 1.9%, 25.6 ± 1.5%, 9.2 ± 1.1%, 39.1 ± 9.8%, and 54.6 ± 1.2% in each group (q > 1.15).
CONCLUSION: The time required for induction of irreversible apoptosis of CT26 cells by EVO is around 24 h and the inhibitory effect was dose-dependent. EVO exhibited a significant synergistic inhibitory effect with RO3306 on CT26 cell proliferation in a sequential treatment regime but not in a simultaneous treatment manner.
- Citation: Cui J, Wu YY, Tan YH, Zhang GX, Du BY, Chang JR. Synergistic killing effect of evodiamine combined with RO3306, a CDK1 inhibitor, on murine colon cancer CT26 cells. Shijie Huaren Xiaohua Zazhi 2011; 19(12): 1244-1250
- URL: https://www.wjgnet.com/1009-3079/full/v19/i12/1244.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v19.i12.1244
结肠癌是消化系常见的恶性肿瘤之一, 随着生活生平的不断提高, 结肠癌发病率有不断上升的趋势. 结肠癌治疗目前仍以手术治疗为主, 但患者术后生活质量差, 复发和转移率高[1-4]. 大量的研究表明, 中医药或中西医结合能提高恶性肿瘤疗效, 同时能减轻放化疗的不良反应. 中药的某些有效成分能有效地抑制肿瘤细胞生长[5-8]. 吴茱萸碱(evodiamine, EVO)为中药吴茱萸的一种有效成分[9-13]. 近年来不少研究发现, EVO对多种恶性肿瘤细胞具有较强的体外杀伤和诱导凋亡作用[14-17], 且起效浓度较低, 具有一定临床应用前景[18-20], 本研究组前期研究表明, EVO能使细胞阻滞于G2/M期, 可能诱导M期阻滞(M-arrest)[21-25]. 最近有研究发现, 对药物诱导下已发生M期阻滞的细胞, 通过加入CDK1抑制剂以诱导其发生M期滑移(M-slippage), 可大大提高肿瘤细胞的凋亡率, 也就是说相继诱导M期阻滞与M期滑移的两种药物可能具有协同性诱导凋亡效应[26-30]. RO3306, 喹啉噻唑啉酮(quinolinyl thiazolinone)的一种衍生物, 是CDK1的特异抑制剂[31-33], 本研究把EVO和RO3306联合且序贯用药, 探讨其能否能协同诱导肿瘤细胞凋亡, 增强EVO对肿瘤细胞的杀伤力.
小鼠结肠癌细胞株CT26购自ATCC; RPMI 1640和2.5 g/L含EDTA的胰酶购自Gibco公司; 小牛和胎牛血清购自PAA公司, 青、链霉素溶液购自杭州吉诺生物技术有限公司; MTT和二甲基亚砜(DMSO)均为Sigma公司产品; Giemsa购自北京鼎国生物技术公司; EVO(批号: 046K1230)和RO3306(批号: L24795)购自 Sigma公司; 碘化丙啶(propidium iodide, PI, 批号: 6155K)购自MP Biomedicals. 荧光倒置显微镜产自Olympus公司; Bio-Rad 680型全自动酶标仪为美国Bio-Rad公司产品; 流式细胞仪为美国Becton Dickinson公司产品.
1.2.1 细胞培养: 肿瘤细胞株按常规方法培养传代, 均培养于RPMI 1640(高糖), 另补充100 mL/L灭活胎牛血清, 2 µmol/L谷氨酰胺, 1.5 g的碳酸氢钠, 1%的青、链霉素溶液.
1.2.2 MTT法检测EVO对CT26杀伤作用的量效关系及其诱导不可逆凋亡的时间转折点: 取对数生长期细胞, 按5×103个细胞/孔接种于96孔板培养, 置37 ℃, 50 mL/L CO2培养箱培养, 待细胞贴壁后, 加入不同浓度的EVO, 同时设阴性对照组、 空白对照组, 每组均设8个复孔. 培养24 h后, 每孔加100 µL MTT(5 g/L无血清培养液, pH7.2), 再培养4 h, 弃上清, 每孔加入150 µL DMSO, 振荡溶解, 酶标仪于570 nm处测光吸收值(A值), 细胞生长抑制率按下列公式进行计算: 抑制率(IR) = (1-实验组平均A值/对照组平均A值)×100%, 半数抑制浓度(IC50)通过SPSS18.0统计分析软件包经Probit回归分析计算.
取对数生长期细胞, 按5×103个细胞/孔接种于96孔板培养, 细胞贴壁后加入终浓度为4 mg/L的EVO. 按EVO作用不同时间(16、20、24、28 h)依次分为EVO 1组、EVO 2组、EVO 3组, EVO 4组, 每组再分别设立EVO撤药前及其相应对照组, EVO撤药再培养12 h(EVO作用不同时间后撤药再用完全培养基培养12 h)及其相应对照组. 处理结束后MTT法测定细胞生长抑制率(方法同上).
1.2.3 MTT法比较EVO和RO3306联合同时用药与序贯用药对CT26细胞的抑制作用: 将细胞接种于2个96孔培养板(5×103个细胞/孔). 置37 ℃, 50 mL/L CO2培养箱培养, 待细胞贴壁后加药. 两板均设立对照组、4 mg/L EVO组、2 mg/L EVO组、15 mg/L RO3306组、2 mg/L EVO组+15 mg/L RO3306联合组、4 mg/L EVO组+15 mg/L RO3306联合组. 其中的一板联合组EVO和RO3306同时加药, 各组EVO和RO3306作用时间均为30 h; 另一板不同的是, 联合组EVO和RO3306序贯用药, 先加入EVO作用24 h后再加入RO3306共同作用6 h, 而RO3306组与联合组一样仅在细胞培养结束前6 h才加RO3306. 处理结束后用MTT法检测细胞生长抑制率.
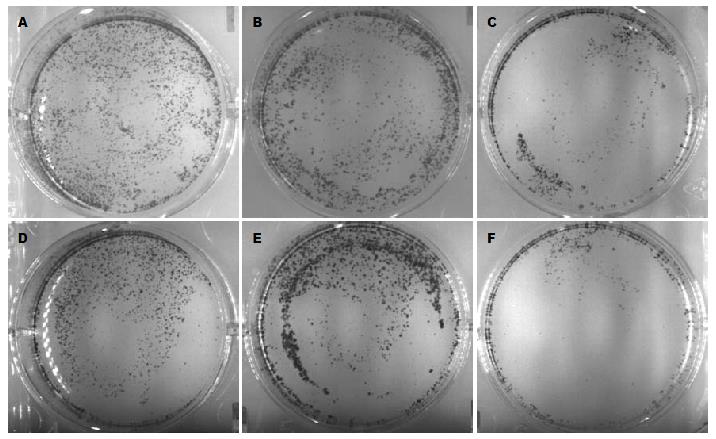
1.2.4 克隆集落形成法检测EVO和RO3306序贯联合用药对CT26细胞集落形成的抑制率: 将细胞接种于6孔培养板, 1×103个细胞/孔. 待细胞贴壁后, 换为无血清培养基培养, 24 h后吸掉无血清培养基后加入完全培养基并进行药物作用. 分组和药物作用方式同1.2.3的序贯用药. 药物作用完毕吸弃培养基, 加入完全培养基继续培养, 共培养8 d. 8 d后吸走上清, 用PBS洗1次, 加入固定液1 mL(甲醇:乙酸 = 3:1)室温固定15 min, 用PBS冲洗1次, 再加入500 µL Giemsa溶液, 室温下染色15 min, 吸掉染液, 用去离子水洗2次. 数码相机拍照并在倒置显微镜下观察计数细胞集落(细胞数>50/每个集落). 按下式计算集落形成抑制率. 集落抑制率(%) = 1-集落数/对照组集落数×100%.
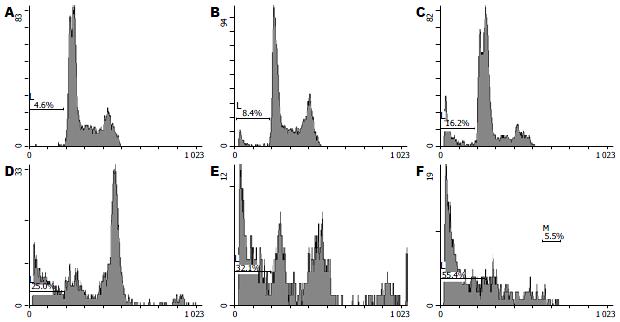
1.2.5 流式细胞PI单染检测EVO和RO3306联合并序贯用药对CT26细胞凋亡率的影响: 细胞以2×105个/孔的密度接种6孔板, 待细胞贴壁后进行药物作用, 分组和用药方式同上. 作用完毕收集细胞, 用PBS洗1次, 将细胞固定于700 mL/L预冷乙醇中过夜, 第2天用PI染色, 4 ℃避光30 min后进行流式细胞术检测. 测定各组晚期凋亡率.
统计学处理 统计学处理采用SPSS18.0统计软件包进行统计学处理. 计量资料以mean±SD表示. 采用金正均q值法[34]判断EVO和RO3306联合用药的协同性, 其中q = 实际联合药效R'(A+B)/理论联合药效R(A+B), R(A+B) = RA+RB-RA×RB, RA、RB为单独用药药效. q<0.85为拮抗, 0.85≤q<1.15为相加, q≥1.15为协同.
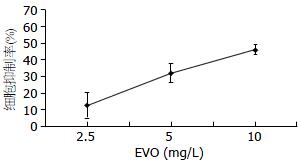
EVO作用于CT26 24 h后镜下观察, 细胞的形态明显改变, 细胞数目减少, MTT结果显示EVO以剂量依赖性方式抑制CT26细胞增殖, 在2.5、5、10 mg/L浓度下作用24 h, EVO对CT26的抑制率分别依次为12.8%±7.7%, 32.1%±5.8%, 46.1%±2.9%, 各浓度组与对照组(0.0%±3.7%)比较均有显著性差异(P<0.01, n = 6). EVO作用24 h对CT26的IC50为10.8 mg/L±4.6 mg/L(图1).
在EVO作用20 h以前的各时间点, 撤药再培养12 h后细胞抑制率均明显比撤药前低, 推测在撤药后培养12 h的过程中有部分细胞经修复机制可恢复正常增殖, 因此可推断此时EVO的凋亡诱导作用是部分可逆的; 而在作用24 h时间点, EVO撤药再培养12 h后细胞抑制率却比撤药前明显增加; 作用>24 h时, EVO撤药再培养12 h后比撤药前抑制率增加更显著(P<0.01), 推测此时EVO诱导的细胞凋亡已经进入不可逆阶段, 因此即使在撤药后再培养12 h, 细胞抑制率却增加了. EVO诱导不可逆凋亡的时间转折点可能在24 h左右(表1).
EVO与RO3306序贯用药联合组的协同杀伤明显优于EVO与RO3306联合同时加药作用30 h的杀伤作用, 采用金正均q值法计算, 序贯用药的q值>1.15, 显示协同性增效作用, 而同时用药联合组的q值均<0.85(表2).
EVO和RO3306各单药组对CT26细胞的集落形成均有抑制作用, EVO与RO3306序贯联合用药各组的细胞集落形成的抑制率大于两成分单独作用组(差异有统计学意义), 联合作用q>1.15, 为协同性作用(表3, 图2).
EVO、RO3306各单药组的凋亡率均大于对照组, 差异具有统计学意义, EVO与RO3306序贯联合用药的各联合组的凋亡率均显著大于两成分单独作用组, 联合作用组q>1.15, 显示二者联合是协同性作用(表4, 图3).
结肠癌是人类主要恶性肿瘤之一, 近年来其发病率呈逐年上升趋势. 但随着科学技术的发展, 近年来其诊断和治疗水平也有了很大提高. 但是中晚期患者目前仍缺少有效的治疗方法. 因此, 寻找新的有效的治疗药物, 对提高结肠整体防治水平有重要意义.
Tao等[26,27]对有丝分裂拮抗剂KSPI进行大量深入实验研究, 发现KSPI能激活纺锤体检验点, 使细胞阻滞于M期并维持一定时间后, 紧接着引发细胞的异常M期滑移, 即在未完全修复已损伤纺锤体的情况下, 细胞突破纺锤体检验点, "滑移"至下一细胞周期的G1期, 形成4倍体的假G1期细胞, 进而下调生存素(Survivin)的表达和激活Bax而诱发凋亡. 进一步的研究发现KSPI诱导凋亡有赖于M期阻滞与异常M期滑移环节的偶联, 两者的相继发生对诱导凋亡是缺一不可的. 此外, 在KSPI诱导细胞发生M期阻滞后, 如果加入另一诱发细胞发生M期滑移的药物, 如CDK1的抑制剂purv, 可协同性提高前者诱导的凋亡率. 该研究结果提示, 能诱导细胞M期阻滞的有丝分裂抑制剂和CDK1抑制剂联合使用诱导肿瘤细胞凋亡, 很可能产生相互协同作用.
本研究组前期研究证明, EVO能使多种肿瘤细胞株(HepG2、人胃癌SGC7901、小鼠肝癌H22等)阻滞于G2/M期, 是细胞水平杀伤力较强的少数几种中药成分之一; EVO诱导的凋亡极有可能是M期凋亡[21-25], 药物作用后细胞染色体聚集于细胞赤道板浓聚成一字或人字形, 是M期细胞形态特征. 提示EVO很可能与KSPI类似, 是有丝分裂拮抗剂.
本研究对EVO作用的不同时间点进行MTT检测, 检测到作用24 h后是EVO诱导CT26细胞凋亡的转折点, 此点前如果撤药细胞将恢复周期运转和生长; 而转折点后大量细胞出现凋亡形态, 如果此时撤药细胞仍将进入不可逆凋亡途径, 因此即使在撤药后培养12 h细胞的抑制率不减反增.
本研究通过MTT法、克隆集落形成法、流式细胞法研究EVO与CDK1特异性抑制剂RO3306联合用药对结肠癌细胞CT26的效应, 结果表明: EVO与RO3306联合并序贯用药, 能够明显地提高EVO对CT26的抑制率, 获得协同效应, 但二者同时联合用药却无类似协同效应. 流式细胞数检测也发现二者联合并序贯用药可显著提高EVO对结肠癌细胞的凋亡诱导作用. 结果提示EVO诱导的细胞凋亡与KSPI类似, 可能不仅与M期阻滞事件相关, 还与异常M期滑移事件的发生相关; EVO和RO3306联合且序贯用药的协同效应, 有可能是通过M期阻滞和M期滑移机制实现. 相关研究国内未见报道, 要明确该分子机制还需更多实验依据.
吴茱萸碱是中药吴茱萸的有效成分之一, 近年研究发现, 吴茱萸碱对多种恶性肿瘤细胞具有较强的体外杀伤和诱导凋亡作用, 且起效浓度较低, 具有一定临床应用前景.
牛英才, 副研究员, 齐齐哈尔医学院医药科学研究所
Tao等最近研究发现, 对药物诱导下已发生M期阻滞的细胞, 通过加入CDK1抑制剂以诱导其发生M期滑移, 可大大提高肿瘤细胞的凋亡率, 也就是说相继诱导M期阻滞与M期滑移的两种药物可能具有协同性诱导凋亡效应.
本文检测到吴茱萸碱(EVO)诱导凋亡的可逆时间约在24 h之后, EVO与RO3306联合并序贯用药, 能够明显地提高EVO对结肠癌细胞CT26的抑制率, 获得协同效应, 但二者同时联合用药却无类似效应.
中药成分用药方式的不同导致协同效应也不同, 提纯和强化主要成分作用的组分配方能否比中药复方制剂更强效, 本文为阐明中药复方药理探索研究方法, 为古方新用, 提高中药临床疗效和抗肿瘤新药开发提供科学依据和参考.
本文选题新颖, 设计合理, 具有一定的理论和应用价值.
编辑: 李薇 电编:何基才
| 1. | Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology. 2010;113:27-34. [PubMed] [DOI] |
| 2. | Yan DW, Li DW, Yang YX, Xia J, Wang XL, Zhou CZ, Fan JW, Wen YG, Sun HC, Wang Q. Ubiquitin D is correlated with colon cancer progression and predicts recurrence for stage II-III disease after curative surgery. Br J Cancer. 2010;103:961-969. [PubMed] [DOI] |
| 3. | Woo HJ, Hwang SK, Park SH, Hwang JH, Hamm IS. Factors related to the local treatment failure of gamma knife surgery for metastatic brain tumors. Acta Neurochir (Wien). 2010;152:1909-1914. [PubMed] [DOI] |
| 4. | Nishigori T, Matsumoto H, Nakano D, Yamaguchi T, Takahashi K, Iwasaki Y, Ohashi M, Nunobe S, Iwanaga T, Nemoto T. [A case of submucosal invasive cancer of the sigmoid colon, recurring as multiple liver metastases one year after the surgery]. Gan To Kagaku Ryoho. 2008;35:2144-2146. [PubMed] |
| 7. | Li J, Sun GZ, Lin HS, Pei YX, Qi X, An C, Yu J, Hua BJ. The herb medicine formula "Yang Wei Kang Liu" improves the survival of late stage gastric cancer patients and induces the apoptosis of human gastric cancer cell line through Fas/Fas ligand and Bax/Bcl-2 pathways. Int Immunopharmacol. 2008;8:1196-1206. [PubMed] [DOI] |
| 8. | Zhao F, Liu PX. [Progress of study on action mechanisms of TCM in anti-tumor and preventing metastasis of tumor]. Zhongguo Zhongxiyi Jiehe Zazhi. 2007;27:178-181. [PubMed] |
| 9. | Wang XN, Han X, Xu LN, Yin LH, Xu YW, Qi Y, Peng JY. Enhancement of apoptosis of human hepatocellular carcinoma SMMC-7721 cells through synergy of berberine and evodiamine. Phytomedicine. 2008;15:1062-1068. [PubMed] [DOI] |
| 10. | Yang J, Wu LJ, Tashiro S, Onodera S, Ikejima T. Nitric oxide activated by p38 and NF-kappaB facilitates apoptosis and cell cycle arrest under oxidative stress in evodiamine-treated human melanoma A375-S2 cells. Free Radic Res. 2008;42:1-11. [PubMed] [DOI] |
| 11. | Yang J, Wu LJ, Tashino S, Onodera S, Ikejima T. Reactive oxygen species and nitric oxide regulate mitochondria-dependent apoptosis and autophagy in evodiamine-treated human cervix carcinoma HeLa cells. Free Radic Res. 2008;42:492-504. [PubMed] [DOI] |
| 12. | Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2010; Aug 2. [Epub ahead of print]. [PubMed] |
| 13. | Chen MC, Yu CH, Wang SW, Pu HF, Kan SF, Lin LC, Chi CW, Ho LL, Lee CH, Wang PS. Anti-proliferative effects of evodiamine on human thyroid cancer cell line ARO. J Cell Biochem. 2010;110:1495-1503. [PubMed] [DOI] |
| 14. | Zhang C, Fan X, Xu X, Yang X, Wang X, Liang HP. Evodiamine induces caspase-dependent apoptosis and S phase arrest in human colon lovo cells. Anticancer Drugs. 2010;21:766-776. [PubMed] [DOI] |
| 15. | Jiang J, Hu C. Evodiamine: a novel anti-cancer alkaloid from Evodia rutaecarpa. Molecules. 2009;14:1852-1859. [PubMed] [DOI] |
| 17. | Liao CH, Pan SL, Guh JH, Chang YL, Pai HC, Lin CH, Teng CM. Antitumor mechanism of evodiamine, a constituent from Chinese herb Evodiae fructus, in human multiple-drug resistant breast cancer NCI/ADR-RES cells in vitro and in vivo. Carcinogenesis. 2005;26:968-975. [PubMed] [DOI] |
| 18. | Yang ZG, Chen AQ, Liu B. Antiproliferation and apoptosis induced by evodiamine in human colorectal carcinoma cells (COLO-205). Chem Biodivers. 2009;6:924-933. [PubMed] [DOI] |
| 19. | Wang C, Li S, Wang MW. Evodiamine-induced human melanoma A375-S2 cell death was mediated by PI3K/Akt/caspase and Fas-L/NF-kappaB signaling pathways and augmented by ubiquitin-proteasome inhibition. Toxicol In Vitro. 2010;24:898-904. [PubMed] [DOI] |
| 20. | Kan SF, Yu CH, Pu HF, Hsu JM, Chen MJ, Wang PS. Anti-proliferative effects of evodiamine on human prostate cancer cell lines DU145 and PC3. J Cell Biochem. 2007;101:44-56. [PubMed] [DOI] |
| 21. | Huang DM, Guh JH, Huang YT, Chueh SC, Chiang PC, Teng CM. Induction of mitotic arrest and apoptosis in human prostate cancer pc-3 cells by evodiamine. J Urol. 2005;173:256-261. [PubMed] [DOI] |
| 22. | Huang YC, Guh JH, Teng CM. Induction of mitotic arrest and apoptosis by evodiamine in human leukemic T-lymphocytes. Life Sci. 2004;75:35-49. [PubMed] [DOI] |
| 26. | Tao W. The mitotic checkpoint in cancer therapy. Cell Cycle. 2005;4:1495-1499. [PubMed] [DOI] |
| 27. | Tao W, South VJ, Zhang Y, Davide JP, Farrell L, Kohl NE, Sepp-Lorenzino L, Lobell RB. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8:49-59. [PubMed] [DOI] |
| 28. | Blagosklonny MV. Mitotic arrest and cell fate: why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle. 2007;6:70-74. [PubMed] [DOI] |
| 29. | Chan YW, Ma HT, Wong W, Ho CC, On KF, Poon RY. CDK1 inhibitors antagonize the immediate apoptosis triggered by spindle disruption but promote apoptosis following the subsequent rereplication and abnormal mitosis. Cell Cycle. 2008;7:1449-1461. [PubMed] [DOI] |
| 30. | Degenhardt Y, Greshock J, Laquerre S, Gilmartin AG, Jing J, Richter M, Zhang X, Bleam M, Halsey W, Hughes A. Sensitivity of cancer cells to Plk1 inhibitor GSK461364A is associated with loss of p53 function and chromosome instability. Mol Cancer Ther. 2010;9:2079-2089. [PubMed] [DOI] |
| 31. | Kojima K, Shimanuki M, Shikami M, Andreeff M, Nakakuma H. Cyclin-dependent kinase 1 inhibitor RO-3306 enhances p53-mediated Bax activation and mitochondrial apoptosis in AML. Cancer Sci. 2009;100:1128-1136. [PubMed] [DOI] |
| 32. | Vassilev LT, Tovar C, Chen S, Knezevic D, Zhao X, Sun H, Heimbrook DC, Chen L. Selective small-molecule inhibitor reveals critical mitotic functions of human CDK1. Proc Natl Acad Sci U S A. 2006;103:10660-10665. [PubMed] [DOI] |
| 33. | Vassilev LT. Cell cycle synchronization at the G2/M phase border by reversible inhibition of CDK1. Cell Cycle. 2006;5:2555-2556. [PubMed] [DOI] |