修回日期: 2009-09-22
接受日期: 2009-09-28
在线出版日期: 2009-11-18
目的: 观察体外大鼠骨髓间充质干细胞(MSCs)对肝星状细胞(HSCs)RhoA信号因子及其细胞周期调控因子P27表达的影响, 探讨MSCs调控HSCs细胞周期G1/S转换机制.
方法: 贴壁筛选法培养、纯化SD大鼠MSCs, 传代至第4代使用; 大鼠肝星状细胞(HSC-T6) 系及纤维原细胞系冻融后传代使用. 应用6孔塑料细胞培养盒, 每孔使用半透膜(transwell insert)建立上下双层细胞共培养体系, 常规培养. 实验分3组: 空白对照组、阴性对照组、MSCs实验组. 用WST-8法对HSCs增殖率进行测定; 流式细胞仪检测细胞周期; RT-PCR、Western blot检测MSCs与HSCs共培养后HSCs内RhoA, P27 mRNA和蛋白的表达.
结果: (1)HSCs与MSCs共培养24 h后, HSCs表现明显增殖抑制(P<0.01), 且呈现时间依赖性. MSCs实验组与空白对照组、阴性对照组比较均有显著性差异(均P<0.01). (2)共培养12 h后, MSCs可阻滞HSCs由G0/G1期向S期转换, 使G0/G1期细胞增多, S期细胞减少, 与空白对照组、阴性对照组比较差异均有统计学意义(P<0.01). (3)共培养12 h后, MSCs实验组RhoA mRNA表达与空白对照组、阴性对照组比较均有统计学差异(P<0.05, P<0.01), 随时间的延长呈递减趋势; 共培养后, MSCs实验组P27 mRNA表达与空白对照组、阴性对照组比较均无统计学差异. (4)共培养12 h后, MSCs实验组RhoA蛋白表达与空白对照组、阴性对照组比较均有统计学差异(均P<0.01), 随时间的延长呈递减趋势; 共培养12 h后, MSCs实验组P27蛋白与空白对照组、阴性对照组比较均有统计学差异(均P<0.01), 随时间的延长呈递增趋势. (5)相关性分析显示: RhoA与P27 mRNA的表达无明显相关(r = -0.105); RhoA与P27蛋白的表达呈显著负相关(r = -0.943, P<0.01).
结论: MSCs抑制HSCs增殖的机制可能是通过RhoA-P27通路调控HSCs细胞周期改变; RhoA活性的下调可能是引起HSCs内P27蛋白表达增加的原因.
引文著录: 苏思标, 姜海行, 王东旭, 覃山羽, 梁梓宇. 骨髓间充质干细胞调控肝星状细胞RhoA、P27的表达. 世界华人消化杂志 2009; 17(32): 3283-3291
Revised: September 22, 2009
Accepted: September 28, 2009
Published online: November 18, 2009
AIM: To investigate the effects of bone marrow mesenchymal stem cells (MSCs) on the mRNA and protein expression of RhoA (Ras homolog gene family member A) and P27 in hepatic stellate cells (HSCs) and explore the mechanisms how MSCs regulate cell cycle progression of HSCs.
METHODS: MSCs were isolated from rat bone marrow and propagated in culture flasks. Meanwhile, HSCs and fibroblasts were thawed and passaged. An indirect co-culture system between MSCs/fibroblasts and HSCs was established using a Transwell membrane system (diameter: 24 mm; pore size: 0.4 μm). HSCs were randomly divided into three groups: blank control group (HSCs alone), negative control group (HSCs plus fibroblasts), and experimental group (HSCs plus MSCs). Cell proliferation was tested by WST-8 assay. Cell-cycle phases were determined by flow cytometry. The mRNA and protein expression of RhoA and P27 in HSCs was determined by reverse transcription-polymerase chain reaction (RT-PCR) and Western blot, respectively.
RESULTS: After 24 h of co-culture, the reduced rate of cell proliferation in the experimental group was significantly higher than those in the blank control group and negative control group co-culture(both P < 0.01). Flow cytometry analysis showed that, after 12 hours of co-culture, the percentage of HSCs in the G0/G1 phase in the experimental group was significantly higher than those in the two control groups (both P < 0.01), while the percentage of HSCs in the S phase in the experimental group was significantly lower than those in the two control groups (both P < 0.01). After 12 h of co-culture, the expression level of RhoA mRNA in the experimental group was significantly lower than those in the two control groups (both P < 0.01), whereas the expression level of P27 mRNA showed no significant differences between the experimental group and the two control groups (both P > 0.05). The expression level of RhoA protein in the experimental group was significantly lower than those in the two control groups (both P < 0.01), whereas the expression level of P27 in the experimental group was significantly higher than those in the two control groups (both P < 0.01). No correlation was noted between the expression of RhoA and P27 mRNAs (r = 0.105). However, a negative correlation was noted between the expression of RhoA and P27 proteins (r = -0.943, P < 0.01).
CONCLUSION: MSCs inhibit the proliferation of HSCs possibly by modulating the RhoA-P27 pathway to alter cell cycle progression of HSCs. The upregulation of P27 protein may be due to the downregulation of RhoA activity.
- Citation: Su SB, Jiang HX, Wang DX, Qin SY, Liang ZY. Bone marrow mesenchymal stem cells modulate the expression of RhoA and P27 in hepatic stellate cells. Shijie Huaren Xiaohua Zazhi 2009; 17(32): 3283-3291
- URL: https://www.wjgnet.com/1009-3079/full/v17/i32/3283.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v17.i32.3283
在真核细胞中, 细胞周期调控的物质基础是细胞周期蛋白(cyclins), 细胞周期蛋白依赖性激酶(cyclins-dependent kinases, CDKs), 细胞周期蛋白依赖性激酶抑制剂(cyclins-dependent kinases inhibitors, CKIs)以及其他中间因子. P27作为CKIs群体中的一员, 在细胞增殖过程中起着关键的调控作用[1-2], 能对细胞周期G1/S期转换起关键的调控作用[3]. RhoA是RhoGTP激酶家族的成员之一, 具有调节细胞骨架动力学、基因转录、细胞周期进程及细胞转化的功能. RhoA与P27是紧密联系的, RhoA的活化可下调P27的水平. RhoA可通过调控cyclinE/CDK2的活性而调控P27蛋白的降解并影响细胞周期G1/S期进程[4-5]. Tammy et al[6]研究发现, RhoA可通过激活PI3K通路而减少P27蛋白的表达, 并能引起其自身DNA合成发生改变, 从而调控细胞的增殖与迁移. Rho通路阻断剂、洛伐他丁或者胞外酶体C3均可增强P27 mRNA的翻译效率[7]. 此外, RhoA还可以通过调控Skp2-P27通路而促进细胞周期G1/S期转换[8]. P27调节细胞迁移是通过调控RhoA的活性而起作用的[9]. 可见, RhoA活性的改变可以影响P27蛋白的表达与降解, 从而影响细胞周期G1/S期转换的进程. 近期研究发现, 骨髓间充质干细胞(bone marrow mesenchymal stem cells, MSCs)可下调RhoA-ROCK信号转导通路活性, 并能抑制肝星状细胞(hepatic stellate cells, HSCs)活化、增殖与迁移, 促进HSCs凋亡[10-11]. 然而, MSCs抑制HSCs增殖的机制尚不十分明确. 我们通过建立MSCs与HSCs共培养体系, 检测共培养后HSCs内RhoA、P27蛋白和mRNA表达水平的改变, 初步探讨MSCs通过RhoA-P27通路调控HSCs细胞周期改变的机制.
健康SD大鼠6只, 周龄6-8 wk, 由广西医科大学实验动物中心提供. 肝星状细胞系(HSC-T6)及纤维原细胞系(由中山大学附属肿瘤医院细胞库提供); DMEM-LG培养液(购于美国Gibco公司); 特级胎牛血清(购于美国Hyclone公司); 小鼠抗 RhoA、P27 mAb(购于美国Santa Cruz公司); TRIzol(购于美国Invitrogen公司); 逆转录试剂盒(购于美国MBI公司); Transwell insert半透膜(购于美国Millipore公司); Cell Counting Kit-8(CCK-8)试剂盒(购于碧云天生物技术研究所); 凯基细胞周期检测试剂盒(购于凯基生物公司); 小鼠重组肝细胞生长因子(HGF)(购于美国Peprotech公司).
1.2.1 MSCs的分离、培养与功能鉴定: 按文献[12]方法在无菌条件下分离SD大鼠股骨骨髓细胞, 于37 ℃、饱和湿度、50 mL/L CO2培养箱中培养, 利用MSCs与其他贴壁细胞的贴壁差异性严格控制传代时胰酶的量和消化时间, 传代纯化MSCs, 显微镜下观察细胞形态. 取第4代细胞(Passege 4, P4), 2.5 g/L胰酶消化, 调整细胞浓度2×105/cm2接种于50 mL一次性培养瓶中, 备试验用. 取第4代细胞, 用特级胎牛血清内添加20 μg/L(终浓度)HGF诱导MSCs, 置于37 ℃、饱和湿度、50 mL/L CO2培养箱中培养, 每3天换液1次, 连续培养14 d, 倒置相差显微镜下观察细胞形态.
1.2.2 HSC-T6的培养、传代与活化鉴定: 大鼠HSC-T6系冻融后传代使用.于L-DMEM培养液(含100 mL/L胎牛血清)、37 ℃, 50 mL/L CO2培养箱中培养, 8 h即可贴壁生长, 2-3 d后细胞80%-90%铺满瓶底即可再次传代, 传至3-4代细胞生长活跃, 增殖明显可用于实验. 采用免疫组织化学法检测α-SMA表达. 倒置相差显微镜下观察活体细胞形态学改变.
1.2.3 纤维原细胞的培养、传代: 纤维原细胞系冻融后传代使用方法同上.
1.2.4 细胞共培养: 参照文献[13-14]方法应用6孔塑料细胞培养盒, 在半透膜(transwell insert) 上层接种MSCs或纤维原细胞(2×105 cells/well), 在下层接种HSC-T6细胞(2×105 cells/well), 建立上下双层细胞共培养体系, 常规培养. 实验分组: (1)空白对照组: HSCs单独培养(其上层只含培养基); (2)阴性对照组: HSCs与纤维原细胞共培养; (3)MSCs实验组: MSCs与HSCs共培养. 以上体系培养观察0、6、12、24、48、72 h. 各时间段于倒置相差显微镜下动态观察活体细胞形态学改变.
1.2.5 WST-8法检测HSCs增殖率: 共培养各时段后用2.5 g/L胰酶消化贴壁细胞, 用细胞计数板计数细胞, 各时段细胞配成细胞浓度为2×105/mL, 吹打, 混匀, 取96孔板, 每孔加入100 μL, 每时段设3个复孔, 每孔加入10 μL CCK-8溶液. 设立空白孔, 加入100 μL无细胞的PBS溶液和10 μL的CCK-8溶液. 在细胞培养箱内继续孵育1 h, 再用酶标仪检测. 选择吸光度为450 nm进行检查. 取各时段A值均数进行比较.
1.2.6 流式细胞仪检测细胞周期: MSCs与HSCs各以2×105 cells/well的量共培养, 取各时间段的细胞, 胰酶消化贴壁细胞, PBS清洗, 70%预冷酒精悬浮固定细胞, 4 ℃过夜, 加入等量PBS再洗2遍, 加100 μL RNaseA 37 ℃水浴30 min, Propidium Iodide(PI)4 ℃避光显色30 min, 流式细胞仪检测, MCYCLE软件分析细胞周期.
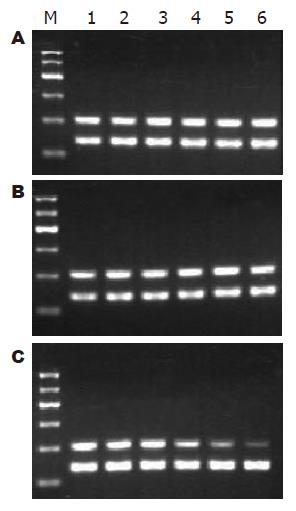
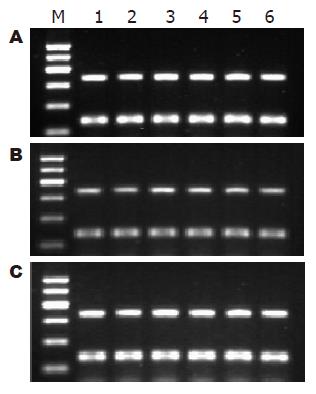
1.2.7 HSCs总RNA提取和RT-PCR: 收集各时段HSCs, 计数, 每5×106个细胞加1 mL的TRIzol, 振动机振荡混匀. TRIzol一步抽提法提取总RNA. 按逆转录试剂盒说明进行逆转录, 并根据以下条件进行目的基因的扩增: 95 ℃预变性5 min进入循环, 95 ℃变性45 s, 55 ℃退火45 s, 72 ℃ 1 min, 共35个循环后, 72 ℃延伸5 min, 以GAPDH为内参照. 扩增引物由上海生工生物工程公司合成. RhoA的上游引物5'-TGGTGATGGAGCTTGTGGTAAG-3', 下游引物 5'-AACATCAGTGTCTGGGTAGGAG-3'; P27的上游引物5'-TGCAACCGACGATTCTTCTACTCAA-3', 下游引物5'-CAAGCAGTGATGTATCTGATAAACAAGGA-3'; GAPDH上游引物: 5'-GCCAGTAGACTCCACGACAT-3', 下游引物5'-GCAAGTTCAACGGCACAG-3'. 取 6 μL PCR产物及6 μL DNA Marker进行1.7%琼脂糖凝胶电泳, 采用凝胶图像分析仪进行吸光度扫描, 观察条带的灰度强弱, 以目的基因/GAPDH的灰度比值表示相对目的基因 mRNA水平.
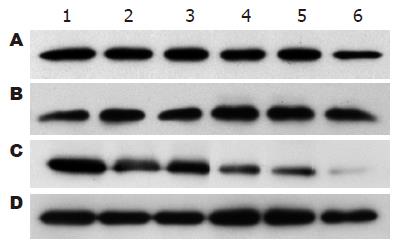
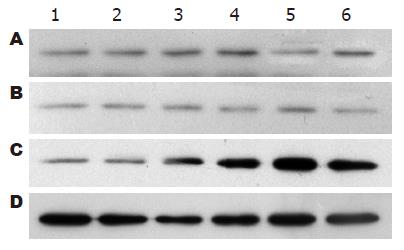
1.2.8 HSCs总蛋白提取和Western blot检测: 用细胞裂解液提取各时段HSCs总蛋白, 考马斯亮蓝比色法测定蛋白含量, 上样量为80 μg, 蛋白进行15% SDS-PAGE凝胶电泳, PVDF转膜, 非特异性封闭; 加入一抗小鼠抗RhoA、P27 mAb(1:500稀释), 4 ℃过夜, 加入辣根过氧化物酶标记的二抗进行杂交. ECL发光剂1-5 min, 曝光、显影、定影. 数码成像分析系统软件对结果进行分析, 以目的蛋白/GAPDH的灰度比值表示相对目的蛋白水平.
统计学处理 数据资料以mean±SD表示并应用统计软件SPSS13.0进行分析, 以P<0.05为有统计学差异, P<0.01为有显著性差异.
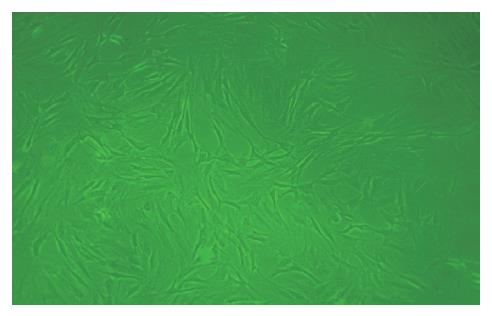
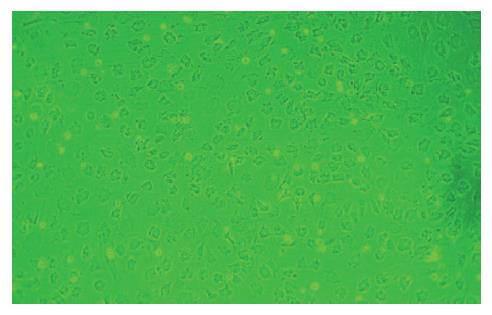
接种24 h后首次换液, 可见少量类圆形细胞贴壁生长. 原代培养3 d后, 镜下可见单个或少量成集落生长贴壁细胞, 形态大多呈短梭形; 7-10 d后, 细胞集落不断扩大并形成融合单层, 细胞形态大多呈长梭形或多角形; 传至P4, MSCs逐渐纯化, 类似成纤维细胞, 呈旋涡状生长(图1); 经HGF诱导的细胞在最初2-3 d形态无明显变化, 约7-10 d由梭形向三角形、椭圆形或多角形变化, 14 d时, 分化细胞基本为椭圆形, 细胞体积增大, 高倍镜下细胞折光性强, 核大而圆, 偶见双核, 类似肝细胞形态(图2).
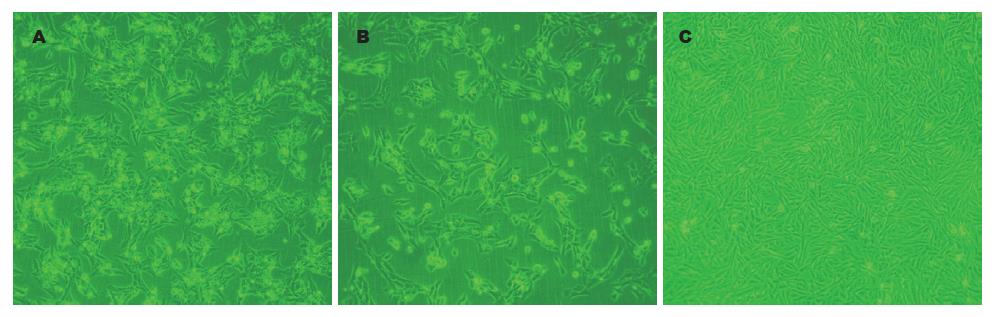
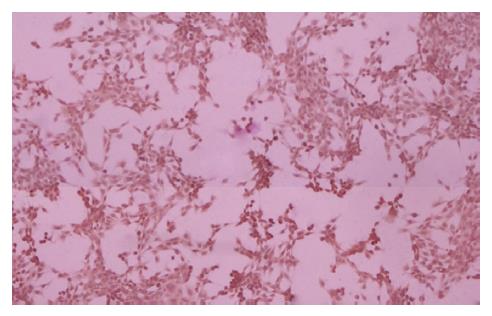
HSCs 12 h在培养瓶中完全贴壁生长, 呈圆形或椭圆形, 胞质内含折光颗粒(图3A), 48 h后发生细胞间融合现象, 细胞变长且分裂增殖, 呈纤维样细胞形状, 胞质内折光颗粒减少(图3B). 消化传代后的HSCs生长速度加快, 48 h成典型的成纤维原细胞形态(图3C). α-SMA是HSCs活化的标志: 免疫组织化学染色显示, HSCs培养48 h后α-SMA表达呈阳性, 胞质被染成棕黄色, 呈细条索状. HSCs呈星形, 胞体大, 膜状伸展. α-SMA阳性率达95%以上(图4).
纤维原细胞培养12 h, 在培养瓶中可见完全贴壁生长各组与成纤维细胞形态上基本相似, 呈梭形或不规则三角形, 放射状或旋涡状走行(图5).
MSCs实验组共培养0至24 h时段, HSCs形态学无明显改变(图6A); 48 h HSCs多呈椭圆形, 膜状伸展减弱, 包体变小, 折光颗粒增多, 细胞贴壁减弱, 细胞数减少(图6B); 72 h HSCs呈圆形或椭圆形, 无膜状伸展, 折光颗粒密集, 贴壁差, 细胞数明显减少(图6C). 共培养后, 空白对照组与阴性对照组HSCs形态学均无明显改变, 呈星形, 胞体大, 膜状伸展, 折光颗粒较少.
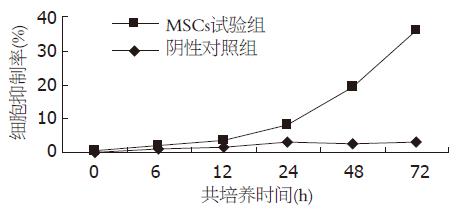
以空白对照组的细胞数作为参照值. 共培养24 h, MSCs实验组HSCs表现轻度的增殖抑制, 其抑制率为5.15%±2.1%, 此后细胞增殖抑制明显增强, 48、72 h各为16.23%±2.35%、32.91%±1.8%, 出现时间依赖性(图7). 24 h后, MSCs实验组与阴性对照组(2.85%±0.12%、2.77%±0.25%、2.89%±0.11%)比较具有显著性差异(P<0.01). 共培养全过程中, 阴性对照组与空白对照组比较无差异.
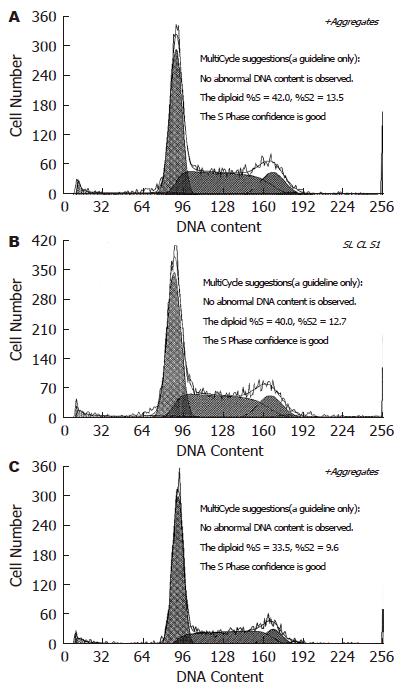
共培养12 h后, MSCs实验组与空白对照组、阴性对照组比较, HSCs停滞在G0/G1期的细胞数明显增多(P<0.01), S期细胞数显著减少(P<0.01); 24、48、72 h, G0/G1期的细胞数分别为49.45%±0.95%、54.28%±0.99%、58.64%±1.10%, S期细胞数分别为38.86%±1.17%、35.42%±0.94%、33.5%±0.78%. 共培养全过程中, 空白对照组与阴性对照组比较无差异(图8).
共培养12 h, MSCs实验组RhoA mRNA表达(0.89±0.02)较空白对照组(1.06±0.02)显著下调(P<0.01), 此后迅速下调, 72 h达到最低水平(0.37±0.05); 共培养各时间段中, RhoA mRNA表达, 阴性对照组(1.07±0.03, 1.03±0.05, 1.06±0.03, 1.04±0.07, 1.01±0.06, 0.96±0.10)与空白对照组(1.08±0.02, 1.04±0.03, 1.06±0.02, 0.96±0.08, 1.00±0.06, 0.92±0.07)比较均无差异(图9); 共培养全过程中, 各组P27 mRNA表达的比较均无差异(图10). RhoA与P27 mRNA表达无明显相关(r = -0.105).
共培养后12 h, MSCs实验组RhoA蛋白表达(0.86±0.07)较空白对照组(1.11±0.12)显著下调(P<0.01), 此后缓慢下降, 72 h达到最低水平(表1, 图11); 共培养后12 h, MSCs实验组P27蛋白表达(0.39±0.03)较空白对照组(0.20±0.04)上调(P<0.05), 至24 h MSCs实验组P27蛋白(0.73±0.07)表达较空白对照组(0.20±0.04)显著上调(P<0.01), 此后持续维持高表达状态(表2, 图12). 阴性对照组与空白对照组RhoA、P27蛋白表达在共培养的各个时间点比较均无差异. RhoA与P27蛋白表达呈显著负相关(r = -0.943, P<0.01).
HSCs是肝纤维化的中心环节, 其活化后可向肌成纤维细胞转型. 活化型星状细胞有较大的胶原合成能力[15], 大量合成细胞外基质(extracellular matrix, ECM), 导致肝内ECM过量积聚, 最终导致肝纤维化和肝硬化形成. MSCs是骨髓来源干细胞[16-17], 具有多向分化潜能. 在体内外研究中, MSCs可成功分化为肝样细胞[18-19]. MSCs通过外周静脉移植可有效逆转肝纤维化或肝硬化, 阻止肝组织病理学变化[20-22]. MSCs定植入肝脏之后, 通过与肝细胞及星状细胞之间的旁分泌及其信号通路传导发挥重要作用[23].Shiotani et al[11]研究发现抑制Rho-ROCK信号通路活化可以阻断HSCs增殖、收缩和迁移, 促进HSCs凋亡. 同时, 我们前期研究[10]发现, Rho家族蛋白在治疗大鼠急慢性肝损伤中发挥重要作用. MSCs可下调RhoA-ROCK信号转导通路活性加速肝脏修复. 在体外MSCs与HSCs共培养研究中, MSCs可抑制HSCs的活性、增殖及其RhoA蛋白的表达. 可以推断, RhoA活性下调可能在MSCs对HSCs增殖抑制过程中起着重要作用.
本研究发现, MSCs与HSCs共培养后, 随着时间的延长, HSCs细胞形态逐渐改变, 由0 h呈星形, 胞体大, 膜状伸展, 折光颗粒较少, 至48 h呈椭圆形, 膜状伸展减弱, 包体变小, 折光颗粒增多, 细胞贴壁减弱, 细胞数减少, 至72 h呈圆形或椭圆形, 无膜状伸展, 折光颗粒密集, 贴壁差, 细胞数明显减少; HSCs细胞增殖率亦下降明显, 这种增殖抑制作用在共培养24 h后更明显; 在对HSCs细胞周期分析时, 我们发现, 共培养12 h后, HSCs停滞在G0/G1期的细胞数增加开始明显, 而S期的细胞数亦开始显著减少, 至72 h时, 这种离散的变化趋势更加显著; 在蛋白水平上, 共培养12 h后, HSCs内RhoA蛋白表达显著减少, 而P27蛋白表达明显增加, P27蛋白与RhoA蛋白表达的变化存在显著的负相关关系. 不难发现, 这些变化表现出明显的时间依赖性, 他们各自的变化存在紧密的联系. 研究证实, RhoA可通过调控cyclinE/CDK2[5]、PI3K通路[6]、Skp2[8]的活性而调控P27蛋白的表达与降解, 最终改变细胞周期的G1/S期进程; RhoA还可调控CIP/KIP家族肿瘤抑制因子(P21、P27、P57)或选择性抑制RhoA或其效应因子mammalian Diaphanous 1和Rho激酶(ROCK), 有效地抑制细胞增殖和细胞周期G1/S期转化[24]. 此外, 在正常细胞中, P27蛋白量在细胞周期G0期是较高的, 而在有丝分裂原诱导细胞进入G1期时P27蛋白量会迅速地减少[25-26]. P27蛋白高表达可使细胞停滞在G1期时段, 抑制P27表达可以增加S期细胞的比率[27]. 可见, MSCs抑制HSCs RhoA的高表达, 而RhoA活性的下调导致P27蛋白降解的减少, P27蛋白在胞内大量聚积, 致使大量HSCs停滞在细胞周期的G0/G1期, 即停滞在细胞周期的DNA合成前期, 最终导致HSCs细胞分裂、增殖减少, 活性减低, 并促进细胞凋亡.
然而, 本研究各组在共培养全过程中, P27 mRNA表达没有明显变化; 在MSCs实验组中, P27蛋白水平却能随着RhoA活性下调而呈增多趋势. 有学者认为, P27在转录水平不受调控[28-29], P27蛋白上调可能与胞质内P27蛋白降解受阻有关. P27蛋白表达的调节方式主要发生在翻译后水平, 即泛素蛋白酶体途径介导的P27蛋白降解[30-31], 该途径的激活需要Skp2蛋白的活化. 当Skp2-P27复合物形成, 即可致使P27蛋白泛素化及降解[32-37], 而RhoA可以通过调控Skp2-P27通路而促进细胞周期G1/S期转换[8].
在共培养模型中, HSCs在0, 6, 12 h各时段内细胞形态、活性、增殖抑制率及其RhoA、P27蛋白和mRNA表达水平均无明显变化, 这可能与两细胞各自通过旁分泌或自分泌某些细胞因子和生长因子有关, 如IL-10、TNF-α、GM-CSF[38]、HGF[19]和NGF[39]等, 这些活性因子可能相互影响, 并导致微环境的改变.
总之, MSCs可能通过RhoA-P27通路调控HSCs的细胞周期G1/S期转换, 抑制HSCs的增殖并促进其凋亡. RhoA活性的下调可能是引起HSCs P27蛋白表达增加的原因. 然而本研究应用的共培养体系所涉及的微环境极为复杂, 深入研究该体系内各种信号因子的改变, 对于阐明MSCs与HSCs作用机制具有深远意义.
MSCs是一类具有自我增殖和分化潜能的多能干细胞, MSCs移植治疗肝纤维化的效果已得到初步肯定, 但其治疗机制尚不明确. 研究体外MSCs与HSCs相互干预的作用机制具有重要的意义.
张凤春, 教授, 上海交通大学医学院附属仁济医院肿瘤中心
近年来, 国内外异体或自体MSCs移植治疗肝病的研究越发增多, 但采用构建MSCs和HSCs共培养的微环境来研究其相互作用机制的研究却极少.
Arnaud et al报道, P27调节细胞迁移是通过调控RhoA的活性而起作用的; 而Shiotani et al研究发现, MSCs可下调RhoA-ROCK信号转导通路活性, 并能抑制HSCs活化、增殖与迁移, 促进HSCs凋亡.
本文首次构建MSCs和HSCs共培养的微环境, 研究MSCs抑制HSCs RhoA活化, 并导致细胞周期的关键调控因子P27胞内表达增加, 阻滞HSCs的增殖、分化. 这可能是MSCs逆转肝纤维化的作用机制之一.
本实验研究MSCs与HSCs共培养后HSCs内RhoA、P27表达的变化, 说明MSCs可能通过RhoA-P27通路调控HSCs的增殖、分化, 为MSCs移植治疗肝纤维化提供新的理论依据.
本文统计学方法正确, 研究内容有一定的创新性.
编辑: 李军亮 电编: 吴鹏朕
| 1. | Polyak K, Lee MH, Erdjument-Bromage H, Koff A, Roberts JM, Tempst P, Massague J. Cloning of p27Kip1, a cyclin-dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell. 1994;78:59-66. [PubMed] [DOI] |
| 2. | Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67-74. [PubMed] [DOI] |
| 3. | Slingerland J, Pagano M. Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol. 2000;183:10-17. [PubMed] [DOI] |
| 4. | Weber JD, Hu W, Jefcoat SC Jr, Raben DM, Baldassare JJ. Ras-stimulated extracellular signal-related kinase 1 and RhoA activities coordinate platelet-derived growth factor-induced G1 progression through the independent regulation of cyclin D1 and p27. J Biol Chem. 1997;272:32966-32971. [PubMed] [DOI] |
| 5. | Hu W, Bellone CJ, Baldassare JJ. RhoA stimulates p27(Kip) degradation through its regulation of cyclin E/CDK2 activity. J Biol Chem. 1999;274:3396-3401. [PubMed] [DOI] |
| 6. | Seasholtz TM, Zhang T, Morissette MR, Howes AL, Yang AH, Brown JH. Increased expression and activity of RhoA are associated with increased DNA synthesis and reduced p27(Kip1) expression in the vasculature of hypertensive rats. Circ Res. 2001;89:488-495. [PubMed] [DOI] |
| 7. | Vidal A, Millard SS, Miller JP, Koff A. Rho activity can alter the translation of p27 mRNA and is important for RasV12-induced transformation in a manner dependent on p27 status. J Biol Chem. 2002;277:16433-16440. [PubMed] [DOI] |
| 8. | Mammoto A, Huang S, Moore K, Oh P, Ingber DE. Role of RhoA, mDia, and ROCK in cell shape-dependent control of the Skp2-p27kip1 pathway and the G1/S transition. J Biol Chem. 2004;279:26323-26330. [PubMed] [DOI] |
| 9. | Besson A, Gurian-West M, Schmidt A, Hall A, Roberts JM. p27Kip1 modulates cell migration through the regulation of RhoA activation. Genes Dev. 2004;18:862-876. [PubMed] [DOI] |
| 11. | Shiotani S, Shimada M, Suehiro T, Soejima Y, Yosizumi T, Shimokawa H, Maehara Y. Involvement of Rho-kinase in cold ischemia-reperfusion injury after liver transplantation in rats. Transplantation. 2004;78:375-382. [PubMed] [DOI] |
| 13. | Parekkadan B, van Poll D, Megeed Z, Kobayashi N, Tilles AW, Berthiaume F, Yarmush ML. Immunomodulation of activated hepatic stellate cells by mesenchymal stem cells. Biochem Biophys Res Commun. 2007;363:247-252. [PubMed] [DOI] |
| 14. | Shi L, Li G, Wang J, Sun B, Yang L, Wang G, Wang D, Mu L, Chen H, Jin L. Bone marrow stromal cells control the growth of hepatic stellate cells in vitro. Dig Dis Sci. 2008;53:2969-2974. [PubMed] [DOI] |
| 15. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] |
| 16. | Jiang Y, Vaessen B, Lenvik T, Blackstad M, Reyes M, Verfaillie CM. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896-904. [PubMed] [DOI] |
| 17. | Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483-3493. [PubMed] [DOI] |
| 18. | Oh SH, Witek RP, Bae SH, Zheng D, Jung Y, Piscaglia AC, Petersen BE. Bone marrow-derived hepatic oval cells differentiate into hepatocytes in 2-acetylaminofluorene/partial hepatectomy-induced liver regeneration. Gastroenterology. 2007;132:1077-1087. [PubMed] [DOI] |
| 19. | Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742-748. [PubMed] [DOI] |
| 20. | Sakaida I, Terai S, Yamamoto N, Aoyama K, Ishikawa T, Nishina H, Okita K. Transplantation of bone marrow cells reduces CCl4-induced liver fibrosis in mice. Hepatology. 2004;40:1304-1311. [PubMed] [DOI] |
| 21. | Zhao DC, Lei JX, Chen R, Yu WH, Zhang XM, Li SN, Xiang P. Bone marrow-derived mesenchymal stem cells protect against experimental liver fibrosis in rats. World J Gastroenterol. 2005;11:3431-3440. [PubMed] |
| 22. | Ueno T, Nakamura T, Torimura T, Sata M. Angiogenic cell therapy for hepatic fibrosis. Med Mol Morphol. 2006;39:16-21. [PubMed] [DOI] |
| 23. | Matsuda-Hashii Y, Takai K, Ohta H, Fujisaki H, Tokimasa S, Osugi Y, Ozono K, Matsumoto K, Nakamura T, Hara J. Hepatocyte growth factor plays roles in the induction and autocrine maintenance of bone marrow stromal cell IL-11, SDF-1 alpha, and stem cell factor. Exp Hematol. 2004;32:955-961. [PubMed] [DOI] |
| 24. | Zhang S, Tang Q, Xu F, Xue Y, Zhen Z, Deng Y, Liu M, Chen J, Liu S, Qiu M. RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res. 2009;7:570-580. [PubMed] [DOI] |
| 25. | Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570-573. [PubMed] [DOI] |
| 26. | Reynisdottir I, Polyak K, Iavarone A, Massague J. Kip/Cip and Ink4 Cdk inhibitors cooperate to induce cell cycle arrest in response to TGF-beta. Genes Dev. 1995;9:1831-1845. [PubMed] [DOI] |
| 27. | Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27Kip1 for restriction point control of the fibroblast cell cycle. Science. 1996;272:877-880. [PubMed] [DOI] |
| 28. | Singh SP, Lipman J, Goldman H, Ellis FH Jr, Aizenman L, Cangi MG, Signoretti S, Chiaur DS, Pagano M, Loda M. Loss or altered subcellular localization of p27 in Barrett's associated adenocarcinoma. Cancer Res. 1998;58:1730-1735. [PubMed] |
| 30. | Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M. Role of the ubiquitin-proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science. 1995;269:682-685. [PubMed] [DOI] |
| 31. | Nakayama KI, Hatakeyama S, Nakayama K. Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem Biophys Res Commun. 2001;282:853-860. [PubMed] [DOI] |
| 32. | Carrano AC, Eytan E, Hershko A, Pagano M. SKP2 is required for ubiquitin-mediated degradation of the CDK inhibitor p27. Nat Cell Biol. 1999;1:193-199. [PubMed] [DOI] |
| 33. | Montagnoli A, Fiore F, Eytan E, Carrano AC, Draetta GF, Hershko A, Pagano M. Ubiquitination of p27 is regulated by Cdk-dependent phosphorylation and trimeric complex formation. Genes Dev. 1999;13:1181-1189. [PubMed] [DOI] |
| 34. | Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. p45SKP2 promotes p27Kip1 degradation and induces S phase in quiescent cells. Nat Cell Biol. 1999;1:207-214. [PubMed] [DOI] |
| 35. | Nakayama K, Nagahama H, Minamishima YA, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N. Targeted disruption of Skp2 results in accumulation of cyclin E and p27(Kip1), polyploidy and centrosome overduplication. EMBO J. 2000;19:2069-2081. [PubMed] [DOI] |
| 36. | Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol. 2001;3:321-324. [PubMed] [DOI] |
| 37. | Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI. A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell. 2001;7:639-650. [PubMed] [DOI] |
| 38. | Yannaki E, Athanasiou E, Xagorari A, Constantinou V, Batsis I, Kaloyannidis P, Proya E, Anagnostopoulos A, Fassas A. G-CSF-primed hematopoietic stem cells or G-CSF per se accelerate recovery and improve survival after liver injury, predominantly by promoting endogenous repair programs. Exp Hematol. 2005;33:108-119. [PubMed] [DOI] |