修回日期: 2008-02-26
接受日期: 2008-03-08
在线出版日期: 2008-03-28
目的: 研究转染乙型肝炎病毒(HBV)的HepG2.2.15细胞株在体外促进肝星状细胞中CTGF和TGF-β1的表达, 进而探讨HBV促肝细胞纤维化的机制.
方法: 将HepG2和HepG2.2.15细胞株分别在体外与肝星状细胞(LX-2)共培养, 以单独培养的肝星状细胞为对照组. 培养24, 48, 72 h后, 以Real-time PCR定量检测肝星状细胞中CTGF和TGF-β1 mRNA的表达, 以Western blot定量检测其蛋白表达.
结果: 与对照组比较, 在24、48、72 h时点, CTGF和TGF-β1 mRNA分别增高约1.7、4.2、9.6倍(P<0.05)和2.2、6.1、8.1倍(P<0.01), 以72 h差异最为显著; 而与HepG2细胞共培养实验组LX-2细胞CTGF和TGF-β1 mRNA在三个时间点分别增高约1.7、1.2、1.3倍(P<0.05)和2.7、1.9、2.1倍(P<0.05). CTGF和TGF-β1蛋白表达量分别增高约2.1、2.6、2.5倍(P<0.05)和1.7、3.3、3.1倍(P<0.01), 以48 h差异最为显著; 而与HepG2细胞共培养实验组LX-2细胞CTGF和TGF-β1蛋白表达量在三个时间点分别增高约1.6、1.1、0.9倍(P<0.05)和1.1、1.4、2.5倍(P<0.05).
结论: 与HepG2.2.15细胞株共培养后, 肝星状细胞中肝纤维化相关因子的表达明显增强. 体外实验证明HBV具有诱导肝细胞纤维化的重要作用.
引文著录: 哈明昊, 饶慧瑛, 刘峰, 潘孝本, 封波, 陈红松, 魏来. 乙型肝炎病毒促进CTGF和TGF-β1在肝星状细胞中的表达. 世界华人消化杂志 2008; 16(9): 924-928
Revised: February 26, 2008
Accepted: March 8, 2008
Published online: March 28, 2008
AIM: To investigate whether HepG2.2.15 cell line induces the expression of connective tissue growth factor (CTGF) and transforming growth factor-β1 (TGF-β1) in hepatic stellate cells and the mechanism of hepatitis B virus (HBV) in inducing fibrogenesis.
METHODS: The hepatic stellate cells (LX-2) were co-cultured with HepG2 or HepG2.2.15 in vitro and the LX-2 cells cultured alone were used as controls. After culturing for 24, 48 and 72 h, real-time polymerase chain reaction (PCR) was performed to detect the expression of CTGF and TGF-β1 mRNA in LX-2 cells. Western-blot analysis was used to measure the expression of CTGF and TGF-β1 proteins in LX-2 cells.
RESULTS: After 24, 48, and 72 h, the expression of CTGF and TGF-β1 mRNA in LX-2 cells co-cultured with HepG2.2.15 were higher than those in the controls (CTGF: 1.7, 4.2, 9.6 times higher, P < 0.05; TGF-β1: 2.2, 6.1, 8.1 times higher, P < 0.01), and the most eminent effect was found at 72 h; however, CTGF and TGF-β1 mRNA expression in LX-2 cells co-cultured with HepG2 were 1.7, 1.2, 1.3 and 2.7, 1.9, 2.1 times higher than those in the controls (all P < 0.05). At the same time point, the protein expression of CTGF and TGF-β1 in LX-2 cells co-cultured with HepG2.2.15 (CTGF: 2.1, 2.6, 2.5 times higher, P < 0.05; TGF-β1: 1.7, 3.3, 3.1 times higher, P < 0.01) or HepG2 (CTGF: 1.6, 1.1, 0.9 times higher, P < 0.05; TGF-β1: 1.1, 1.4, 2.5 times higher, P < 0.05) were also higher than those in the control cells.
CONCLUSION: The expression of fibrosis-related factors in hepatic stellate cells are increased significantly after co-culturing with HepG2.2.15, which proves that HBV can induce fibrogenesis in vitro.
- Citation: Ha MH, Rao HY, Liu F, Pan XB, Feng B, Chen HS, Wei L. Hepatitis B virus induces expression of connective tissue growth factor and transforming growth factor-β1 in hepatic stellate cells. Shijie Huaren Xiaohua Zazhi 2008; 16(9): 924-928
- URL: https://www.wjgnet.com/1009-3079/full/v16/i9/924.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i9.924
乙型肝炎病毒(hepatitis B virus, HBV)感染呈世界性流行, 但不同地区HBV感染的流行强度差异很大. 全世界有3.5亿慢性HBV感染者, 每年约有50-120万人死于HBV感染相关的并发症[1-4]. HBV的感染导致肝细胞的损伤和炎症, 与肝纤维化和肝细胞癌的发生呈强相关性[5-8].
肝纤维化的发生是由于细胞外基质(extracellular matrix, ECM)的过多积聚, 构成ECM的主要成分是胶原纤维Ⅰ和胶原纤维Ⅲ[9-13]. 激活的肝星状细胞(hepatic stellate cell, HSC)是合成ECM的主要效应细胞[16-18]; 转化生长因子(transforming growth factor, TGF)-β1是促纤维化合成最重要的细胞因子, 能强有力的促进ECM的合成和积聚[19-21]; 结蒂组织生长因子(connective tissue growth factor, CTGF)是在TGF-β1诱导作用下, 由人上皮细胞合成分泌, 作为TGF-β1下游调控因子调控纤维化反应[22-25].
虽然研究表明HBV与肝细胞纤维化、肝硬化呈强相关性; 但是HBV致肝细胞纤维化的机制仍未阐明. 特别是感染HBV的肝细胞是否促进了肝细胞纤维化的发生, 如果感染了HBV的肝细胞促进了肝细胞纤维化的发生, 其机制及调控通路是什么. 本研究利用体外细胞共培养技术对这一课题进行研究.
DMEM(dulbecco's modified eagle's medium)高糖培养基以及胎牛血清(fetal bovine serum, FBS)购自美国Hyclone公司; RNA反转录试剂, 荧光定量PCR反应扩增仪TaKaRa Ex Taq R-PCR Version.2.1均购自TaKaRa公司; 人肝星状细胞系LX-2, 由Friedman教授和徐列明教授(上海中医药大学肝病研究所)惠赠, HepG2和HepG2.2.15细胞系为本所保存细胞系, Transwell细胞共培养系统购自Corning公司, 小鼠抗人CTGF和TGF-β1一抗购自R&D公司, PBS由北京大学人民医院生产. BCATM蛋白定量试剂盒和ECL Western印染发光试剂购自Pierce公司, 硝酸纤维素膜购自Gelman公司.
1.2.1 细胞共培养: 以含100 mL/L FBS DMEM培养基分别培养LX-2、HepG2和HepG2.2.15细胞, 选取生长状态良好的细胞, 按照HepG2和HepG2.2.15细胞与LX-2细胞比例分别为4∶1接种于Transwell细胞共培养系统中. HepG2和HepG2.2.15细胞接种于可渗透性滤膜上层; LX-2细胞接种于可渗透性滤膜下层. LX-2细胞接种密度为5×107个/L, HepG2和HepG2.2.15细胞接种密度为2×108个/L. 对照组LX-2细胞按照5×107个/L密度接种于普通6孔板, 无其他细胞与其共培养. 等待细胞生长至50%融合度时以PBS漂洗三遍, 加入含2 mL/L FBS DMEM培养基培养至72 h.
1.2.2 引物设计: 利用Bioedit软件设计引物. CTGF上游引物序列: TGCGACTCCAC CCTCCAGC,下游引物序列: GGCGGTC ATGGTTGGCACTG; TGF-β1上游引物序列: TTGCTCCCTCCCTGCCCC, 下游引物序列: CAGGAGACAGGCCGGGGATG.
1.2.3 RNA逆转录: 逆转录体系为: Total RNA 1.5 μg, MgCl2 2 μL, 10×RT Buffer 1 μL, dNTP 1 μL, RNase Inhibitor 0.25 μL, AMV Rverse Transcriptase 0.5 μL, Oligo dT primer 0.5 μL, RNase free ddH2O 3.75 μL. 反应按照以下过程进行: 30℃ 10 min, 42℃ 50 min, 95℃ 5 min, 4℃ 5 min.
1.2.4 Real-time PCR(Sybr Green法): 反应体系为: TaKaRa Ex Taq 0.25 μL, PCR Forward Primer 0.5 μL, PCR Reverse Primer 0.5 μL, dNTP Mixture 0.75 μL, 模板1 μL, Mg2+ Solution 0.5 μL, 5×Real time PCR Buffer(Mg2+ Free)5 μL, Even green 2.5 μL, 加ddH2O至总体系25 μL. PCR反应条件: 95℃ 120 s, 95℃ 15 s, 60℃ 20 s, 72℃ 20 s. 将预实验的PCR产物按照测A值后以10倍浓度梯度进行稀释, 选择1/1000, 1/10 000, 1/100 000, 1/1 000 000浓度的稀释产物作为标准品模版, 进行荧光定量PCR反应并同时在荧光定量PCR仪中输入以上4个浓度梯度的浓度数值. 通过这4个标准品生成的反应数据, 软件Rotor-Gene 6.0根据反应的荧光实时监控数据和标准品的浓度关系, 生成标准曲线. 通过此标准曲线来计算在标准曲线所划定的CT值时的样品浓度. 通过所得到的浓度可计算出样品在此刻的拷贝数.
1.2.5 Western blot: 参照Bio-Rad公司提供的配胶方案配制12%分离胶和5%积层胶. 积层胶聚合时, 蛋白样品与2×上样缓冲液等体积混合, 100℃煮沸5 min使蛋白变性. 用微量移液器按预定顺序加样, 每孔上样50 μg总蛋白. 上样后, 200 V稳压条件下进行电泳, 直至溴酚兰到达分离胶底部(约45 min), 关闭电源结束电泳. 转膜后, 把硝酸纤维素膜转移到杂交袋中室温下摇床预杂交3 h按0.1 mL/cm2膜面积加入5% Blotto预杂交液及分别加入1∶200至1∶1000稀释度的小鼠抗CTGF和TGF-β1抗体, 4℃摇床过夜. 5% Blotto预杂交液漂洗3次, 每次15 min. 按0.1 mL/cm2膜面积加入5% Blotto预杂交液及分别加入1∶1000稀释度的辣根过氧化物酶标记的抗鼠IgG抗体, 室温下摇床杂交45 min. 5% Blotto预杂交液漂洗1次, 15 min, 0.05% TBS-T漂洗2次, 每次10 min, 1×TBS漂洗1次, 10 min. ECL显影, 用Umax2100XL扫描仪以及Quantity One图像分析软件测定条带的吸光度值(A), 以CTGF和TGF-β1的光密度值/β-actin的光密度值代表CTGF和TGF-β1蛋白的相对表达量.
统计学处理 数据均以mean±SD表示, 用SPSS11.0统计软件, 组间差异采用单因素方差分析.
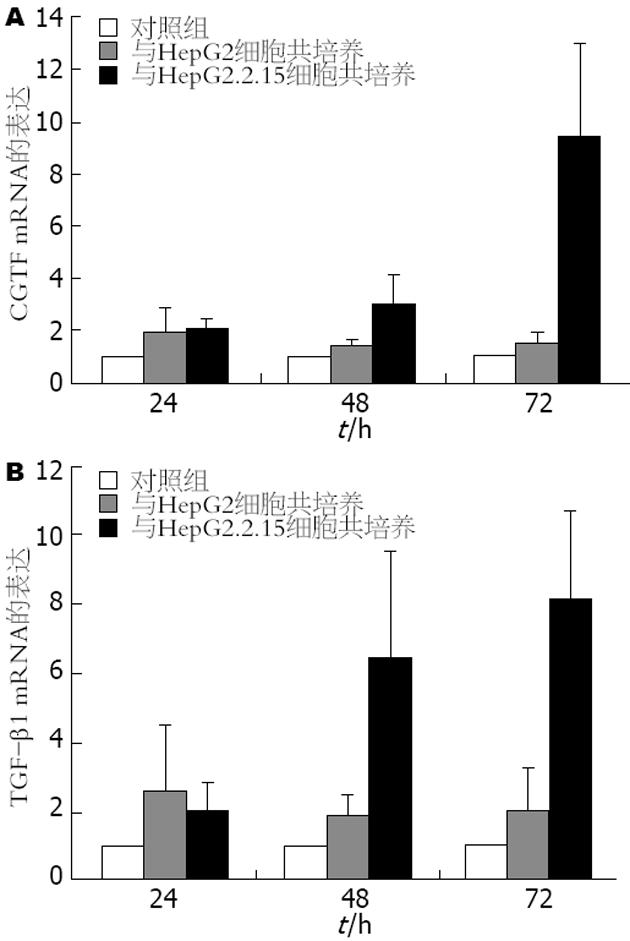
各实验组均取同样条件下培养的3份标本进行检测, 取其平均值. 将单独培养组LX-2细胞中CTGF和TGF-β1 mRNA的表达量设定为1倍, 将共培养实验组LX-2细胞中CTGF和TGF-β1 mRNA表达量与其比较. 发现, 与HepG2细胞共培养实验组LX-2细胞CTGF和TGF-β1 mRNA在24、48、72 h时间点分别增高约1.7、1.2、1.3倍(P<0.05)和2.7、1.9、2.1倍(P<0.05); 与HepG2.2.15细胞共培养实验组LX-2细胞CTGF和TGF-β1 mRNA在24、48、72 h时间点分别增高约1.7、4.2、9.6倍(P<0.05)和2.2、6.1、8.1倍(P<0.01). 其中以72 h与HepG2.2.15共培养组LX-2细胞中CTGF和TGF-β1 mRNA表达差异最为显著(图1).
各实验组均取同样条件下培养的3份标本进行检测, 取其平均值. 发现与正常对照组相比, 与HepG2细胞共培养实验组LX-2细胞CTGF和TGF-β1蛋白表达量在24、48、72 h时间点分别增高约1.6、1.1、0.9倍(P<0.05)和1.1、1.4、2.5倍(P<0.05); 与HepG2.2.15细胞共培养实验组LX-2细胞CTGF和TGF-β1蛋白表达量在24、48、72 h时间点分别增高约2.1、2.6、2.5倍(P<0.05)和1.7、3.3、3.1倍(P<0.01). 其中以48 h与HepG2.2.15共培养组LX-2细胞中CTGF和TGF-β1蛋白表达量增高最为显著(图2).
多种研究表明, 抑制HSC的表达明显减少肝纤维化基质的沉积[26-27]. 至目前为止, 尚未有研究表明HBV能够感染HSC, 也未有感染了HBV的HSC细胞建系成功. 缺乏HBV直接感染HSC的证据, 对于研究HBV致肝细胞纤维化的机制提出新的难题. 转染了HBV的HepG2.2.15细胞上清中含有HBV的各种蛋白及HBV的复制中间体[28], 是目前研究HBV的有效模型. 我们对HepG2.2.15细胞与HSC共培养后能否促进HSC纤维化表达增强进行了研究.
在实验中我们利用了细胞共培养技术. Transwell细胞共培养系统的优点是: 利用聚碳酸酯膜将两种细胞分隔开, 避免了两种细胞的直接作用, 而直径小的多的细胞因子可以自由通过膜, 在细胞间发挥调控作用.
TGF-β1在肝纤维化发生过程中具有重要作用. TGF-β1诱导ECM的合成和积聚, 是肝纤维化反应的中心调控因子. TGF-β1还能促进胶原纤维的合成并下调金属基质蛋白的表达[29-31].
本文对与HepG2和HepG2.2.15细胞共培养后的LX-2细胞中CTGF和TGF-β1的表达, 从基因水平和蛋白水平进行了检测. 本研究发现, 同对照组相比, 与HepG2和HepG2.2.15细胞共培养后的LX-2细胞中CTGF和TGF-β1的表达水平均有明显升高, 与HepG2.2.15细胞共培养后效应更为显著. 其中与HepG2.2.15细胞共培养后CTGF和TGF-β1 mRNA的表达在72 h升高最为明显; CTGF和TGF-β1蛋白的表达量在48 h亦有明显升高.
本研究利用体外细胞共培养技术, 证明了与HepG2细胞相比, HepG2.2.15细胞能明显促进HSC中CTGF和TGF-β1的表达. 而CTGF和TGF-β1在肝纤维化的过程中具有重要促进作用, 我们的实验证明HBV可能通过促进HSC中肝纤维化相关调控蛋白的表达, 发挥重要的致肝细胞纤维化的作用.
乙型肝炎病毒(HBV)感染呈世界性流行, HBV的感染导致肝细胞的损伤和炎症, 与肝纤维化和肝细胞癌的发生呈强相关性.
党双锁, 副教授, 西安交通大学第二医院感染科.
本研究以体外共培养实验证明HBV通过感染肝细胞释放促肝纤维化因子诱导肝纤维化的形成.
本文证明HBV感染后致肝纤维化的调控途径, 明确了HBV促进释放的重要促纤维化因子, 为临床治疗HBV感染后肝纤维化指明靶点.
细胞共培养技术: 是指以Transwell细胞共培养系统, 利用聚碳酸酯膜将两种细胞分隔开, 避免了两种细胞的直接作用, 而直径小的多的细胞因子可以自由通过膜,在细胞间发挥调控作用.
本研究实用性强, 方法先进, 结果可靠, 是一篇好的论文.
编辑: 程剑侠 电编:何基才
| 1. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [DOI] |
| 2. | Steffen M, Cornberg M, Buggisch P. Treatment of chronic hepatitis C with consensus interferon in relapsers and non-responders to interferon-based therapy. Hepatogastroenterology. 2007;54:2368-2372. [PubMed] |
| 3. | Kogo M, Sato N, Yoneyama K, Imawari M, Kiuchi Y. Bleeding index after the first course of endoscopic treatment for esophageal varices in liver cirrhotic patients with and without hepatocellular carcinoma. Hepatogastroenterology. 2007;54:2049-2054. [PubMed] |
| 4. | Delgado JS. Evolving trends in nonalcoholic fatty liver disease. Eur J Intern Med. 2008;19:75-82. [PubMed] [DOI] |
| 5. | Santambrogio R, Costa M, Barabino M, Opocher E. Laparoscopic radiofrequency of hepatocellular carcinoma using ultrasound-guided selective intrahepatic vascular occlusion. Surg Endosc. 2008;. [PubMed] [DOI] |
| 6. | Raoul JL. Natural history of hepatocellular carcinoma and current treatment options. Semin Nucl Med. 2008;38:S13-S18. [PubMed] [DOI] |
| 7. | Chen YB, Sun YA, Gong JP. Effects of rapamycin in liver transplantation. Hepatobiliary Pancreat Dis Int. 2008;7:25-28. [PubMed] |
| 8. | Kishor S, Turner ML, Borg BB, Kleiner DE, Cowen EW. Cutaneous sarcoidosis and primary biliary cirrhosis: A chance association or related diseases? J Am Acad Dermatol. 2008;58:326-335. [PubMed] [DOI] |
| 9. | Lau JY, Wright TL. Molecular virology and pathogenesis of hepatitis B. Lancet. 1993;342:1335-1340. [PubMed] [DOI] |
| 10. | Hopfner M, Schuppan D, Scherubl H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol. 2008;14:1-14. [PubMed] [DOI] |
| 11. | Andersson KL, Chung RT. Hepatic schistosomiasis. Curr Treat Options Gastroenterol. 2007;10:504-512. [PubMed] [DOI] |
| 12. | McCrudden R, Iredale JP. Liver fibrosis, the hepatic stellate cell and tissue inhibitors of metalloproteinases. Histol. Histopathol. 2000;15:1159-1168. [PubMed] |
| 13. | Knittel T, Kobold D, Saile B, Grundmann A, Neubauer K, Piscaglia F, Ramadori G. Rat liver myofibroblasts and hepatic stellate cells: different cell populations of the fibroblast lineage with fibrogenic potential. Gastroenterology. 1999;117:1205-1221. [PubMed] [DOI] |
| 14. | Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247-2250. [PubMed] [DOI] |
| 15. | Fonseca JC. Natural history of chronic hepatitis B. Rev Soc Bras Med Trop. 2007;40:672-677. [PubMed] [DOI] |
| 16. | Bissell DM. Chronic liver injury, TGF-beta, and cancer. Exp Mol Med. 2001;33:179-190. [PubMed] [DOI] |
| 17. | Gressner AM, Weiskirchen R, Breitkopf K, Dooley S. Roles of TGF-beta in hepatic fibrosis. Front Biosci. 2002;7:d793-d807. [PubMed] [DOI] |
| 18. | Zhang LJ, Yu JP, Li D, Huang YH, Chen ZX, Wang XZ. Effects of cytokines on carbon tetrachloride-induced hepatic fibrogenesis in rats. World J Gastroenterol. 2004;10:77-81. [PubMed] |
| 19. | Paradis V, Perlemuter G, Bonvoust F, Dargere D, Parfait B, Vidaud M, Conti M, Huet S, Ba N, Buffet C. High glucose and hyperinsulinemia stimulate connective tissue growth factor expression: a potential mechanism involved in progression to fibrosis in nonalcoholic steatohepatitis. Hepatology. 2001;34:738-744. [PubMed] [DOI] |
| 20. | Terziyski K, Andonov V, Marinov B, Kostianev S. Exercise performance and ventilatory efficiency in patients with mild and moderate liver cirrhosis. Clin Exp Pharmacol Physiol. 2008;35:135-140. [PubMed] |
| 21. | Chan HL, Tse CH, Mo F, Koh J, Wong VW, Wong GL, Lam Chan S, Yeo W, Sung JJ, Mok TS. High viral load and hepatitis B virus subgenotype ce are associated with increased risk of hepatocellular carcinoma. J Clin Oncol. 2008;26:177-182. [PubMed] [DOI] |
| 22. | Nagase H. Activation mechanisms of matrix metalloproteinases. Biol Chem. 1997;378:151-160. [PubMed] |
| 23. | Sato H, Seiki M. Membrane-type matrix metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem. 1996;119:209-215. [PubMed] [DOI] |
| 24. | Sebastiani G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepat. 2008;15:212-218. [PubMed] [DOI] |
| 25. | La Villa G, Gentilini P. Hemodynamic alterations in liver cirrhosis. Mol Aspects Med. 2008;29:112-118. [PubMed] [DOI] |
| 26. | Roingeard P, Lu SL, Sureau C, Freschlin M, Arbeille B, Essex M, Romet-Lemonne JL. Immunocytochemical and electron microscopic study of hepatitis B virus antigen and complete particle production in hepatitis B virus DNA transfected HepG2 cells. Hepatology. 1990;11:277-285. [PubMed] [DOI] |
| 27. | Meda F, Zuin M, Invernizzi P, Vergani D, Selmi C. Serum autoantibodies: a road map for the clinical hepatologist. Autoimmunity. 2008;41:27-34. [PubMed] [DOI] |
| 28. | Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159-1170. [PubMed] [DOI] |
| 29. | Kuiper JJ, de Man RA, van Buuren HR. Review article: Management of ascites and associated complications in patients with cirrhosis. Aliment Pharmacol Ther. 2007;26 Suppl 2:183-193. [PubMed] [DOI] |
| 30. | Gao B, Jeong WI, Tian Z. Liver: An organ with predominant innate immunity. Hepatology. 2008;47:729-736. [PubMed] [DOI] |
| 31. | Arteel GE. Silencing a killer among us: ethanol impairs immune surveillance of activated stellate cells by natural killer cells. Gastroenterology. 2008;134:351-353. [PubMed] [DOI] |