修回日期: 2008-04-08
接受日期: 2008-04-21
在线出版日期: 2008-04-28
目的: 分析幽门螺杆菌(H. pylori)与人胃腺癌上皮细胞(human gastric adenocarcinoma epithelial, AGS)的相互作用蛋白质组学差异.
方法: 采用双向凝胶电泳分离H. pylori 26695株与AGS细胞相互作用0.5、2、4 h时间点的细胞和黏附菌的全蛋白样品, 考马斯亮兰染色, ImageMster 2D图像分析软件比较分析, 识别差异蛋白, 将差异蛋白点进行胶内酶解, 经基质辅助电离解析飞行时间质谱(MALDI-TOF-TOF/MS)获得肽质量指纹图谱(PMF), 通过搜寻蛋白质数据库完成对差异蛋白的鉴定.
结果: 对0.5、2、4 h时间点图谱差异蛋白点分析共发现主要差异蛋白点66个, 对其中34个(共对应16种蛋白)最终完成准确鉴定, 2 h后8种蛋白开始上调, 其中2种是细胞来源, 6种H. pylori来源; 4 h时4种细胞来源蛋白下调, 并有4种新的蛋白开始被观察到. 在共感染的早期, 细胞的琥珀酸脱氢酶、H. pylori的抗氧化酶类、黏附素以及HSP 60表达上调. 感染晚期主要是细胞来源的亲环素A、新生多肽相关复合物α多肽开始表达, H. pylori来源的尿素酶、非血红素铁蛋白等蛋白明显出现在黏附菌成分中.
结论: H. pylori与AGS细胞相互作用过程中, 细菌和细胞蛋白表达均存在明显时序性变化, 早期相互作用则主要表现为以与黏附等有关的蛋白表达变化, 后期向有利细菌存活和增殖的方向发展, 并呈现出与免疫逃逸和病理损伤相关的变化.
引文著录: 宋衍燕, 赵飞, 肖迪, 马广源, 孟凡亮, 何丽华, 张建中. 幽门螺杆菌与胃上皮细胞不同作用时间点蛋白质组的差异分析. 世界华人消化杂志 2008; 16(12): 1260-1265
Revised: April 8, 2008
Accepted: April 21, 2008
Published online: April 28, 2008
AIM: To analyze the differential expression of proteome in human gastric adenocarcinoma epithelial AGS cells co-cultured with H. pylori.
METHODS: The samples of AGS cells co-cultured with H. pylori 26695 strain at three time-points (0.5, 2 and 4 h) were collected and separated by 2-dimensional polyacrylamide gel electrophoresis (2-DE) technique and computer-assisted image analysis was used to analyze the differential proteomic expression. The significantly differentially expressed proteins were recognized and identified with the 4700 proteomics discovery system.
RESULTS: There were 66 protein spots that were significantly differentially expressed at different time-points, 34 (corresponding to 16 kinds of proteins) of which were identified with matrix-assisted laser desorption/ionization time-of-flight/time-of-flight (MALDI-TOF-TOF). At 2-h time-point, 8 kinds of proteins were up-regulated obviously, of which 2 originated from the cells and 6 from H. pylori. At the 4-h time-point, 4 kinds of cell-originated proteins were down-regulated, and 4 kinds of new proteins were observed. Succinate dehydrogenase iron-sulfur subunit, HpaA, HSP 60 and peroxiredoxin were up-regulated at the early stage of co-infection. However, at the late stage of co-infection, two cell-originated proteins (cyclophilin A, nascent-polypeptide-associated complex alpha polypeptide) and two H. pylori-originated proteins (urease, non-heme iron protein) were found to express.
CONCLUSION: During the interaction between AGS cells and H. pylori, the expression of proteins is associated with adhesion change in the early stage, followed by a favorable alteration in the survival and proliferation of H. pylori as well as immunologic escape and pathological erosion.
- Citation: Song YY, Zhao F, Xiao D, Ma GY, Meng FL, He LH, Zhang JZ. Sequential proteomic characteristics of AGS cells co-cultured with H. pylori strain. Shijie Huaren Xiaohua Zazhi 2008; 16(12): 1260-1265
- URL: https://www.wjgnet.com/1009-3079/full/v16/i12/1260.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v16.i12.1260
幽门螺杆菌(H. pylori)是一种可在人胃黏膜定植, 并与慢性活动性胃炎、萎缩性胃炎、胃溃疡、十二指肠溃疡、胃癌和胃黏膜相关淋巴组织淋巴瘤[1-2]等疾病发生相关的革兰氏阴性菌, 是WHO确认的唯一与癌症发生密切相关的细菌, 研究较多, 但结果千差万别[3-6], 具体的H. pylori的致癌机制仍不清楚. 人胃腺癌上皮细胞(human gastric adenocarcinoma epithelial, AGS)是目前最为常用的研究H. pylori和胃癌相关的细胞模型, 本实验拟应用H. pylori国际标准菌株26695和AGS细胞共同孵育, 在不同时间点取样, 以期发现二者相互作用时对蛋白表达影响的时序性变化, 为更好的理解H. pylori感染和致病机制提供有意义的线索.
H. pylori 26695菌株, 全基因组测序国际标准菌株, 美国华盛顿大学医学院分子生物学系惠赠, 中国疾病预防控制中心传染病预防控制所传染病诊断室保存. AGS细胞(ATCCCRL1739), 北京大学公共卫生学院惠赠. Bradford试剂盒购自Biorad公司.
1.2.1 蛋白样品制备: AGS细胞于100 mL/L FBS 1640培养基, 50 mL/L CO2气体培养环境, 37℃培养48 h; H. pylori 26695接种于含50 mL/L绵羊全血哥伦比亚琼脂平板上, 置50 mL/L O2, 100 mL/L CO2, 850 mL/L N2的微需氧气体环境中37℃培养72 h后刮取菌苔用PBS洗3次, 5000 g离心10 min, 去上清, 充分悬浮细菌, 测定A600值, 按照A600 = 2.2×108, 细胞每培养瓶计数2×10 , 细胞与细菌的比例按照1:300, 二者分别相互作用0.5, 2, 4 h, 弃去细胞培养液体, 预冷的PBS洗3次, 细胞刮刮取细胞培养皿底部的细胞, PBS液10 mL, 4000 r/min, 4℃离心10 min, 计3次. 每皿细胞平均加入1.5 mL的裂解液, 冰浴30 kHz超声, 超声2 s间隔2 s直至完全溶解; 加入蛋白酶、核酸酶抑制剂, 10 μL/mL; 室温1 h, 12 000 r/min, 4℃离心30 min, 取上清[7]. 蛋白定量方法按Bradford[8]方法进行.
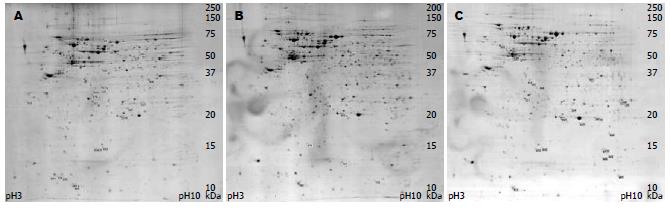
1.2.2 2-DE: 等电聚焦[9-10](IEF)使用24 cm pH3-pH10非线性干胶条(GE Healthcare), 设置聚焦仪(Amersham Biosciences产品)程序: 20℃表面温度, 电流强度50 μA/胶条, 30 V, 6 h; 60 V, 6 h; 100 V, 30 min; 300 V, 1 h; 600 V, 1 h; 1000 V, 1 h; 8000 V, 10 h; 聚焦至VhT 90 000左右. 等电聚焦结束后, 将干胶条分别在130 mmol/L的DTT(Amersham Biosciences产品)和135 mmol/L的碘乙酰氨(Amersham Biosciences产品)中振荡平衡15 min后转至125 g/L聚丙烯酰胺凝胶, 使用垂直电泳仪(Ettan DALTtwelve System, GE Healthcare, Uppsala, Sweden)跑二向电泳, 25℃, 自动泵, 2.5 W/胶, 30 min, 18 W/胶, 4.5 h. 考马斯亮兰染色[11], 扫描仪采集图像, ImageMaster 2D Platinum 5.0分析软件分析图像, 以增加或降低1.5倍以上做为标准, 确定差异蛋白点. 用自动切点仪(SpotPicker, Amersham Biosciences产品)切下各差异点, 胶粒用300 mL/L乙腈的0.1 mol/L NH4HCO3脱色, 采用胰酶(Sigma产品)进行胶上蛋白酶解.
1.2.3 质谱鉴定: ABI4700时间飞行质谱仪进行质谱分析[12], Nd: YAG激光器, 335 nm, 200 Hz激光激发, 应用GSP Explorer Workstation软件搜索NCBInr数据库.
H. pylori与AGS细胞共孵育0.5 h 2-DE图谱中识别蛋白点691个, 2 h蛋白点693个, 4 h蛋白点710个(图1). 蛋白图谱展示AGS细胞和黏附在细胞上的H. pylori菌体全蛋白, 应用ImageMaster 2D Platinum 5.0分析软件分析以volume≥1.5倍以上作为差异点, 将2 h和4 h图谱分别与0.5 h图谱分析, 共发现差异蛋白点66个, 差异点的情况如图2所示.
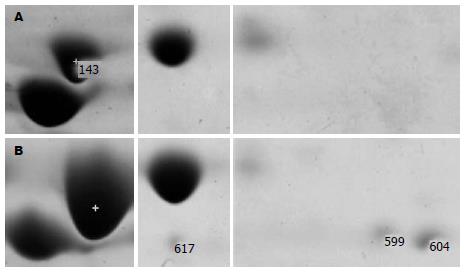
差异点切下, 酶解后经过MALDI-TOF/TOF-MS鉴定, 鉴定出34个蛋白, 鉴定蛋白的等电点和分子量基本与理论值相符, 质谱鉴定结果如表1所示. 共有16种, 其中8种蛋白自2 h开始上调, H. pylori来源的6种, 分别是热休克蛋白(heat shock protein, HSP)60、硫氧还蛋白、烷基过氧化物还原酶、神经氨酰乳糖结合原纤维血凝素(neuraminyllactose-binding hemagglutinin homolog, NLBH, HpaA)、巯基过氧化氢还原酶、γ-谷氨酰转肽酶, 细胞来源的2种, 分别是琥珀酸脱氢酶、泛醌-细胞色素C还原酶; 4种蛋白下调, 全部是细胞来源, 包括ATP合成酶、肌动蛋白、过氧化物酶6(peroxiredoxin 6, PDX6), 未命名蛋白; 有4种蛋白在4 h蛋白图谱提示开始表达: 细胞来源的亲环素A(cyclophilinA, CyPA)、新生多肽相关复合物α多肽(nascent-polypeptide-associated complex alpha polypeptide, NAC)以及H. pylori来源的尿素酶、非血红素铁蛋白.
| Spot No. | Accession No. | Protein | PI/MW(Da) |
| 0.5 h-143 | gi5644643 | chaperonin GroEL (H. pylori) | 5.55/58 227.5 |
| 0.5 h-200 | gi114644226 | mitochondrial ATP synthase, H+ transporting F1 complex beta subunit (Homo sapiens) | 4.95/48 083 |
| 0.5 h-290 | gi164451511 | actin | 5.3/41 796.9 |
| 0.5 h-284 | gi157111829 | actin | 5.29/41 615.8 |
| 0.5 h-193 | gi90077396 | unnamed protein product | 8.46/29 151.2 |
| 0.5 h-478 | gi15644820 | succinate dehydrogenase iron-sulfur subunit (H. pylori 26695) | 5.34/27 633.4 |
| 0.5 h-513 | gi55775699 | V protein [Simian parainfluenza virus 5] | 7.55/23 920 |
| 0.5 h-517 | gi114565485 | peroxiredoxin 6 isoform 1 (Pantroglodytes) | 5.69/23 205.2 |
| 0.5 h-547 | gi54036561 | cytochrome b-c1 complex subunit Rieske | 8.78/29 746.5 |
| 0.5 h-579 | gi11139093 | GrpE-like protein cochaperone (Homo sapiens) | 8.29/24 132.9 |
| 0.5 h-578 | gi15646170 | alkyl hydroperoxide reductase(H. pylori 26695) | 5.88/22 221.4 |
| 0.5 h-689 | gi15611830 | thioredoxin (H. pylori J99) | 5.16/11 847.1 |
| 4 h-205 | gi89574029 | mitochondrial ATP synthase, H+ transporting F1 complex beta subunit (Homo sapiens) | 5.8/57 919.4 |
| 4 h-289 | gi1703127 | actin, cytoplasmic type 8 | 5.31/41 820.8 |
| 4 h-287 | gi1703123 | actin, cytoplasmic type 5 | 5.3/ 41 822.8 |
| 4 h-399 | gi149029725 | nascent-polypeptide-associated complex alpha | 4.87/15 006.8 |
| 4 h-400 | gi149029725 | nascent-polypeptide-associated complex alpha | 4.87/15 006.8 |
| 4 h-515 | gi55775699 | V protein [Simian parainfluenza virus 5] | 7.55/23 920 |
| 4 h-466 | gi15645574 | hypothetical protein HP0958 (H. pylori 26695) | 8.73/29 673.6 |
| 4 h-724 | gi54301401 | alkyl hydroperoxide reducatase (H. pylori) | 5.88/22 222.4 |
| 4 h-600 | gi15646170 | alkyl hydroperoxide reductase (H. pylori 26695) | 5.88/22 221.4 |
| 4 h-652 | gi18482498 | non-heme ferritin protein (H. pylori) | 5.51/19 242.6 |
| 4 h-711 | gi15611830 | thioredoxin (H. pylori J99) | 5.16/11 847.1 |
| 4 h-599 | gi15646170 | alkyl hydroperoxide reductase (H. pylori 26695) | 5.88/22 221.4 |
| 4 h-604 | gi15646170 | alkylhydroperoxide reductase (H. pylori 26695) | 5.88/22 221.4 |
| 4 h-559 | gi54036561 | cytochrome b-c1 complex subunit Rieske | 8.78/29 746.5 |
| 4 h-535 | gi15645038 | putative neuraminyllactose-binding hemagglutinin homolog (hpaA) (H. pylori 26695) | 5.19/53 905.2 |
| 4 h-520 | gi15644703 | bifunctional urease subunit gamma/beta (H. pylori 26695) | 8.46/26 522.9 |
| 4 h-617 | gi54301431 | alkyl hydroperoxide reducatase (H. pylori) | 6.08/22 221.5 |
| 4 h-666 | gi13937981 | peptidylprolyl isomerase A (cyclophilin A) (Homo sapiens) | 7.68/17 999.9 |
| 4 h-656 | gi15645018 | adhesin-thiol peroxidase (H. pylori 26695) | 7.68/18 280.8 |
| 4 h-634 | gi15645732 | amma-glutamyltranspeptidase (H. pylori 26695) | 9.27/61 112.9 |
| 2 h-6041 | gi15646170 | alkyl hydroperoxide reductase (H. pylori 26695) | 5.88/22 221.4 |
| 2 h-760 | gi15611830 | thioredoxin (H. pylori J99) | 5.16/11 847.1 |
胃癌的发生发展是多阶段、很多因素参与这个过程, H. pylori慢性感染与此密切相关, 具体机制尚未清楚. 本实验结果显示0.5、2、4 h三个时间点H. pylori与AGS相互作用的动态变化主要是三羧酸循环代谢相关、抗氧化相关酶类、黏附素以及致病机制相关蛋白变化.
感染早期细菌来源的琥珀酸脱氢酶、H. pylori来源的抗氧化相关酶类、黏附素以及HSP60开始表达上调. 琥珀酸脱氢酶是三羧酸循环代谢相关酶类, 此酶升高表示细胞代谢在早期旺盛, 但随二者相互作用时间延长, H. pylori逐渐占据优势, H. pylori的抗氧化作用相关酶类表达逐渐增强上调, 如硫氧还蛋白和烷基过氧化氢还原酶硫氧蛋白泛醌-细胞色素C还原酶等. 这可能是两个方面的作用结果, 一是H. pylori与AGS共孵育时在有氧环境中, 而H. pylori培养的环境是微需氧的环境, 为适应环境生存H. pylori的代谢改变; 也可能是AGS作为弱抗原提呈细胞感染细菌后引发杀菌机制, 产生依氧杀菌机制, 从而引发氧化酶和其他酶类的活化, 产生多种具有效杀伤活性的活性氧中介物和活性氮中介物, 后者是否参与变化尚需验证. 黏附素HpaA感染2 h开始上调, 并逐渐增强. HpaA是H. pylori的主要黏附素之一, 能够与AGS细胞特异性受体结合, 使二者紧密黏附. 实验结果提示H. pylori和AGS相互作用2 h开始受体配体方式的紧密黏附逐渐加强. 近来研究表明HSP在多种肿瘤细胞表达增强, 与肿瘤的增殖、侵袭力、转移性及凋亡等生物学行为以及与肿瘤免疫、肿瘤耐药性等有关[13-14]. HSP60在浅表性胃炎不同分型的肠化胃黏膜组织、胃癌组织中的表达阳性率呈逐渐增高趋势[15]. 李波清 et al对胃癌和非胃癌来源的菌株进行蛋白质学分析, 发现HSP60存在丙氨酸和苏氨酸的变异, 推测这一结构域可能是区分胃癌与非胃癌菌株的分子特征之一(资料待发表). 本研究中HSP60自2 h开始上调, 表明此蛋白在感染早期就开始发挥作用.
共培养4 h出现4种差异蛋白, 其中H. pylori来源的非血红素铁蛋白是一大类含铁但不含血红素的蛋白, 核苷酸还原酶和脂加氧酶均属于此类蛋白, 前者活性增高可引起DNA的合成增加, 后者在生物膜磷脂氧化性降解起到关键的作用, 这类蛋白的表达提示感染后期细菌的增生旺盛以及对细胞膜的破坏性增强. H. pylori尿素酶A/B亚单位在晚期开始表达, 与H. pylori适应酸性环境代谢相关, 也和H. pylori致病相关. 细胞来源的CyPA是一种在生物界广泛存在、高度保守的蛋白质, 具有肽基脯氨酰顺/反异构酶活性, 参与蛋白质折叠、装配与运输; 在细胞内能与环孢酶素A结合, 参与免疫抑制; 还可介导胆固醇转运, 能发挥前炎性因子的功能, 并在信号转导中具有重要作用, 而且是一种氧化应激诱导分泌的生长因子[16-23]. 实验提示CyPA在H. pylori致病过程中可能起到很重要的作用, 如炎症刺激以及免疫逃逸, 参与H. pylori的持续性感染. 而NAC是与种系发育进化相关的高度保守的蛋白, 与核糖体结合参与转录调节, 生物学功能多样; 有研究发现其参与很多生物学过程并和肝炎、脑恶性肿瘤等多种临床疾病相关, 可参与信号转导[24]、抑制凋亡[25]过程, 过度表达可以扰乱细胞分化[26]等. 在H. pylori的感染晚期出现此蛋白的表达上调, 提示其在致病机制中发挥相应的作用. 66个差异蛋白点共鉴定出34个, 未鉴定出蛋白主要是由于目前国际上的数据库(肽库)的积累尚不充分, 这部分尚需今后通过蛋白测序来确认.
不同时间点的蛋白图谱为我们提供了一个动态变化的H. pylori与AGS相互作用过程序贯性特点. 相互作用过程, 细菌最终占据优势, 整个蛋白图谱出现明显下调的蛋白都是细胞来源的, 细胞上调的2种蛋白主要与病理变化相关, 均在4 h蛋白图谱中开始表达, 提示这个时间点是H. pylori致病机制启动的关键. 本研究结果为进一步研究H. pylori的致病机制提供很重要的线索.
人胃腺癌上皮细胞(AGS)是目前最为常用的研究H. pylori和胃癌相关的细胞模型, 本实验拟应用H. pylori国际标准菌株26695和AGS细胞共同孵育, 在不同时间点取样, 以期发现二者相互作用时对蛋白表达影响的时序性变化, 为更好的理解
H. pylori感染和致病机制提供有意义的线索.
同行评价者
李玉明, 教授, 江苏省南通市第一人民医院消化内科.
H. pylori与人胃腺癌上皮细胞相互作用是研究的经典思路, 但具体致病机制目前尚不清楚.
李波清 et al对胃癌和非胃癌来源的菌株进行蛋白质学分析, 发现HSP60存在丙氨酸和苏氨酸的变异, 推测这一结构域可能是区分胃癌与非胃癌菌株的分子特征之一.
本研究观察细胞变化的在同时观察了细菌蛋白质组学的连续性时间点的变化.
本研究有利于H. pylori的致病机制和治疗靶点的发现, 为更好的理解H. pylori感染机制提供有意义的线索.
本研究设计合理, 结果可信, 具有一定的参考价值.
编辑: 李军亮 电编:何基才
| 1. | Weel JF, van der Hulst RW, Gerrits Y, Roorda P, Feller M, Dankert J, Tytgat GN, van der Ende A. The interrelationship between cytotoxin-associated gene A, vacuolating cytotoxin, and Helicobacter pylori-related diseases. J Infect Dis. 1996;173:1171-1175. [PubMed] |
| 2. | Pasceri V, Patti G, Cammarota G, Pristipino C, Richichi G, Di Sciascio G. Virulent strains of Helicobacter pylori and vascular diseases: a meta-analysis. Am Heart J. 2006;151:1215-1222. [PubMed] [DOI] |
| 3. | Gygi SP, Corthals GL, Zhang Y, Rochon Y, Aebersold R. Evaluation of two-dimensional gel electrophoresis-based proteome analysis technology. Proc Natl Acad Sci U S A. 2000;97:9390-9395. [PubMed] |
| 4. | Grünenfelder B, Rummel G, Vohradsky J, Röder D, Langen H, Jenal U. Proteomic analysis of the bacterial cell cycle. Proc Natl Acad Sci U S A. 2001;98:4681-4686. [PubMed] [DOI] |
| 5. | Nilsson I, Utt M, Nilsson HO, Ljungh A, Wadström T. Two-dimensional electrophoretic and immunoblot analysis of cell surface proteins of spiral-shaped and coccoid forms of Helicobacter pylori. Electrophoresis. 2000;21:2670-2677. [PubMed] [DOI] |
| 6. | Pereira DR, Martins D, Winck FV, Smolka MB, Nishimura NF, Rabelo-Gonçalves EM, Hara NH, Marangoni S, Zeitune JM, Novello JC. Comparative analysis of two-dimensional electrophoresis maps (2-DE) of Helicobacter pylori from Brazilian patients with chronic gastritis and duodenal ulcer: a preliminary report. Rev Inst Med Trop Sao Paulo. 2006;48:175-177. [PubMed] [DOI] |
| 7. | Görg A, Obermaier C, Boguth G, Harder A, Scheibe B, Wildgruber R, Weiss W. The current state of two-dimensional electrophoresis with immobilized pH gradients. Electrophoresis. 2000;21:1037-1053. [PubMed] [DOI] |
| 9. | Larsson T, Bergström J, Nilsson C, Karlsson KA. Use of an affinity proteomics approach for the identification of low-abundant bacterial adhesins as applied on the Lewis(b)-binding adhesin of Helicobacter pylori. FEBS Lett. 2000;469:155-158. [PubMed] [DOI] |
| 10. | Enroth H, Akerlund T, Sillén A, Engstrand L. Clustering of clinical strains of Helicobacter pylori analyzed by two-dimensional gel electrophoresis. Clin Diagn Lab Immunol. 2000;7:301-306. [PubMed] [DOI] |
| 12. | Kimmel B, Bosserhoff A, Frank R, Gross R, Goebel W, Beier D. Identification of immunodominant antigens from Helicobacter pylori and evaluation of their reactivities with sera from patients with different gastroduodenal pathologies. Infect Immun. 2000;68:915-920. [PubMed] [DOI] |
| 13. | Hauet-Broere F, Wieten L, Guichelaar T, Berlo S, van der Zee R, Van Eden W. Heat shock proteins induce T cell regulation of chronic inflammation. Ann Rheum Dis. 2006;65 Suppl 3:iii65-iii68. [PubMed] [DOI] |
| 14. | Ehrenfried JA, Herron BE, Townsend CM Jr, Evers BM. Heat shock proteins are differentially expressed in human gastrointestinal cancers. Surg Oncol. 1995;4:197-203. [PubMed] |
| 16. | Handschumacher RE, Harding MW, Rice J, Drugge RJ, Speicher DW. Cyclophilin: a specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544-547. [PubMed] [DOI] |
| 17. | Gasser CS, Gunning DA, Budelier KA, Brown SM. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci U S A. 1990;87:9519-9523. [PubMed] [DOI] |
| 18. | Ryffel B, Woerly G, Greiner B, Haendler B, Mihatsch MJ, Foxwell BM. Distribution of the cyclosporine binding protein cyclophilin in human tissues. Immunology. 1991;72:399-404. [PubMed] |
| 19. | Smart EJ, Ying Y, Donzell WC, Anderson RG. A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem. 1996;271:29427-29435. [PubMed] [DOI] |
| 20. | Thériault Y, Logan TM, Meadows R, Yu L, Olejniczak ET, Holzman TF, Simmer RL, Fesik SW. Solution structure of the cyclosporin A/cyclophilin complex by NMR. Nature. 1993;361:88-91. [PubMed] [DOI] |
| 21. | Jin ZG, Melaragno MG, Liao DF, Yan C, Haendeler J, Suh YA, Lambeth JD, Berk BC. Cyclophilin A is a secreted growth factor induced by oxidative stress. Circ Res. 2000;87:789-796. [PubMed] |
| 22. | Jin ZG, Lungu AO, Xie L, Wang M, Wong C, Berk BC. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:1186-1191. [PubMed] [DOI] |
| 23. | Kim SH, Lessner SM, Sakurai Y, Galis ZS. Cyclophilin A as a novel biphasic mediator of endothelial activation and dysfunction. Am J Pathol. 2004;164:1567-1574. [PubMed] |
| 24. | Neuhof A, Rolls MM, Jungnickel B, Kalies KU, Rapoport TA. Binding of signal recognition particle gives ribosome/nascent chain complexes a competitive advantage in endoplasmic reticulum membrane interaction. Mol Biol Cell. 1998;9:103-115. [PubMed] |
| 25. | Stilo R, Liguoro D, di Jeso B, Leonardi A, Vito P. The alpha-chain of the nascent polypeptide-associated complex binds to and regulates FADD function. Biochem Biophys Res Commun. 2003;303:1034-1041. [PubMed] [DOI] |