修回日期: 2007-01-04
接受日期: 2007-01-20
在线出版日期: 2007-03-28
目的: 研究hFXYD6基因反义核酸对人胆管癌QBC939细胞体外增殖和侵袭能力的影响.
方法: 构建hFXYD6基因反义核酸真核表达载体pcDNA3.1(-)/hFXYD6(-)并转染人胆管癌QBC939细胞, 同时设pcDNA3.1(-)空载体对照组和空白对照组. SYBR Green I荧光定量RT-PCR和免疫组化分别检测hFXYD6 mRNA及蛋白表达; MTT、平板克隆形成实验检测细胞体外增殖活性; 流式细胞仪检测细胞周期; Transwell侵袭小室模型检测细胞体外侵袭能力.
结果: 与空白组和空载组比较, 反义组细胞hFXYD6 mRNA及蛋白表达量降低; 细胞群体倍增时间增加(46.8 h vs 34.5 h, 35.3 h), 细胞克隆形成率降低(24.3%±5.3% vs 61.0%±8.5%, 58.0%±5.6%, P<0.001); 细胞周期中G1期细胞比例明显升高(66.4%±2.9% vs 33.5%±2.3%, 39.4%±3.7%, P<0.001), S期比例明显减少(18.6%±1.6% vs 36.2%±2.1%, 34.1%±1.6%, P<0.001); Transwell侵袭小室中24 h穿膜细胞数无显著改变. 空白组和空载组细胞间均无明显差异.
结论: hFXYD6反义核酸抑制人胆管癌QBC939细胞体外增殖能力, 但对其侵袭能力无明显作用.
引文著录: 窦春青, 岳鑫, 周宁新. hFXYD6反义核酸对胆管癌细胞体外增殖和侵袭能力的影响. 世界华人消化杂志 2007; 15(9): 929-935
Revised: January 4, 2007
Accepted: January 20, 2007
Published online: March 28, 2007
AIM: To investigate the effect of human antisense FXYD6 (FXYD domain containing ion transport regulator 6) on the proliferation and invasion of human cholangiocarcinoma cell line QBC939 in vitro.
METHODS: Human cholangiocarcinoma cell line QBC939 was transfected with the plasmid expressing human antisense FXYD6. Meanwhile, the empty vector and non-transfection group were designed. The mRNA transcription level of hFXYD6 was assayed by real-time reverse transcriptase polymerase chain reaction (RT-PCR) with SYBR Green I, and the expression of hFXYD6 protein was detected by immunohistochemistry. MTT and the colony-forming assay were used to measure the ability of cell growth. The cell cycle distribution was analyzed by flow cytometry and Transwell chamber model was employed to test the ability of cell invasion in vitro.
RESULTS: In comparison with the cells transfected with empty vector or without transfection, QBC939 cells transfected with antisense hFXYD6 had a significant decrease in mRNA transcription and protein expression. The cell doubling time was augmented (46.8 h vs 34.5 h, 35.3 h), whereas the colony formation was decreased (24.3% ± 5.3% vs 61.0% ± 8.5%, 58.0% ± 5.6%; P < 0.001). The G1-phase cell population was obviously increased (66.4% ± 2.9% vs 33.5% ± 2.3%, 39.4% ± 3.7%, P < 0.001), but the S-phase one was decreased (18.6% ± 1.6% vs 36.2% ± 2.1%, 34.1% ± 1.6%, P < 0.001). The cells moved from the upper chamber into the lower one in Transwell chamber assay had no marked difference. Between the cells without transfection and transfected with empty vector, there were no significant differences in cell doubling time, colony forming ability, cell cycle distribution and the ability of cell invasion in vitro.
CONCLUSION: Transfection of antisense hFXYD6 can inhibit the ability of cell proliferation, but it has no effect on the ability of cell invasion in human cholangiocarcinoma cell line QBC939 in vitro.
- Citation: Dou CQ, Yue X, Zhou NX. Effect of human antisense FXYD6 on the proliferation and invasion of human cholangiocarcinoma cells in vitro. Shijie Huaren Xiaohua Zazhi 2007; 15(9): 929-935
- URL: https://www.wjgnet.com/1009-3079/full/v15/i9/929.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i9.929
FXYD6基因全称是包含FXYD结构的离子转运调节子6(FXYD domain containing ion transport regulator 6, FXYD6), 是一功能尚不明确的新基因. 在胆管癌的差异显示PCR中, 我们发现, FXYD6基因与胆管癌的分化相关, 随分化程度降低FXYD6基因表达递增[1-4]. 原位RT-PCR结果进一步证实: 低分化胆管癌FXYD6 mRNA表达高于高分化胆管癌. FXYD6为FXYD蛋白家族组成之一. 已证实FXYD3在胰腺癌[5]、乳腺癌[6]及前列腺癌[7]等异常高表达, 降低FXYD3表达可抑制肿瘤细胞的增殖能力; 而FXYD5在胃癌[8]、结肠癌[9]、食管鳞状细胞癌[10]、胰腺癌[11]、舌癌[12]、宫颈癌[13]等异常高表达, 降低FXYD5表达则抑制肿瘤细胞的侵袭能力. 本实验通过hFXYD6反义核酸真核表达载体的构建及对胆管癌细胞QBC939的基因转染, 并利用SYBR Green I荧光定量RT-PCR、免疫组化、MTT、流式细胞术和体外侵袭模型等方法, 研究了hFXYD6反义核酸对胆管癌细胞QBC939体外增殖和侵袭能力的影响.
人胆管癌细胞系QBC939购自中南大学人类干细胞国家工程研究中心细胞库; 内切酶EcoRⅠ, KpnⅠ及T4 DNA连接酶(Promega公司); DMEM培养基、胎牛血清、Lipofectamine 2000、G418、MTT和TRIzol均购自美国Gibco公司; 细胞在含100 mL/L胎牛血清的培养基中置于37 ℃, 50 mL/L CO2孵箱内常规培养; 人FXYD6真核细胞表达载体pcDNA3.1(+)/hFXYD6由我室前期构建; 空载体pcDNA3.1(-)由我室保存; 兔抗人FXYD6多克隆抗体由我室制备; 羊抗兔IgG荧光抗体(北京中杉金桥生物技术有限公司); Transwell侵袭小室(美国Costar公司); Matrigel人工基质胶(美国BD).
1.2.1 hFXYD6反义核酸真核表达载体构建: 用限制性核酸内切酶EcoRⅠ/KpnⅠ双酶切pcDNA3.1(-)空载体和pcDNA3.1(+)/hFXYD, 所得pcDNA3.1(+)/hFXYD6酶切产物小片段(300 bp)和pcDNA3.1(-)空载体经切胶回收纯化后以T4 DNA连接酶连接, 经测序鉴定后命名为pcDNA3.1(-)/hFXYD6(-).
1.2.2 细胞转染: pcDNA3.1(-)空载体及所得pcDNA3.1(-)/hFXYD6(-)经Bgl Ⅱ酶切线性化并纯化后用于细胞转染. G418预筛选确定胆管癌细胞QBC939基因转染后G418抗性筛选浓度. 采用Lipofectamine 2000介导基因的转染(按说明书进行), 并按已确定的G418浓度进行抗性筛选. 3-4 wk后, 见有抗性细胞集落形成时, 挑取细胞克隆并放大培养. 分别称为反义组(细胞转染hFXYD6反义核酸)、空载组(细胞仅转染空载体)和空白组(细胞未转染反义核酸及空载体)细胞.
1.2.3 SYBR Green I荧光定量RT-PCR法检测hFXYD6 mRNA表达: TRIzol一步法提取细胞总RNA(按试剂盒说明)并纯化除去DNA. 取1 mg RNA, 以Oligo-dT为引物反转录合成cDNA. 实时荧光定量RT-PCR: 50 mL反应体系中含有25 mL 2×SYBR Green I qPCR mix, 2 mL cDNA模板, hFXYD6或hGAPDH上下游引物各1 mL. hFXYD6上游引物序列5'-AACAGAGCCCCAGAAAGCAGA -3', 下游引物序列5'-CCGGTTTTCTTAAGCATCGACA-3'; hGAPDH上游引物序列5'-TCCTCTGACTTCAACAGCGACA-3', 下游引物序列5'-CCCTGTT GCTGTAGCCAAATTC-3'. 同时设无模板对照、无RT对照和阳性对照. 扩增条件: 95 ℃ 10 min, 95 ℃ 15 s, 60 ℃ 15 s, 72 ℃ 20 s, 低于溶解温度2 ℃时检测荧光, 共40个循环. 扩增完毕进行溶解曲线分析. 采用相对定量法, 以hGAPDH为参照, 利用Ct值计算hFXYD6的相对量.
1.2.4 免疫组化检测hFXYD6表达: 采用荧光免疫组织化学法, 主要操作步骤如下: 将无菌盖玻片置培养皿中, 细胞按5×107/L接种于培养皿中制备细胞爬片. 细胞爬片以PBS洗2次, 3 min/次; 40 g/L多聚甲醛室温下固定30 min; PBS洗2次, 3 min/次; 30 mL/L H2O2室温孵育10 min; PBS洗2次, 5 min/次; 10 mL/L正常山羊血清封闭30 min; 加1:100兔抗人FXYD6多克隆抗体, 4 ℃孵育过夜; PBS洗3次, 5 min/次; 加荧光素标记二抗, 37 ℃孵育60 min; PBS洗3次, 5 min/次, 封片. PBS代替一抗作为阴性对照. 激光共聚焦显微镜观察摄片.
1.2.5 MTT法绘制细胞生长曲线: 将3组细胞分别以1×104/孔接种于96孔板, 共5块板, 每块板各组细胞分别接种3孔. 次日开始每日取一块96孔板做MTT测定. 测定时每孔加入5 g/L MTT液20 μL, 37 ℃作用4 h, 弃MTT后, 每孔加入100 μL DMSO, 振摇10 min后酶标仪测定A540值, 以只加培养液不加细胞的阴性对照孔调零. 绘制细胞生长曲线并按文献[14]方法计算细胞群体倍增时间.
1.2.6 平板克隆形成实验检测细胞克隆形成能力: 将对数生长期的3组细胞分别以2.5 g/L胰蛋白酶消化成单个细胞悬液, 并作梯度倍比稀释, 按每孔50个细胞接种于24孔板, 每组细胞各接种6孔. 静止培养2-3 wk, 当培养板出现肉眼可见的克隆时终止培养. PBS清洗后加纯甲醇1 mL固定15 min, 吉姆萨液染色. 将培养板置于显微镜低倍数下计数大于50个细胞的克隆数, 按公式计算克隆形成率. 克隆形成率(%) = 克隆数/接种数×100%
1.2.7 流式细胞仪检测细胞周期: 将对数生长期的3组细胞分别以2.5 g/L胰蛋白酶消化成单个细胞悬液, 离心重悬后700 mL/L乙醇固定, 4 ℃保存. PBS清洗后加入碘化丙啶(PI)染液, 混匀, 4 ℃放置30 min, 调整细胞浓度为1×109/L, 流式细胞仪检测并软件分析结果, 每组重复3次.
1.2.8 Transwell侵袭小室模型测定细胞体外侵袭能力: 将浓度为0.5 g/L Matrigel人工基质胶20 μL铺于Transwell侵袭小室聚碳酯微孔膜(孔径8 μm)的上表面, 置37 ℃ 30 min使其聚成凝胶. Transwell上室中分别加入已消化重悬的各组细胞100 μL (1×108/L), 下室中加入600 μL含趋化因子无血清DMEM培养基, 培养24 h后取出, PBS清洗, 棉签去除滤膜上层细胞, 将已经侵入并贴附于微孔膜下层的细胞固定并吉姆萨液染色, 显微镜下直接观察穿过膜的细胞数. 随机计数5个视野, 计数每个视野内穿过8 μm微孔的细胞数. 以侵袭细胞的相对数目来表示肿瘤细胞的侵袭能力.
统计学处理 以SPSS13.0软件进行统计分析, 所有计量资料以mean±SD表示, 采用单因素方差分析, 两组间比较行SNK-q检验; P<0.05为统计学差异显著.
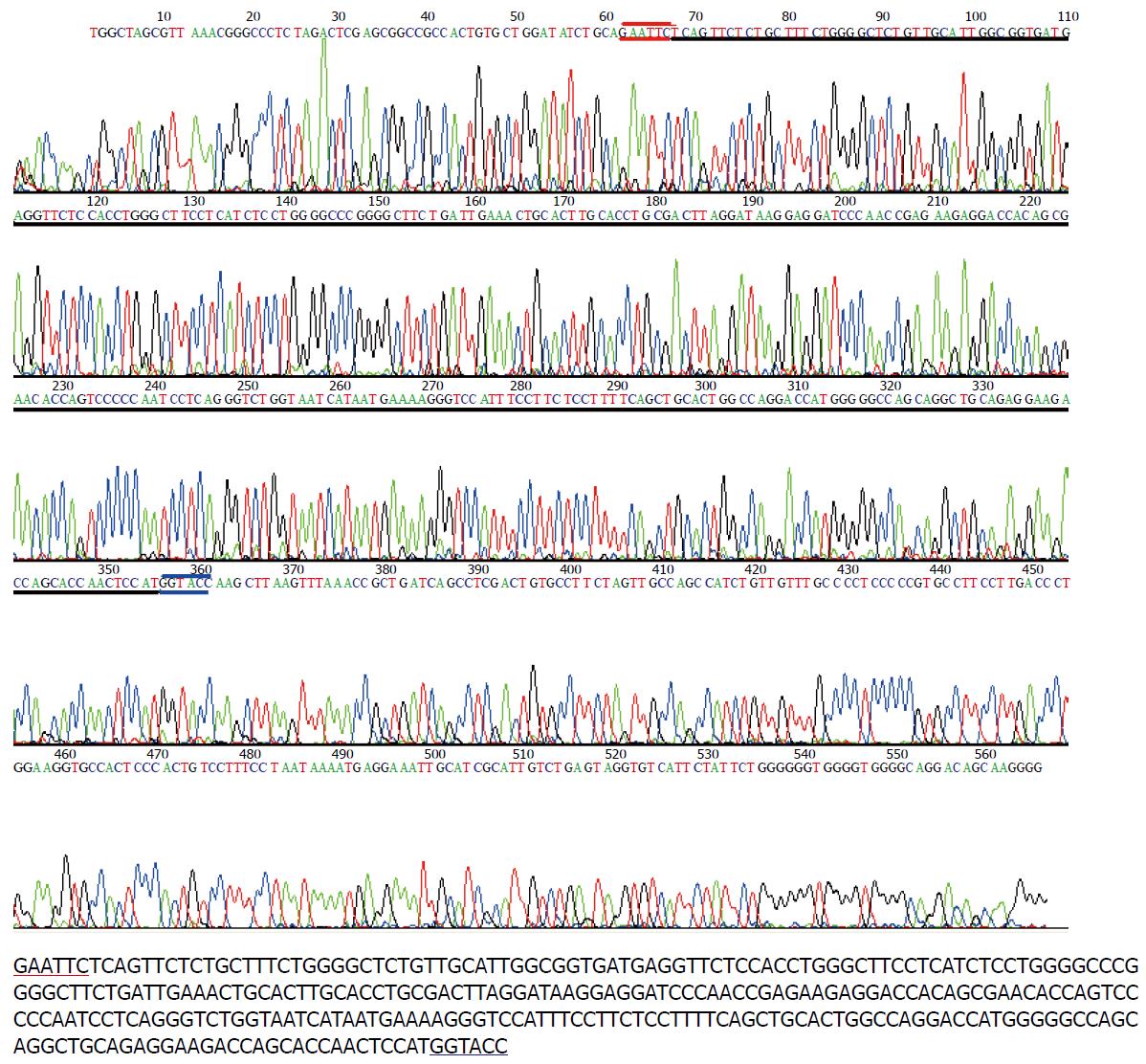
成功构建人类FXYD6反义核酸真核表达载体pcDNA3.1(-)/hFXYD6(-), 其测序结果如图1.
筛选出的3株反义组细胞hFXYD6 mRNA相对含量分别为0.6, 0.7和0.9; 空白组细胞为11.2; 空载组细胞为11.8. 反义组细胞hFXYD6 mRNA相对量低于空白组及空载组细胞; 空白组及空载组细胞间无明显差异. 在转录水平表明, hFXYD6反义核酸转染成功.
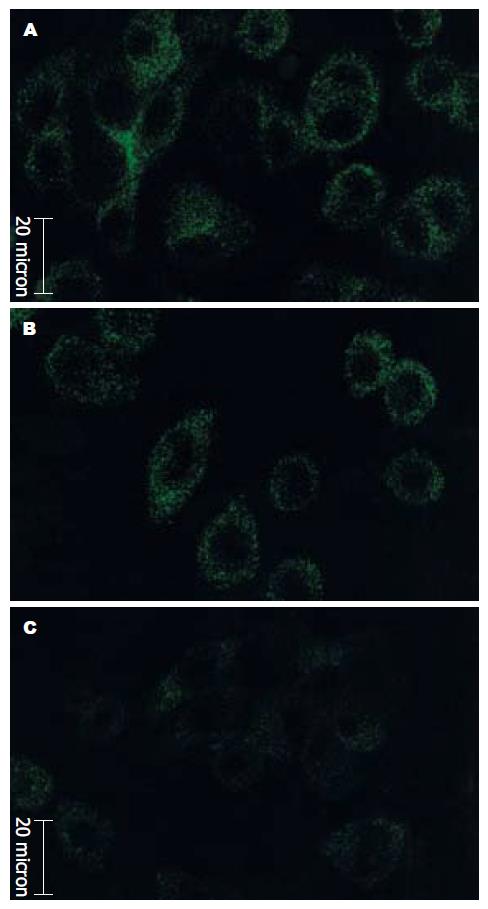
3组细胞胞质中均出现绿色荧光, 提示各组细胞中均有hFXYD6蛋白表达, 但反义组细胞表达强度明显较空白组和空载组减弱, 空白组和空载组间无显著差别(图2). 在翻译水平表明, hFXYD6反义核酸转染成功.
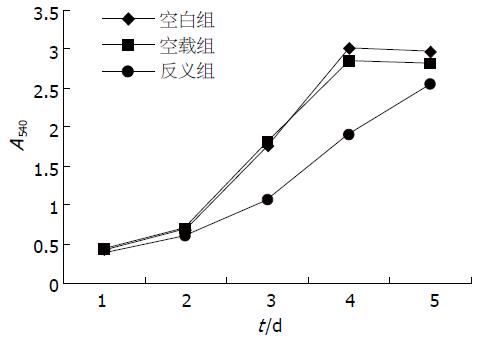
反义组细胞生长速率明显低于空白组和空载组细胞, 而空白组和空载组细胞间生长速率无明显差异(图3). 空白组、空载组及反义组细胞群体倍增时间分别为34.5, 35.3和46.8 h. 表明反义hFXYD6基因转染抑制QBC939细胞生长速率.
3组细胞2 wk后均有克隆形成. 反义组细胞克隆形成率为24.3%±5.3%; 空白组细胞为61.0%±8.5%; 空载组细胞为58.0%±5.6%. 反义组细胞克隆形成率较空白组和空载组明显减小, 差异有显著意义(P均<0.001); 空白组与空载组比较无差异(P = 0.442), 表明反义hFXYD6基因转染降低QBC939细胞克隆形成能力.
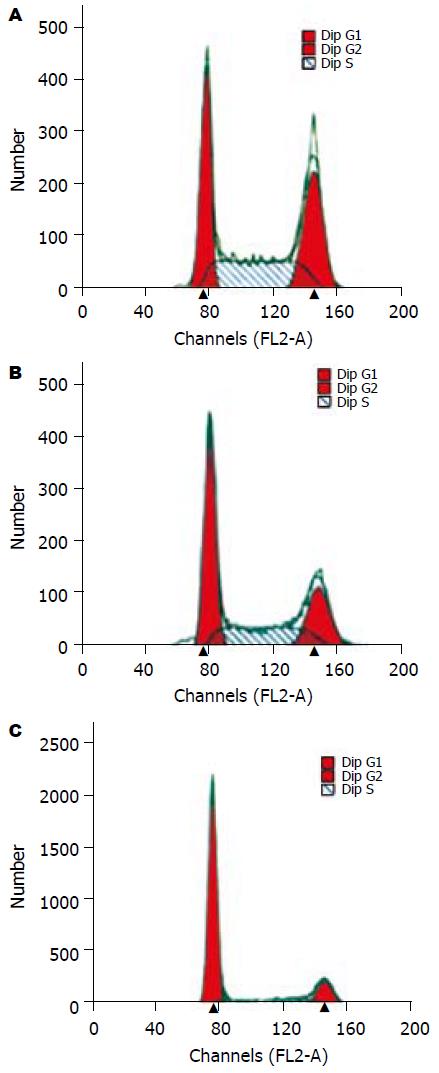
反义组细胞G1期和S期细胞比例分别为66.4%±2.9%, 24.3%±5.3%; 空白组细胞分别为33.5%±2.3%, 61.0%±8.5%; 空载组细胞分别为39.4%±3.7%, 58.0%±5.6%. 与空白组及空载组细胞相比, 反义组G1期细胞比例明显升高(P值均<0.001), 而S期细胞所占比例明显降低(P值均<0.001)(图4); 空白组和空载组间G1期和S期细胞均无显著差异(分别为P = 0.199, P = 0.056). 提示反义hFXYD6基因转染后QBC939细胞DNA合成能力受限.
反义组细胞在Transwell侵袭小室中24 h穿膜细胞数为32.8±6.2; 空白组细胞为34.4±5.3; 空载组细胞为29.4±5.2. 3组间比较无明显差异(分别为P = 0.659, P = 0.355, P = 0.183). 表明反义hFXYD6基因转染对QBC939细胞体外侵袭能力无显著影响.
本课题组前期的差显PCR结果发现, FXYD6 mRNA表达与胆管癌分化程度呈负性相关. 随后的原位RT-PCR半定量实验证实: 从正常胆管上皮细胞、高分化胆管癌细胞到低分化胆管癌细胞, FXYD6 mRNA含量依次递增. 染色体比较基因组杂交结果表明, 11号染色体长臂是包括胆管癌在内的多种肿瘤细胞最常见的异常区域[15], 而FXYD6基因正是定位于11号染色体长臂(11q23.3). 那么FXYD6对于胆管癌细胞的增殖转移有何影响? 通过反义核酸技术, 本实验进行了初步探讨.
首先利用pcDNA3.1(+)和pcDNA3.1(-)质粒EcoRⅠ与KpnⅠ酶切位点顺序相反的特点, 通过双酶切pcDNA3.1(+)/hFXYD6获得hFXYD6 ORF区序列后反向插入pcDNA3.1(-)空载的方法, 我们构建了hFXYD6反义核酸真核表达载体pcDNA3.1(-)/hFXYD6(-), 随后的测序结果证实载体构建成功. 细胞转染后通过SYBR Green I实时荧光定量RT-PCR和免疫组化, 分别在转录及翻译水平证实反义核酸转染成功.
通过MTT法绘制细胞生长曲线并计算细胞群体倍增时间、平板克隆形成实验检测细胞克隆形成能力、流式细胞仪检测细胞周期及Transwell侵袭小室模型测定细胞体外侵袭能力, 发现与空白组及空载组细胞相比, 转染hFXYD6反义核酸的胆管癌细胞群体倍增时间增加, 平板克隆形成率降低, S期细胞减少而G1期细胞增多; 而在体外侵袭实验中穿膜细胞数并无显著差异.
总之, 本实验结果表明, hFXYD6反义核酸抑制人胆管癌QBC939细胞体外增殖能力, 但对其侵袭能力无明显作用. 目前对FXYD6的初步认识是Na+/K+-ATPase调节蛋白[16-21]. Na+/K+-ATPase活性的抑制可导致EGFR的持续激活[22-25], 而胆管癌细胞的EGFR表达较正常胆管上皮细胞明显提高, 且EGFR激活可促进肿瘤细胞增殖[26-31]. FXYD6是否通过抑制Na+/K+-ATPase活性而激活EGFR以促进细胞增殖? 具体机制有待于进一步研究.
胆管癌分化程度是影响预后的重要因素之一. 高低分化癌相比, 低分化癌细胞往往具有更强的增殖和侵袭能力. 基于现有理论及治疗方法, 筛选胆管癌分化相关基因, 能提高诊断灵敏性及特异性, 同时还能揭示胆管癌发生、发展的分子机制.
FXYD6为FXYD蛋白家族组成之一. 因FXYD2为Na+/K+-ATPaseg亚基, 故FXYD蛋白家族也称为Na+/K+-ATPaseg亚基蛋白家族. 已发现的FXYD家族蛋白有7个, 目前证实FXYD3与FXYD5为肿瘤相关抗原, 其中FXYD3在胰腺癌、乳腺癌及前列腺癌等异常高表达, 降低FXYD3表达可抑制肿瘤细胞的增殖能力; 而FXYD5在胃癌、结肠癌、食管鳞状细胞癌、胰腺癌、舌癌、宫颈癌等异常高表达, 降低FXYD5表达则抑制肿瘤细胞的侵袭能力. 这为研究FXYD6与胆管癌细胞增殖侵袭开拓了思路.
本文采用反义核酸技术, 首次证实胆管癌分化相关基因FXYD6可促进胆管癌细胞增殖, 而与细胞侵袭无明显关系.
本研究通过体外实验探讨FXYD6反义核酸对胆管癌细胞的抑制作用, 试图找到一种抑制胆管癌生长和转移的新途径, 为FXYD6反义核酸在临床治疗胆管癌提供实验依据.
FXYD蛋白家族: 是近年来发现的Na+/K+-ATPase组织特异性调节子, 是小分子(66氨基酸-178氨基酸)不溶于水的I型膜蛋白, 其结构上包含有相似的细胞外固定FXYD序列的单跨膜片段, 并按发现时间顺序分别命名为FXYD1-7. 该家族蛋白在哺乳动物中广泛分布, 所有蛋白在胚胎早期均有表达, 成年后则主要在转运流动性液体和溶质的组织器官中表达, 如肾、乳腺、胰腺、前列腺、结肠、肝、肺和胎盘; 在电兴奋组织, 如神经系统和肌肉中亦有表达.
本文实验设计合理, 选题新颖, 采用方法得当、有创新, 统计学结果正确, 在结果和结论中有新的见解, 是一篇较好的论著
编辑: 王晓瑜 电编:张敏
| 5. | Kayed H, Kleeff J, Kolb A, Ketterer K, Keleg S, Felix K, Giese T, Penzel R, Zentgraf H, Büchler MW. FXYD3 is overexpressed in pancreatic ductal adenocarcinoma and influences pancreatic cancer cell growth. Int J Cancer. 2006;118:43-54. [PubMed] [DOI] |
| 6. | Morrison BW, Moorman JR, Kowdley GC, Kobayashi YM, Jones LR, Leder P. Mat-8, a novel phospholemman-like protein expressed in human breast tumors, induces a chloride conductance in Xenopus oocytes. J Biol Chem. 1995;270:2176-2182. [PubMed] [DOI] |
| 7. | Grzmil M, Voigt S, Thelen P, Hemmerlein B, Helmke K, Burfeind P. Up-regulated expression of the MAT-8 gene in prostate cancer and its siRNA-mediated inhibition of expression induces a decrease in proliferation of human prostate carcinoma cells. Int J Oncol. 2004;24:97-105. [PubMed] [DOI] |
| 8. | Shimada Y, Yamasaki S, Hashimoto Y, Ito T, Kawamura J, Soma T, Ino Y, Nakanishi Y, Sakamoto M, Hirohashi S. Clinical significance of dysadherin expression in gastric cancer patients. Clin Cancer Res. 2004;10:2818-2823. [PubMed] [DOI] |
| 9. | Aoki S, Shimamura T, Shibata T, Nakanishi Y, Moriya Y, Sato Y, Kitajima M, Sakamoto M, Hirohashi S. Prognostic significance of dysadherin expression in advanced colorectal carcinoma. Br J Cancer. 2003;88:726-732. [PubMed] [DOI] |
| 10. | Shimada Y, Hashimoto Y, Kan T, Kawamura J, Okumura T, Soma T, Kondo K, Teratani N, Watanabe G, Ino Y. Prognostic significance of dysadherin expression in esophageal squamous cell carcinoma. Oncology. 2004;67:73-80. [PubMed] [DOI] |
| 11. | Shimamura T, Yasuda J, Ino Y, Gotoh M, Tsuchiya A, Nakajima A, Sakamoto M, Kanai Y, Hirohashi S. Dysadherin expression facilitates cell motility and metastatic potential of human pancreatic cancer cells. Cancer Res. 2004;64:6989-6995. [PubMed] [DOI] |
| 12. | Nakanishi Y, Akimoto S, Sato Y, Kanai Y, Sakamoto M, Hirohashi S. Prognostic significance of dysadherin expression in tongue cancer: immunohistochemical analysis of 91 cases. Appl Immunohistochem Mol Morphol. 2004;12:323-328. [PubMed] [DOI] |
| 13. | Wu D, Qiao Y, Kristensen GB, Li S, Troen G, Holm R, Nesland JM, Suo Z. Prognostic significance of dysadherin expression in cervical squamous cell carcinoma. Pathol Oncol Res. 2004;10:212-218. [PubMed] [DOI] |
| 14. | Yin W, Cheepala S, Roberts JN, Syson-Chan K, DiGiovanni J, Clifford JL. Active Stat3 is required for survival of human squamous cell carcinoma cells in serum-free conditions. Mol Cancer. 2006;5:15. [PubMed] [DOI] |
| 15. | Rijken AM, Hu J, Perlman EJ, Morsberger LA, Long P, Kern SE, Hruban RH, Yeo CJ, Griffin CA. Genomic alterations in distal bile duct carcinoma by comparative genomic hybridization and karyotype analysis. Genes Chromosomes Cancer. 1999;26:185-191. [PubMed] [DOI] |
| 16. | Li C, Grosdidier A, Crambert G, Horisberger JD, Michielin O, Geering K. Structural and functional interaction sites between Na,K-ATPase and FXYD proteins. J Biol Chem. 2004;279:38895-38902. [PubMed] [DOI] |
| 17. | Geering K. Function of FXYD proteins, regulators of Na, K-ATPase. J Bioenerg Biomembr. 2005;37:387-392. [PubMed] [DOI] |
| 18. | Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol. 2006;290:F241-F250. [PubMed] [DOI] |
| 19. | Garty H, Karlish SJ. Role of FXYD proteins in ion transport. Annu Rev Physiol. 2006;68:431-459. [PubMed] [DOI] |
| 20. | Lindzen M, Aizman R, Lifshitz Y, Füzesi M, Karlish SJ, Garty H. Domains involved in the interactions between FXYD and Na,K-ATPase. Ann N Y Acad Sci. 2003;986:530-531. [PubMed] [DOI] |
| 21. | Geering K, Béguin P, Garty H, Karlish S, Füzesi M, Horisberger JD, Crambert G. FXYD proteins: new tissue- and isoform-specific regulators of Na,K-ATPase. Ann N Y Acad Sci. 2003;986:388-394. [PubMed] [DOI] |
| 22. | Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832-27837. [PubMed] [DOI] |
| 23. | Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694-18702. [PubMed] [DOI] |
| 24. | Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249-15256. [PubMed] [DOI] |
| 25. | Mohammadi K, Kometiani P, Xie Z, Askari A. Role of protein kinase C in the signal pathways that link Na+/K+-ATPase to ERK1/2. J Biol Chem. 2001;276:42050-42056. [PubMed] [DOI] |
| 26. | Javle MM, Yu J, Khoury T, Chadha KS, Iyer RV, Foster J, Kuvshinoff BW, Gibbs JF, Geradts J, Black JD. Akt expression may predict favorable prognosis in cholangiocarcinoma. J Gastroenterol Hepatol. 2006;21:1744-1751. [PubMed] [DOI] |
| 27. | Yoon JH, Gwak GY, Lee HS, Bronk SF, Werneburg NW, Gores GJ. Enhanced epidermal growth factor receptor activation in human cholangiocarcinoma cells. J Hepatol. 2004;41:808-814. [PubMed] [DOI] |
| 28. | Tsubouchi H. Sustained activation of epidermal growth factor receptor in cholangiocarcinoma: a potent therapeutic target? J Hepatol. 2004;41:859-861. [PubMed] [DOI] |
| 31. | 顾 广玉, 朱 明华, 王 文亮, 詹 镕洲. 表皮生长因子受体在肝细胞癌及胆管细胞癌中的表达. 临床与实验病理学杂志. 1999;15:385-386. |