修回日期: 2007-01-03
接受日期: 2007-01-10
在线出版日期: 2007-03-18
目的: 探讨保加利亚乳酸杆菌(lactobacillus bulgaricus, LBG)对幽门螺旋杆菌悉尼株脂多糖(H. pylori SS1-LPS)作用下SGC-7901细胞的p38有丝分裂原活化蛋白激酶(p38MAPK)磷酸化水平和凋亡率的影响.
方法: 使用LBG(1×1013 CFU/L)或p38MAPK通路阻滞剂SB203580(10 mmol/L)预处理SGC-7901细胞, 1 h后分别加入2.5×103, 2.5×104, 2.5 ×105 EU/L的H. pylori SS1-LPS, 干预2 h后使用免疫细胞化学方法检测各组细胞磷酸化p38MAPK(P-p38MAPK)的水平, 4、5、6 h后使用MTT法检测细胞活性, 6 h后使用流式细胞仪检测各组细胞凋亡率.
结果: 与对照组比较, H. pylori SS1-LPS直接干预后, SGC-7901细胞的活性明显下降(0.164±0.028 vs 0.622±0.068, P<0.05), 凋亡率(10.000%±0.510% vs 4.175%±0.206%, P<0.05)和P-p38MAPK水平明显上升(79.771±1.424 vs 4.075±0.135, P<0.01), 呈剂量依赖性; LBG预处理各组的细胞凋亡率和P-p38MAPK水平无明显改变; SB203580预处理各组的细胞活性和细胞凋亡率无明显改变.
结论: H. pylori SS1-LPS可诱导SGC-7901细胞凋亡, 其机制可能包括诱导生成P-p38MAPK; 而LBG能对抗H. pylori SS1-LPS的促凋亡作用, 其机制可能抑制H. pylori SS1-LPS诱导生成P-p38MAPK.
引文著录: 周超, 马洪升. 乳酸杆菌对幽门螺旋杆菌脂多糖作用下的SGC-7901细胞p38MAPK磷酸化水平和凋亡率的影响. 世界华人消化杂志 2007; 15(8): 807-812
Revised: January 3, 2007
Accepted: January 10, 2007
Published online: March 18, 2007
AIM: To investigate effects of lactobacillus bulgaricus (LBG) on the levels of phosphorylated p38 mitogen-activated protein kinase (P-p38MAPK) and apoptosis index (AI) in gastric cancer cell line SGC-7901 treated with lipopolysaccharide of H. pylori Sydney strain 1 (H. pylori SS1-LPS).
METHODS: Human gastric cancer cell line SGC-7901 was treated with H. pylori SS1-LPS at the concentration of 2.5×103, 2.5×104, 2.5 ×105 EU/L, respectively, after pretreatment for 1 h with 10 mmol/L SB203580 (blocker of p38MAPK) or 1×1013 CFU/L LBG. The level of P-p38MAPK was analyzed by immunocytochemistry after 2 h of H. pylori SS1-LPS treatment. The cell activity was detected by MTT assay after 4、5 and 6 h of treatment, and the apoptosis was measured by flow cytometry at the 6th hour.
RESULTS: H. pylori SS1-LPS inhibited cell activity (0.164 ± 0.028 vs 0.622 ± 0.068, P < 0.05) and up-regulated the level of P-p38MAPK (79.771 ± 1.424 vs 4.075 ± 0.135, P < 0.01) and AI value (10.000% ± 0.510% vs 4.175% ± 0.206%, P < 0.05) in a dose-dependent manner. The level of P-p38MAPK and AI value in SGC-7901 cells were not significantly different between LBG pretreatment group and the controls, and the cell activity and AI value were not markedly different between SB203580 pretreatment group and the controls.
CONCLUSION: H. pylori SS1-LPS may induce the apoptosis of SGC-7901 cells by activating the phosphorylation of p38MAPK, while LBG can prevent H. pylori SS1-LPS-induced apoptosis of SGC-7901 cells by inhibiting the phosphorylation of p38MAPK.
- Citation: Zhou C, Ma HS. Effects of lactobacillus on phosphorylated p38 mitogen-activated protein kinase and apoptosis in SGC-7901 cells treated with lipopolysaccharide of Helicobacter pylori. Shijie Huaren Xiaohua Zazhi 2007; 15(8): 807-812
- URL: https://www.wjgnet.com/1009-3079/full/v15/i8/807.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i8.807
体内体外的研究显示[1-16]: H. pylori感染能诱导胃黏膜上皮细胞凋亡, 进而削弱黏膜屏障功能, 促进胃炎、胃溃疡的发生, 并增加胃黏膜上皮细胞的丢失, 促进向萎缩性胃炎的演进, 上调上皮细胞的代偿性增殖, 增加发生胃腺癌的风险. 幽门螺旋杆菌脂多糖(lipopolysaccharide of Helicobacter pylori, H. pylori-LPS)其中起了重要作用, 他主要通过激活p38MAPK通路, 生成P-p38MAPK, 进而诱导胃黏膜上皮细胞凋亡[15,17-19]. 最近研究显示[20-27], 乳酸杆菌能明显抑制H. pylori的生长繁殖、黏附定植和毒素生成能力, 抑制H. pylori诱导的细胞因子和炎症介质的生成. 但是, 目前尚未见到关于乳酸杆菌是否能阻止H. pylori-LPS对胃黏膜上皮细胞的诱导凋亡作用的研究报道. 本研究旨在通过体外试验, 研究LBG是否能抑制H. pylori-LPS诱导的胃黏膜上皮细胞凋亡以及p38MAPK磷酸化途径在其中所起的作用.
H. pylori SS1, LBG及人胃腺癌上皮细胞株SGC7901均由四川大学公共卫生学院医检教研室提供. MRS培养基粉、空肠弯曲菌培养基粉购自江苏宜兴市永信生物有限公司. RPMI1640培养液购自成都哈里公司. LPS提取试剂盒购自捷瑞生物工程(上海)有限公司(intron biotechnology公司生产); LPS定量检测试剂盒购自上海伊华临床医学科技公司. SB203580购自晶美生物(Alexis公司). 抗P-p38MAPK抗体购自基因公司(Cell Signaling公司生产), 为兔抗人; 链亲和素(streptavidin, SP)法免疫化学试剂盒购自武汉博士德公司.
1.2.1 SGC-7901细胞的培养: 常规复苏细胞后, 悬液转入25 cm2培养瓶, 使用生长液(100 mL RPMI培养液中, 加入100 kU/L青霉素、100 mg/L链霉素, 并加入小牛血清至100 mL/L浓度制得)于含50 mL/L CO2的37 ℃细胞培养箱中培养, 约48 h换液一次, 细胞生长至覆盖约70%-80%培养瓶底面时传代.
1.2.2 LBG的培养: 常规解冻复苏菌种后, 使用MRS肉汤于37 ℃烛缸中培养24-48 h; 收集MRS肉汤, 5000 r/min离心10 min, 弃上清, PBS重悬, 比浊管调浓度到1×1010 CFU/mL, 5000 r/min离心10 min洗涤两次后, 等量单纯RPMI1640重悬, 为LBG菌液.
1.2.3 H. pylori SS1的培养: 常规解冻复苏菌种后, 接种于含H. pylori液体培养基的带侧口三角烧瓶中, 使用换气法使瓶内为含50 mL/L O2、100 mL/L CO2和850 mL/L N2的混合气, 置于振荡培养箱中于37 ℃下, 约100 r/min振荡培养48 h.
1.2.4 H. pylori SS1-LPS的制备: 完成1.2.3中步骤后, 收集液体培养基, 4 ℃下, 10000 g离心10 min, 弃上清, 所得沉淀菌团通过湿涂片及革兰氏染色片的镜下观察和尿素酶、H2O2触酶及氧化酶的生化反应鉴定, 确定为H. pylori, 其后参照LPS提取试剂盒说明书操作, 制得H. pylori SS1-LPS原液.
1.2.5 H. pylori SS1-LPS原液浓度的测定: 参照LPS定量检测试剂盒说明书操作, 为基质显色法; 用单纯RPMI1640稀释H. pylori SS1-LPS原液, 获得不同浓度LPS工作液.
1.2.6 实验分组及各组预处理和干预过程: 1 mg SB203580溶于133 mL DMSO中, 为20000 mmol/L, 用单纯RPMI1640按1:2000稀释, 为10 mmol/L SB工作液. 采用3-4代培养细胞, 常规消化后, 分别接种于96孔板、六孔板(内含盖玻片)、6 cm培养皿, 培养24 h后, 换用维持液同步化24 h后进行实验. 分为: NC组(对照组, 不加任何预处理及干预措施, 与其余各组同时测定实验数据)、LPS1组、LPS2组及LPS3组(此3组中, 干预所用H. pylori SS1-LPS的工作浓度分别为2.5×103, 25×103和250×103 EU/L)、LBG-LPS1组、LBG-LPS2组及LBG-LPS3组(此3组中, 上述LBG菌液预处理细胞1 h后, 再使用H. pylori SS1-LPS干预, 工作浓度分别为2.5×103, 25×103和250×103 EU/L)、SB-LPS1组、SB-LPS2组及SB-LPS3组(此3组中, 上述SB工作液预处理细胞1 h后, 再使用H. pylori SS1-LPS干预, 工作浓度分别为2.5×103, 25×103和250×103 EU/L).
1.2.7 MTT法检测细胞活性: LPS1, LPS2及LPS3, SB-LPS1, SB-LPS2及SB-LPS3各组均干预4 h, 5 h和6 h, 各组96孔板均在干预终止前4 h加入MTT液50 mL/每孔继续培养, 干预终止时, 吸掉原有液体, 每孔加入DMSO 100 mL, 振荡10 min, 酶标仪490 nm波长测量A值.
1.2.8 免疫细胞化学检测各组细胞P-p38MAPK的水平: 干预2 h后, 使用40 g/L PBS多聚甲醛固定各组六孔板中的细胞爬片, 室温30 min, SP法检测P-p38MAPK.
1.2.9 流式细胞术检测各组细胞凋亡率: 干预6 h后, 消化各组6 cm培养皿中细胞, 收集细胞悬液, 1000 r/min离心5 min洗涤2次, 1800 r/min离心3 min洗涤2次, 加入4 ℃预冷的700 mL/L乙醇固定30 min, 使用碘化丙啶(Propidium Iodide, PI)溶液, 流式细胞仪上, 488 nm氩离子激光下, 对样本进行检测, Multcycle DNA软件观察凋亡峰, 测定凋亡峰的面积分析凋亡细胞百分数.
统计学处理 所有数据输入计算机利用SPSS13.0软件进行统计分析. 计量资料用均数±标准差表示, 主要用多个样本均数间两两比较的One-Way ANOVA的LSD法及Tukey法. 检验水准a = 0.05, 以P<0.05时, 认为具有统计学意义.
以A值代表细胞活性. 在同一时间点, H. pylori SS1-LPS直接干预各组活性明显低于NC组(P<0.05), 且LPS1, LPS2, LPS3 3组活性逐渐降低(P<0.05), 各组活性随干预时间的延长而逐渐降低(P<0.05); SB203580预处理各组活性与同一时间点的NC组活性相比, 均无明显变化(P>0.05)(表1).
H. pylori SS1-LPS直接干预各组AI明显高于NC组(P<0.05), LPS1, LPS2和LPS3 3组AI逐渐升高(P<0.05); LBG预处理各组及SB203580预处理各组AI与NC组AI相比, 均无明显变化(P>0.05)(表2).
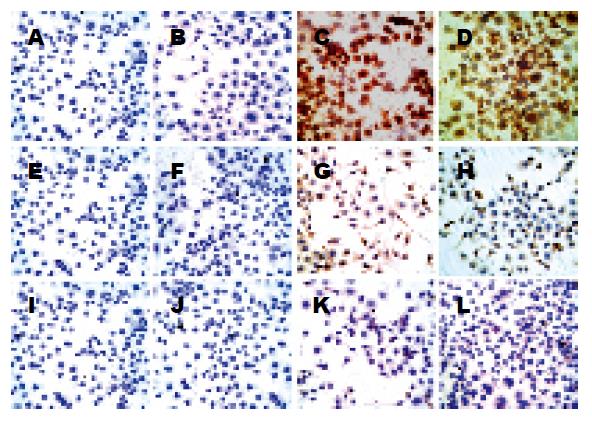
各组每张爬片随机选取5个视野照像, 每张照片使用Image-Pro Plus 5.0软件测量IOD值(integrated optical density, 积分吸光度), 以照片IOD值的均数±标准差形式表示各组P-p38MAPK水平. H. pylori SS1-LPS直接干预各组P-p38MAPK水平明显高于NC组(P<0.05), LPS1, LPS2, LPS3 3组P-p38MAPK水平逐渐升高(P<0.05); LBG预处理各组P-p38MAPK水平与NC组相比, 均无明显变化(P>0.05)(表3, 图1).
既往的研究[17]已经证实, H. pylori-LPS结合于胃黏膜上皮细胞表面的Toll样受体-4(Toll-like receptor-4, TLR-4), 进而顺序激活白细胞介素-1受体相关激酶(interleukin-1 receptor associated kinase, IRAK)、TNF受体相关因子-6(tumor necrosis factor receptor associated factor-6, TRAF-6)、转化生长因子β活化激酶1(transforming growth factor-β-activated kinase 1, TAK1)、TAK1结合蛋白2/1(TAK1-binding protein 1/2, TAB2/1)、MAPK激酶3/6(mitogen-activated protein kinase kinase 3/6, MKK3/6), 最终磷酸化p38MAPK. Kawahara et al[18]的研究显示, H. pylori-LPS诱导生成的P-p38MAPK能激活caspase-8, 进而促进细胞色素C的释放, 再激活caspase-9、3, 诱导豚鼠胃黏膜上皮细胞凋亡. p38MAPK通路抑制剂SB203580并不阻断上游激酶磷酸化p38MAPK, 而是结合于P-p38MAPK的106位苏氨酸位点, 使P-p38MAPK丧失结合ATP的能力, 进而不能磷酸化其下游因子来发挥作用. SB203580预处理各组的P-p38MAPK水平与对应各干预组相比, 并无明显差异.
本研究显示, H. pylori SS1-LPS通过促进P-p38MAPK的生成进而抑制细胞活性及诱导细胞凋亡. 这与之前的报道[15,18-19]一致. 在本实验中, P-p38MAPK通过何种下游因子和机制介导了SGC-7901细胞凋亡有待于进一步的研究. 本研究还显示, LBG通过抑制H. pylori-LPS诱导P-p38生成的能力而抑制其诱导细胞凋亡的作用. Yan et al[28]的研究表明, 乳酸杆菌活菌及其培养上清液能阻止TNF-α, 干扰素g(interferon-g, IFN-γ)和IL-1α诱导小鼠结肠黏膜上皮细胞株YAMC细胞发生凋亡, 其机制包括抑制3者诱导P-p38MAPK生成的能力; 在乳酸杆菌的培养肉汤中, 存在分子量分别为80 kDa和42 kDa的两种蛋白质, 他们能阻止TNF-α、IFN-γ和IL-1α诱导生成P-p38MAPK. 晚近的研究[29-30]证明了上述机制的存在. 那么, LBG抑制P-p38MAPK生成的机制是什么呢? 是LBG分泌物或菌体蛋白与H. pylori SS1-LPS竞争TLR-4上的结合位点, 还是通过激活其他通路进而抑制IRAK, TRAF-6, TAK1, TAB2/1及MKK3/6等位点? 还有待于进一步的研究.
进一步的研究应着眼于动物模型, 观察乳酸杆菌在体内的作用, 并探讨与胃黏膜炎症的关系; 并进行LBG培养上清液、热灭活LBG等的相关作用研究, 进一步了解乳酸杆菌抑制p38MAPK磷酸化的机制, 并能由此继续探讨乳酸杆菌有益作用的分子生物学机制.
正常胃黏膜上皮细胞自我更新极快, 并始终维持凋亡与增殖的动态平衡, 以保持黏膜上皮的正常结构与功能. H. pylori感染诱导上皮细胞过度凋亡, 是H. pylori促使胃黏膜发生一系列病理变化的重要途径. 其中, H. pylori-LPS起了重要作用, 他主要通过激活p38MAPK通路, 生成P-p38-MAPK, 进而诱导胃黏膜上皮细胞凋亡.
近年来, 乳酸杆菌、幽门螺杆菌等组成的胃内微生态系统是研究的热点, 乳酸杆菌制剂能从多个方面对抗H. pylori感染, 但对于其中所涉及的细胞信号传导, 尤其是MAPK通路的研究较少, 这亦是目前研究的热点之一.
相关报道显示, pylori-LPS合于胃黏膜上皮细胞表面的TLR-4, 激活相关信号转导, 诱导生成P-p38MAPK, 通过细胞色素C途径促使细胞凋亡. 而最近报道显示, 乳酸杆菌菌体蛋白或可溶性分泌物能抑制TNF-α等细胞因子诱导的肠上皮细胞凋亡, 其机制包括抑制P-p38MAPK的生成.
本文有助于进一步研究乳酸杆菌抑制H. pylori-LPS诱导凋亡作用的位点及其具体成分.
p38MAPK: p38有丝分裂原活化蛋白激酶, 是有丝分裂原活化蛋白激酶家族的一员, 分子量为38kDa, 应激、多种炎症介质和细胞因子及LPS等通过各种信号途径使p38MAPK磷酸化, P-p38MAPK可转移进入细胞核, 磷酸化多种转录因子, 作用涉及细胞因子的生成、细胞的生长发育、增殖分化和凋亡等.
本研究所做的工作在体外水平证实了H. pylori SS1-LPS可诱导SGC-7901细胞凋亡, 并对其机制在已有的实验数据基础上进行了推测, 具有一定的学术意义.
编辑: 王晓瑜 电编:李琪
| 1. | Wagner S, Beil W, Westermann J, Logan RP, Bock CT, Trautwein C, Bleck JS, Manns MP. Regulation of gastric epithelial cell growth by Helicobacter pylori: offdence for a major role of apoptosis. Gastroenterology. 1997;113:1836-1847. [PubMed] [DOI] |
| 2. | Wu YY, Tsai HF, Lin WC, Chou AH, Chen HT, Yang JC, Hsu PI, Hsu PN. Helicobacter pylori enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in human gastric epithelial cells. World J Gastroenterol. 2004;10:2334-2339. [PubMed] [DOI] |
| 3. | Fan X, Crowe SE, Behar S, Gunasena H, Ye G, Haeberle H, Van Houten N, Gourley WK, Ernst PB, Reyes VE. The effect of class II major histocompatibility complex expression on adherence of Helicobacter pylori and induction of apoptosis in gastric epithelial cells: a mechanism for T helper cell type 1-mediated damage. J Exp Med. 1998;187:1659-1669. [PubMed] [DOI] |
| 4. | Bland DA, Suarez G, Beswick EJ, Sierra JC, Reyes VE. H. pylori receptor MHC class II contributes to the dynamic gastric epithelial apoptotic response. World J Gastroenterol. 2006;12:4689-4693. [PubMed] [DOI] |
| 5. | Suzuki H, Seto K, Mori M, Suzuki M, Miura S, Ishii H. Monochloramine induced DNA fragmentation in gastric cell line MKN45. Am J Physiol. 1998;275:G712-G716. [PubMed] |
| 6. | Shirin H, Sordillo EM, Oh SH, Yamamoto H, Delohery T, Weinstein IB, Moss SF. Helicobacter pylori inhibits the G1 to S transition in AGS gastric epithelial cells. Cancer Res. 1999;59:2277-2281. [PubMed] |
| 7. | Jones NL, Day AS, Jennings HA, Sherman PM. Helicobacter pylori induces gastric epithelial cell apoptosis in association with increased Fas receptor expression. Infect Immun. 1999;67:4237-4242. [PubMed] |
| 8. | Kim JM, Kim JS, Jung HC, Song IS, Kim CY. Apoptosis of human gastric epithelial cells via caspase-3 activation in response to Helicobacter pylori infection: possible involvement of neutrophils through tumor necrosis factor alpha and soluble Fas ligands. Scand J Gastroenterol. 2000;35:40-48. [PubMed] [DOI] |
| 9. | Houghton J, Macera-Bloch LS, Harrison L, Kim KH, Korah RM. Tumor necrosis factor alpha and interleukin 1beta up-regulate gastric mucosal Fas antigen expression in Helicobacter pylori infection. Infect Immun. 2000;68:1189-1195. [PubMed] [DOI] |
| 10. | Fan X, Gunasena H, Cheng Z, Espejo R, Crowe SE, Ernst PB, Reyes VE. Helicobacter pylori urease binds to class II MHC on gastric epithelial cells and induces their apoptosis. J Immunol. 2000;165:1918-1924. [PubMed] [DOI] |
| 11. | Igarashi M, Kitada Y, Yoshiyama H, Takagi A, Miwa T, Koga Y. Ammonia as an accelerator of tumor necrosis factor alpha-induced apoptosis of gastric epithelial cells in Helicobacter pylori infection. Infect Immun. 2001;69:816-821. [PubMed] [DOI] |
| 12. | Kuck D, Kolmerer B, Iking-Konert C, Krammer PH, Stremmel W, Rudi J. Vacuolating cytotoxin of Helicobacter pylori induces apoptosis in the human gastric epithelial cell line AGS. Infect Immun. 2001;69:5080-5087. [PubMed] [DOI] |
| 13. | Zhang ZW, Dorrell N, Wren BW, Farthingt MJ. Helicobacter pylori adherence to gastric epithelial cells: a role for non-adhesin virulence genes. J Med Microbiol. 2002;51:495-502. [PubMed] [DOI] |
| 14. | Zhang ZW, Patchett SE, Farthing MJ. Role of Helicobacter pylori and p53 in regulation of gastric epithelial cell cycle phase progression. Dig Dis Sci. 2002;47:987-995. [PubMed] [DOI] |
| 15. | Slomiany BL, Slomiany A. Disruption in gastric mucin synthesis by Helicobacter pylori lipopolysaccharide involves ERK and p38 mitogen-activated protein kinase participation. Biochem Biophys Res Commun. 2002;294:220-224. [PubMed] [DOI] |
| 16. | Bagchi D, McGinn TR, Ye X, Bagchi M, Krohn RL, Chatterjee A, Stohs SJ. Helicobacter pylori-induced oxidative stress and DNA damage in a primary culture of human gastric mucosal cells. Dig Dis Sci. 2002;47:1405-1412. [PubMed] [DOI] |
| 17. | Slomiany BL, Piotrowski J, Slomiany A. Up-regulation of endothelin-converting enzyme-1 in gastric mucosal inflammatory responses to Helicobacter pylori lipopolysaccharide. Biochem Biophys Res Commun. 2000;267:801-805. [PubMed] [DOI] |
| 18. | Kawahara T, Teshima S, Kuwano Y, Oka A, Kishi K, Rokutan K. Helicobacter pylori lipopolysaccharide induces apoptosis of cultured guinea pig gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol. 2001;281:G726-G734. [PubMed] |
| 19. | Bhattacharyya A, Pathak S, Datta S, Chattopadhyay S, Basu J, Kundu M. Mitogen-activated protein kinases and nuclear factor-kappaB regulate Helicobacter pylori-mediated interleukin-8 release from macrophages. Biochem J. 2002;368:121-129. [PubMed] [DOI] |
| 20. | Cruchet S, Obregon MC, Salazar G, Diaz E, Gotteland M. Effect of the ingestion of a dietary product containing Lactobacillus johnsonii La1 on Helicobacter pylori colonization in children. Nutrition. 2003;19:716-721. [PubMed] [DOI] |
| 21. | Pantoflickova D, Corthésy-Theulaz I, Dorta G, Stolte M, Isler P, Rochat F, Enslen M, Blum AL. Favourable effect of regular intake of fermented milk containing Lactobacillus johnsonii on Helicobacter pylori associated gastritis. Aliment Pharmacol Ther. 2003;18:805-813. [PubMed] [DOI] |
| 22. | Sheu BS, Wu JJ, Lo CY, Wu HW, Chen JH, Lin YS, Lin MD. Impact of supplement with Lactobacillus- and Bifidobacterium-containing yogurt on triple therapy for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2002;16:1669-1675. [PubMed] [DOI] |
| 23. | Sun WH, Ou XL, Cao DZ, Yu Q, Yu T, Hu JM, Zhu F, Sun YL, Fu XL, Su H. Efficacy of omeprazole and amoxicillin with either clarithromycin or metronidazole on eradication of Helicobacter pylori in Chinese peptic ulcer patients. World J Gastroenterol. 2005;11:2477-2481. [PubMed] [DOI] |
| 24. | Armuzzi A, Cremonini F, Bartolozzi F, Canducci F, Candelli M, Ojetti V, Cammarota G, Anti M, De Lorenzo A, Pola P. The effect of oral administration of Lactobacillus GG on antibiotic-associated gastrointestinal side-effects during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther. 2001;15:163-169. [PubMed] [DOI] |
| 25. | Mukai T, Asasaka T, Sato E, Mori K, Matsumoto M, Ohori H. Inhibition of binding of Helicobacter pylori to the glycolipid receptors by probiotic Lactobacillus reuteri. FEMS Immunol Med Microbiol. 2002;32:105-110. [PubMed] [DOI] |
| 26. | Oh Y, Osato MS, Han X, Bennett G, Hong WK. Folk yoghurt kills Helicobacter pylori. J Appl Microbiol. 2002;93:1083-1088. [PubMed] [DOI] |
| 27. | Aiba Y, Suzuki N, Kabir AM, Takagi A, Koga Y. Lactic acid-mediated suppression of Helicobacter pylori by the oral administration of Lactobacillus salivarius as a probiotic in a gnotobiotic murine model. Am J Gastroenterol. 1998;93:2097-2101. [PubMed] [DOI] |
| 28. | Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem. 2002;277:50959-50965. [PubMed] [DOI] |
| 29. | Tao Y, Drabik KA, Waypa TS, Musch MW, Alverdy JC, Schneewind O, Chang EB, Petrof EO. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018-C1030. [PubMed] [DOI] |
| 30. | Resta-Lenert S, Barrett KE. Probiotics and commensals reverse TNF-alpha- and IFN-γamma-induced dysfunction in human intestinal epithelial cells. Gastroenterology. 2006;130:731-746. [PubMed] [DOI] |