修回日期: 2006-12-01
接受日期: 2006-12-01
在线出版日期: 2007-02-18
目的: 探讨NS-398对食管癌细胞的生物学效应及可能的作用机制.
方法: 常规方法培养食管癌Eca-109和TE-13细胞, 以不同浓度NS-398(5, 10, 20, 40, 80 μmol/L)处理24, 48, 72 h. 采用四甲基唑蓝法(MTT)检测NS-398对Eca-109和TE-13细胞生长的抑制作用; 流式细胞仪(FCM)检测细胞凋亡及COX-2, Bcl-2, Bax蛋白的表达; TUNEL法检测2种细胞凋亡情况; 用放射免疫分析(RIA)检测培养液上清中前列腺素E2(PGE2)含量.
结果: NS-398可抑制2种细胞的生长, 并随药物浓度的增高及作用时间的延长抑制率逐渐增高, 并使2种细胞产生的PGE2明显降低. NS-398使2种细胞G0/G1期细胞显著增多, S期细胞显著减少(Eca-109: F = 22.39, P<0.01; TE-13: F = 46.99, P<0.01), 并引起了明显的细胞凋亡. NS-398使2种细胞COX-2和Bcl-2表达显著减少, 而Bax表达显著增高. COX-2和Bcl-2的表达呈显著正相关(Eca-109: r = 0.925, P<0.01; TE-13: r = 0.925, P<0.01), COX-2和Bax表达呈显著负相关(Eca-109: r = -0.937, P<0.01; TE-13: r = -0.703, P<0.01)、Bax和bcl-2表达呈显著负相关(Eca-109: r = -0.926, P<0.01; TE-13: r = -0.753, P<0.01).
结论: NS-398可抑制食管癌细胞的增殖并可诱导其凋亡, 应用COX-2选择性抑制剂对食管癌进行化学预防或辅助治疗具有可能性.
引文著录: 金春亭, 鲁蓓, 李海军, 李玉珍, 武欣, 范婕, 张林西. 环氧化酶-2选择性抑制剂抑制人食管癌细胞的生长及其诱导凋亡. 世界华人消化杂志 2007; 15(5): 440-446
Revised: December 1, 2006
Accepted: December 1, 2006
Published online: February 18, 2007
AIM: To investigate the biological effects of NS-398, a cyclooxygenase-2 (COX-2) selective inhibitor, on esophageal carcinoma cell lines and the possible mechanism.
METHODS: Esophageal carcinoma cell lines Eca-109 and TE-13 were cultured by routine method, and then treated with different concentrations of NS-398 (5, 10, 20, 40, and 80 μmol/L) for 24, 48 and 72 hours. The growths of Eca-109 and TE-13 cells were measured by MTT assay. The expression of COX-2, Bcl-2 and Bax in the cells were detected by flow cytometry (FCM). TUNEL and FCM methods were also used to detect the NS-398-induced apoptosis of the cells. The content of prostaglandin E2 in the supernatants of cell culture was measured by radioimmunoassay (RIA).
RESULTS: NS-398 inhibited the proliferations of Eca-109 and TE-13 cells in concentration- and time-dependent manners, and also decreased the production of PGE2. Meanwhile, NS-398 treatment resulted in an increase of G0/G1-phase cells and a decrease of S-phase ones in both kinds of cells (Eca-109: F = 22.39, P < 0.01; TE-13: F = 46.99, P < 0.01). The apoptosis of Eca-109 and TE-13 cells was also obviously increased after NS-398 treatment. COX-2 and Bcl-2 protein expression were down-regulated, while Bax protein expression was up-regulated in Eca-109 and TE-13 cells after NS-398 treatment. There was a positive correlation between COX-2 and Bcl-2 expression in the two cell lines (Eca-109: r = 0.925, P < 0.01; TE-13: r = 0.925, P < 0.01), but a negative correlation between COX-2 and Bax expression (Eca-109: r = -0.937, P < 0.01; TE-13: r = -0.703, P < 0.01) as well as between Bax and Bcl-2 expression (Eca-109: r = -0.926, P < 0.01; TE-13: r = -0.753, P < 0.01).
CONCLUSION: COX-2 selective inhibitor NS-398 may inhibit the proliferation and induce the apoptosis of esophageal carcinoma cell lines through the inactivation of COX-2, down-regulation of Bcl-2 expression, and up-regulation of Bax experssion. It may be used in the chemoprevention or adjuvant treatment of esophageal carcinomas.
- Citation: Jin CT, Lu B, Li HJ, Li YZ, Wu X, Fan J, Zhang LX. Effects of cyclooxygenase-2 selective inhibitor NS-398 on the proliferation and apoptosis of human esophageal carcinoma cell lines. Shijie Huaren Xiaohua Zazhi 2007; 15(5): 440-446
- URL: https://www.wjgnet.com/1009-3079/full/v15/i5/440.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i5.440
环氧化酶(cyclooxygenase, COX)是前列腺素(prostaglandin, PG)合成过程中的重要限速酶, 在哺乳动物至少有2种同工酶: COX-1和COX-2. COX-1在多种细胞中呈常规保守性表达, 其产物PG在肾的血流调节及胃黏膜上皮细胞保护中可能有重要作用; 而COX-2在正常情况下绝大多数组织中检测不到, 但可被许多因素如细胞因子、癌基因、促癌剂、胆汁酸等诱导表达[1], 参与多种病理生理过程. 研究发现, COX-2在食管癌[2-6]中表达显著增高. 在人及动物实验性肿瘤中发现, PGE2含量增高[7-9], 而PGE2是由COX产生的. 提示可应用COX-2抑制剂对食管癌进行预防及辅助治疗. 关于应用非类固醇类抗炎药(nonsteroidal anti-inflammatory drugs, NSAIDs)预防食管癌变的机制仍不清楚. 我们采用COX-2选择性抑制剂NS-398对2个食管癌细胞株进行研究, 试图探讨COX-2选择性抑制剂对食管癌细胞的生物学效应及可能的作用机制, 为NSAIDs对食管癌高发区人群进行化学预防奠定理论基础.
COX-2为兔IgG多抗、Bax, Bcl-2为小鼠IgG单抗; 羊抗兔FITC-IgG、羊抗鼠FITC-IgG购自Santa Cruz公司. NS-398为Cayman Chemical公司产品. 原位细胞凋亡检测试剂盒购自华美生物工程公司; H-PGE2放射免疫(RIA)检测试剂盒购自解放军总医院放免研究所.
食管癌细胞株Eca-109购自第四军医大学实验动物中心; TE-13由河北医科大学第四医院科研中心惠赠. Eca-109和TE-13分别用含100 mL/L胎牛血清(FBS)的RPMI1640培养液在37℃含50 mL/L CO2及950 mL/L空气饱和湿度温箱中常规培养. 取对数生长期的单层培养细胞, 用2.5 g/L Trypsin+0.2 g/L EDTA对单层细胞进行消化、分离成单细胞悬液, 进行正常传代、收获细胞做相关实验. 应用NS-398分别对2个细胞株进行实验. 观测3个作用时间点即24, 48和72 h. 药物浓度依次为: 5, 10, 20, 40, 80 μmol/L. 在给予不同浓度的NS-398对培养细胞作用一定时间(24, 48及72 h)后, 分别收获细胞. 首先吸取培养液进行离心, 1200 r/min离心5 min, 然后吸取培养液上清1 mL至无菌Ep管中, 立即密封-20℃冰箱冻存, 留取做PGE2放射免疫分析. 离心管底部的细胞与收获的贴壁细胞收集到一起后, 洗涤、固定并送检.
1.2.1 NS-398对人食管癌细胞生长的影响: 细胞增殖抑制实验, 采用四甲基唑蓝(MTT)法. 单细胞悬液常规接种96孔板. 细胞贴壁生长后, 同时换液并施加不同浓度NS-398. 每浓度重复6孔. 同时设滴加单细胞悬液而不加药物的正常对照及只滴加培养液的空白对照. 3个培养板分别于药物作用24, 48及72 h时终止培养. 在酶标仪上选择490 nm滤光片测定A值, 并记录结果. 生长抑制率(IR)(%) = (实验组A490值-对照组A490值)/对照组A490值×100%
1.2.2 细胞周期及凋亡的FCM检测: 20 μmol/L NS-398对培养细胞作用一定时间(24, 48及72 h)后, 分别收获细胞. 对收获的细胞用碘化丙啶一步插入性DNA荧光染色方法进行DNA染色, 具体步骤见文献[10]. 应用相关软件对细胞周期及凋亡进行分析. 在二倍体细胞(G0/G1)峰前出现一个亚二倍体峰位判定为凋亡细胞峰, 以凋亡指数(apoptotic index, AI)表示凋亡状态, 公式为: AI = [细胞总数-(G0/G1+S+G2/M)各时相细胞数]/细胞总数×100%
1.2.3 COX-2, Bcl-2, Bax基因蛋白表达的FCM检测: 对上述收获的细胞同时进行COX-2, Bcl-2, Bax表达的定量检测. 具体操作见参考文献[10].
1.2.4 原位细胞凋亡检测: 对培养液进行离心, 收集已经悬浮的细胞, 再常规消化收获贴壁细胞, 合并后离心成细胞团块进行石蜡包埋. 具体方法根据文献[11]进行操作. 细胞凋亡检测采用TUNEL试剂盒. 对于细胞蜡块切片, 先进行脱蜡至水化, 然后按试剂盒说明书进行. DAB显色, 显微镜下控制显色, 自来水终止显色, 苏木精对比染色, 常规脱水、封片. 以细胞核呈显著棕黄色颗粒状为阳性凋亡细胞, 在光镜下(×400)随机选取5个视野对凋亡细胞进行计数, 并以AI表示凋亡细胞的多少, 即100个完整细胞中平均凋亡细胞数的百分数表示.
1.2.5 PGE2放射免疫法测定: 对Eca-109和TE-13 两个细胞株培养液中PGE2含量进行测定. 应用NS-398进行药物干预实验, 在药物分别作用24, 48及72 h后收集培养液. 具体操作步骤如下: 吸取培养瓶中培养液入离心管中, 1200 r/min离心5 min, 吸取上清液1.5 mL入无菌Ep管中, 立即放入-20℃冰箱中冻存. 测定时按放免检测试剂盒说明书进行操作, 液体闪烁计数器进行检测.
统计学处理 采用SPSS11.0统计软件进行统计分析. 对流式细胞仪检测细胞周期分布、凋亡及COX-2, Bax和Bcl-2的表达的统计分析, 采用单因素方差分析及组间的两两比较. 并对COX-2, Bax和Bcl-2的表达进行相关性分析.
Eca-109细胞和TE-13细胞常规培养下呈单层贴壁生长. Eca-109细胞给予NS-398后继续培养24 h, 20 μmol/L培养瓶中细胞增殖不明显, 少量细胞变圆呈球形; NS-398作用48 h后, 20 μmol/L培养瓶中较多细胞呈球形, 贴壁生长细胞较少. 对于TE-13细胞的形态改变与Eca-109细胞相似.
NS-398对Eca-109细胞和TE-13细胞的生长抑制作用都比较显著. 随NS-398浓度的增高, 其细胞生长抑制作用逐渐增强, 对Eca-109细胞10 μmol/L NS-398作用24 h后其抑制率为26.99%, 40 μmol/L NS-398作用24 h后其抑制率为40.14%. 随药物作用时间的延长, 其抑制率也不断增高, 如对TE-13细胞10 μmol/L NS-398作用24 h后其抑制率为20.00%, 48 h后其抑制率为46.47%, 72 h后其抑制率为73.72%(表1).
| 药物浓度 (μmol/L) | 24 h | 48 h | 72 h | |||
| A490 | IR(%) | A490 | IR(%) | A490 | IR(%) | |
| Eca-109 | ||||||
| 0 | 0.289±0.012 | 0.363±0.041 | 0.689±0.028 | |||
| 5 | 0.269±0.011 | -6.92 | 0.352±0.021 | -3.03 | 0.492±0.022 | -28.59 |
| 10 | 0.254±0.027 | -12.11 | 0.291±0.034 | -19.83 | 0.293±0.031 | -57.47 |
| 20 | 0.211±0.019 | -26.99 | 0.245±0.033 | -32.51 | 0.154±0.017 | -77.65 |
| 40 | 0.173±0.014 | -40.14 | 0.107±0.046 | -70.52 | 0.102±0.023 | -85.20 |
| 80 | 0.129±0.033 | -55.36 | 0.093±0.031 | -74.38 | 0.088±0.012 | -87.23 |
| TE-13 | ||||||
| 0 | 0.380±0.020 | 0.624±0.016 | 0.860±0.073 | |||
| 5 | 0.319±0.015 | -16.05 | 0.567±0.024 | -9.13 | 0.748±0.062 | -13.02 |
| 10 | 0.304±0.036 | -20.00 | 0.334±0.027 | -46.47 | 0.226±0.027 | -73.72 |
| 20 | 0.097±0.018 | -74.47 | 0.029±0.005 | -95.35 | 0.016±0.005 | -98.14 |
| 40 | 0.065±0.008 | -82.89 | 0.048±0.007 | -92.31 | 0.027±0.007 | -96.86 |
| 80 | 0.069±0.006 | -81.84 | 0.075±0.011 | -87.98 | 0.052±0.007 | -93.95 |
NS-398使Eca-109细胞和TE-13细胞G0/G1期细胞显著增多(Eca-109细胞: F = 28.85, P<0.01; TE-13细胞: F = 191.62, P<0.01); 而S期细胞显著减少(Eca-109细胞: F = 22.39, P<0.01; TE-13细胞: F = 46.99, P<0.01). 20 μmol/L NS-398分别对2种细胞都引起了显著的细胞凋亡(Eca-109细胞: F = 229.29, P<0.01; TE-13细胞: F = 191.62, P<0.01). 由于NS-398作用时间的不同, 凋亡率范围为8.18%-11.57%(表2).
| 分组 | 细胞周期分布 | AI | ||
| G0/G1 | S | G2/M | ||
| Eca-109细胞株 | ||||
| 对照 | 66.46±1.33b | 17.64±1.73b | 15.91±1.46b | 1.40±0.53b |
| 24 h | 73.89±2.27 | 12.52±1.13 | 13.60±1.21 | 8.97±0.46 |
| 48 h | 76.00±1.94 | 11.22±0.64 | 12.78±1.44 | 8.61±0.34 |
| 72 h | 72.90±1.83 | 13.25±1.91 | 13.85±0.26 | 8.37±0.88 |
| TE-13细胞株 | ||||
| 对照 | 61.08±4.27b | 21.03±2.65b | 17.89±2.37 | 1.80±0.35b |
| 24 h | 68.38±4.39 | 13.04±2.23 | 18.58±2.20 | 8.18±0.79 |
| 48 h | 74.09±1.72 | 9.60±0.69 | 16.31±1.77 | 8.88±1.05 |
| 72 h | 74.75±2.06 | 10.54±1.13 | 14.71±1.40 | 11.57±0.54 |
Eca-109及TE-13在正常生长时都有较强的COX-2表达, NS-398作用一定时间后, COX-2表达显著减少(Eca-109细胞: F = 79.72, P<0.01; TE-13细胞: F = 60.06, P<0.01); 2种细胞在正常生长情况下Bax表达很接近, NS-398作用一定时间后, Bax表达显著增高(Eca-109细胞: F = 76.96, P<0.01; TE-13细胞: F = 46.87, P<0.01); 而Bcl-2在正常生长时都有较高表达, NS-398作用不同时间后, 其表达显著减少(Eca-109细胞: F = 208.77, P<0.01; TE-13细胞: F = 65.97, P<0.01)(表3).
对于2个细胞株, COX-2和Bcl-2的表达呈显著正相关(Eca-109细胞: r = 0.925, P<0.01; TE-13细胞: r = 0.925, P<0.01), COX-2和Bax表达呈显著负相关(Eca-109细胞: r = -0.937, P<0.01; TE-13细胞: r = -0.703, P<0.01)、Bax和Bcl-2表达呈显著负相关(Eca-109细胞: r = -0.926, P<0.01; TE-13细胞: r = -0.753, P<0.01).
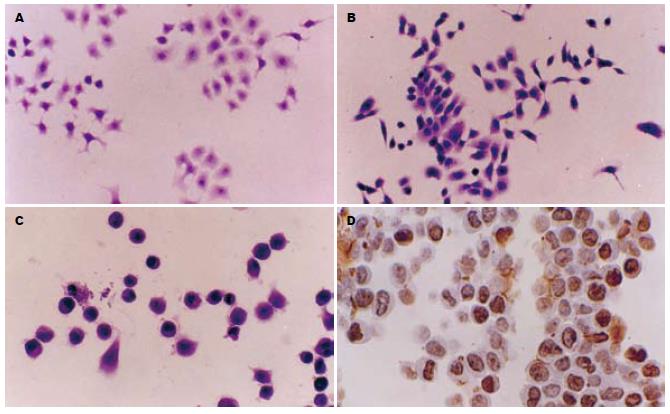
常规培养的Eca-109细胞及TE-13细胞HE染色可见癌细胞呈单层贴壁生长(图1A-B). 原位细胞凋亡检测显示, 40 μmol/L NS-398对Eca-109作用24 h后只有少数细胞发生凋亡, 48 h后HE染色可见部分癌细胞固缩呈球形、浓染(图1C), 凋亡细胞数目明显增多, 药物作用72 h后可见有较多细胞发生凋亡(图1D). NS-398分别对Eca-109和TE-13两细胞株的凋亡情况见表4.
| 细胞株 | 时间 | ||
| 24 h | 48 h | 72 h | |
| Eca-109 | 4.2±1.3 | 17.9±2.7 | 36.7±3.3 |
| TE-13 | 2.9±1.8 | 15.9±2.3 | 36.5±3.8 |
NS-398对Eca-109及TE-13细胞在生长过程中产生的PGE2都有明显的抑制作用. 随着NS-398浓度的增高, PGE2产量明显减少, 尤其是当浓度≥20 μmol/L时; 随着药物作用时间的延长, 与正常生长癌细胞相比, PGE2产量也显著减少(表5).
| 药物浓度(μmol/L) | 24 h | 48 h | 72 h |
| Eca-109细胞株 | |||
| 0 | 2011 | 5967 | 8104 |
| 10 | 2009 | 2518 | 1606 |
| 20 | 1963 | 1802 | 1596 |
| 40 | 1475 | 1802 | 1236 |
| 80 | 803 | 1502 | 765 |
| TE-13细胞株 | |||
| 0 | 4195 | 5647 | 7634 |
| 10 | 1244 | 715 | 1011 |
| 20 | 1030 | 503 | 592 |
| 40 | 653 | 500 | 585 |
| 80 | 547 | 464 | 488 |
食管癌的形成是一个多步骤、多阶段过程, 在其形成及浸润进展过程中, 可有多种基因发生突变或表达异常, 其中发现多数食管癌组织中有COX-2基因表达增高[12-16], COX-2基因作为诱导表达基因, 可能参与食管癌早期阶段的形成[17-19], 而COX-1基因呈常规低水平表达, 可能不参与食管癌的形成. 因此, COX-2基因可作为食管癌化学治疗或进行有效预防的一个新靶点, 应用COX-2选择性抑制剂有可能延缓或阻断食管癌的形成及进展. 目前, 关于COX-2选择性抑制剂对食管癌等癌细胞的作用机制还不清楚. 本研究应用COX-2选择性抑制剂NS-398在2个食管癌细胞系中进行了研究. 研究发现, NS-398能够显著的抑制2个细胞株的增殖活性, 呈药物剂量依赖模式和时间依赖模式, 即随药物浓度的增加其抑制作用逐渐增强及随药物作用时间的延长而抑制作用明显加强. 从细胞形态上可见, 原来呈多边形贴壁生长的细胞部分变为圆形, 不再进行分裂增殖. 对细胞周期的分析发现, 经NS-398作用一定时间后, 2种细胞株G0/G1期细胞显著增多, 而S期及G2/M期细胞明显减少. 可见NS-398使食管癌细胞的生长停滞于G0/G1期. 这与Zhi et al[17]的研究结果一致.
人食管癌细胞系Eca-109和TE-13都是食管鳞状细胞癌. Eca-109及TE-13正常生长情况下都有明显的COX-2表达, 20 μmol/L NS-398作用一段时间后, COX-2表达都显著降低. 对细胞培养液中PGE2含量的检测发现, PGE2产量随药物浓度的增高而显著降低. 提示, NS-398对癌细胞生长的抑制可能是通过对COX-2酶活性的抑制, 从而使PGE2产量明显减少[20-21], 使较多癌细胞停滞于G0/G1期, 从而抑制细胞的生长. 关于COX-2选择性抑制剂对食管癌细胞的作用多有报道[22-24], 但结果不一. 本研究中, NS-398对2个细胞株不但有明显的生长抑制, 还可诱导癌细胞发生凋亡. 凋亡细胞的多少随药物作用时间的延长而增多. 关于COX-2抑制剂可诱导食管癌细胞发生凋亡已有报道[20,25-26], 但其作用机制仍不清楚. Eca-109及TE-13在正常生长时都有明显的Bcl-2表达, Bcl-2可以抑制由多种因素引起的细胞凋亡. 2种细胞由于其COX-2酶活性可以产生较多的PGE2. 在给予一定浓度的NS-398作用一段时间后, PGE2产量明显减少, Bcl-2表达也显著降低, 部分细胞发生了凋亡. 同时, Bax表达显著增高, 其自身能够形成同源二聚体诱导细胞凋亡[27]. Tsujii et al[28]对上皮细胞的研究发现, COX-2高表达细胞中有Bcl-2水平的升高, 而无COX-2表达的细胞中则没有发现Bcl-2表达; Sheng et al[29]发现, PGE2可以抑制由SC-58125诱发的结肠癌细胞凋亡并诱导Bcl-2表达, 说明PGE2可通过Bcl-2抑制细胞凋亡. 提示NS-398诱导食管癌细胞凋亡可能是通过对COX-2酶活性的抑制而起作用的, 同时使抗凋亡蛋白Bcl-2表达降低, 而促凋亡蛋白Bax表达上调, 促进了癌细胞凋亡.
应用COX-2抑制剂对甲基苄基亚硝胺(NMBA)诱发的大鼠食管癌模型中的肿瘤形成有明显预防作用已有报道[30-31]. 因此, 有可能应用COX-2选择性抑制剂对食管癌的形成进行有效的化学预防或辅助治疗, 探索出对我国食管癌防治的新途径.
食管癌是我国常见恶性肿瘤之一, 且食管癌的分布具有明显的地区差异, 寻找有效的化学预防药物是食管癌防治的重要任务之一, COX-2选择性抑制剂对食管癌细胞的生长抑制及诱导癌细胞凋亡使其用于食管癌等肿瘤的预防及临床辅助治疗成为可能.
近年流行病学研究发现, 长期服用阿司匹林、消炎痛等非类固醇类抗炎药(NSAIDs)可降低食管、胃及结肠等部位癌的危险性. 但是, 关于COX-2与癌变的关系及NSAIDs对癌细胞的作用机制还不太清楚, 是近年的研究热点之一.
本文用多种方法对2个食管癌细胞株进行研究, 对细胞生长、细胞周期、细胞凋亡及凋亡相关蛋白等多指标、多方法进行检测. 并应用RIA(放射免疫分析)法检测培养液中PGE2含量.
本文探讨了NS-398对食管癌细胞的生物学效应及可能的作用机制,关于NS-398对肿瘤的生物学效应及凋亡已有较多的报道, 但本文应用多种方法就NS-398对多个细胞株的生物学效应进行研究, 少见报道, 方法新颖, 结果可靠, 具有一定的指导意义.
电编: 张敏 编辑:王晓瑜
| 1. | Coffey RJ, Hawkey CJ, Damstrup L, Graves-Deal R, Daniel VC, Dempsey PJ, Chinery R, Kirkland SC, DuBois RN, Jetton TL. Epidermal growth factor receptor activation induces nuclear targeting of cyclooxygenase-2, basolateral release of prostaglandins, and mitogenesis in polarizing colon cancer cells. Proc Natl Acad Sci USA. 1997;94:657-662. [PubMed] |
| 2. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 3. | Ratnasinghe D, Tangrea J, Roth MJ, Dawsey S, Hu N, Anver M, Wang QH, Taylor PR. Expression of cyclooxygenase-2 in human squamous cell carcinoma of the esophagus; an immunohistoche-mical survey. Anticancer Res. 1999;19:171-174. [PubMed] |
| 4. | Shamma A, Yamamoto H, Doki Y, Okami J, Kondo M, Fujiwara Y, Yano M, Inoue M, Matsuura N, Shiozaki H. Up-regulation of cyclooxygenase-2 in squamous carcinogenesis of the esophagus. Clin Cancer Res. 2000;6:1229-1238. [PubMed] |
| 5. | Kuo KT, Chow KC, Wu YC, Lin CS, Wang HW, Li WY, Wang LS. Clinicopathologic significance of cyclooxygenase-2 overexpression in esophageal squamous cell carcinoma. Ann Thorac Surg. 2003;76:909-914. [PubMed] |
| 6. | Kawabe A, Shimada Y, Uchida S, Maeda M, Sato F, Itami A, Imamura M. Expression of cyclooxygenase-2 is associated with carcinogenesis of the lower part of thoracic esophageal squamous cell carcinoma and p53 expression. Oncology. 2002;62:46-54. [PubMed] |
| 7. | Eberhart CE, Coffey RJ, Radhika A, Giardiello FM, Ferrenbach S, DuBois RN. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183-1188. [PubMed] |
| 8. | Pugh S, Thomas GA. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994;35:675-678. [PubMed] |
| 9. | Boolbol SK, Dannenberg AJ, Chadburn A, Martucci C, Guo XJ, Ramonetti JT, Abreu-Goris M, Newmark HL, Lipkin ML, DeCosse JJ. Cyclooxygenase-2 overexpression and tumor formation are blocked by sulindac in a murine model of familial adenomatous polyposis. Cancer Res. 1996;56:2556-2560. [PubMed] |
| 12. | Yang GZ, Li L, Ding HY, Zhou JS. Cyclooxygenase-2 is over-expressed in Chinese esophageal squamous cell carcinoma, and correlated with NF-kappaB: an immunohistochemical study. Exp Mol Pathol. 2005;79:214-218. [PubMed] |
| 13. | Jiang JG, Tang JB, Chen CL, Liu BX, Fu XN, Zhu ZH, Qu W, Cianflone K, Waalkes MP, Wang DW. Expression of cyclooxygenase-2 in human esophageal squamous cell carcinomas. World J Gastroenterol. 2004;10:2168-2173. [PubMed] |
| 14. | Li Y, Wo JM, Ray MB, Jones W, Su RR, Ellis S, Martin RC. Cyclooxygenase-2 and epithelial growth factor receptor up-regulation during progression of Barrett's esophagus to adenocarcinoma. World J Gastroenterol. 2006;12:928-934. [PubMed] |
| 16. | Nozoe T, Ezaki T, Kabashima A, Baba H, Maehara Y. Significance of immunohistochemical expression of cyclooxygenase-2 in squamous cell carcinoma of the esophagus. Am J Surg. 2005;189:110-115. [PubMed] |
| 17. | Zhi H, Wang L, Zhang J, Zhou C, Ding F, Luo A, Wu M, Zhan Q, Liu Z. Significance of COX-2 expression in human esophageal squamous cell carcinoma. Carcinogenesis. 2006;27:1214-1221. [PubMed] |
| 18. | Lagorce C, Paraf F, Vidaud D, Couvelard A, Wendum D, Martin A, Flejou JF. Cyclooxygenase-2 is expressed frequently and early in Barrett's oesophagus and associated adenocarcinoma. Histopathology. 2003;42:457-465. [PubMed] |
| 19. | Yu HP, Xu SQ, Liu L, Shi LY, Cai XK, Lu WH, Lu B, Su YH, Li YY. Cyclooxygenase-2 expression in squamous dysplasia and squamous cell carcinoma of the esophagus. Cancer Lett. 2003;198:193-201. [PubMed] |
| 20. | Liu JF, Jamieson GG, Drew PA, Zhu GJ, Zhang SW, Zhu TN, Shan BE, Wang QZ. Aspirin induces apoptosis in oesophageal cancer cells by inhibiting the pathway of NF-kappaB downstream regulation of cyclooxygenase-2. ANZ J Surg. 2005;75:1011-1016. [PubMed] |
| 21. | Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, Krishnadath KK, Lutzke LS, Burgart LJ. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002;122:1101-1112. [PubMed] |
| 22. | Kase S, Osaki M, Honjo S, Takeda A, Adachi K, Araki K, Ito H. A selective cyclooxygenase-2 inhibitor, NS398, inhibits cell growth and induces cell cycle arrest in the G2/M phase in human esophageal squamous cell carcinoma cells. J Exp Clin Cancer Res. 2004;23:301-307. [PubMed] |
| 23. | Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, Ohta T, Koichi M. A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis. 2005;26:565-570. [PubMed] |
| 24. | Tuynman JB, Buskens CJ, Kemper K, ten Kate FJ, Offerhaus GJ, Richel DJ, van Lanschot JJ. Neoadjuvant selective COX-2 inhibition down-regulates important oncogenic pathways in patients with esophageal adenocarcinoma. Ann Surg. 2005;242:840-849, discussion 849-850. [PubMed] |
| 25. | Yu HP, Shi LY, Lu WH, Su YH, Li YY, Xu SQ. Expression of cyclooxygenase-2 (COX-2) in human esophageal cancer and in vitro inhibition by a specific COX-2 inhibitor, NS-398. J Gastroenterol Hepatol. 2004;19:638-642. [PubMed] |
| 26. | Vona-Davis L, Riggs DR, Jackson BJ, McFadden DW. Antiproliferative and apoptotic effects of rofecoxib on esophageal cancer in vitro(1). J Surg Res. 2004;119:143-148. [PubMed] |
| 27. | Sermadiras S, Dumas M, Joly-Berville R, Bonte F, Meybeck A, Ratinaud MH. Expression of Bcl-2 and Bax in cultured normal human keratinocytes and melanocytes: relationship to differentiation and melanogenesis. Br J Dermatol. 1997;137:883-889. [PubMed] |
| 28. | Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overex-pressing prostaglandin endoperoxide synthase 2. Cell. 1995;83:493-501. [PubMed] |
| 29. | Sheng H, Shao J, Morrow JD, Beauchamp RD, DuBois RN. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998;58:362-366. [PubMed] |
| 30. | Carlton PS, Gopalakrishnan R, Gupta A, Liston BW, Habib S, Morse MA, Stoner GD. Piroxicam is an ineffective inhibitor of N-nitrosomethyl-benzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376-4382. [PubMed] |
| 31. | Stoner GD, Qin H, Chen T, Carlton PS, Rose ME, Aziz RM, Dixit R. The effects of L-748706, a selective cyclooxygenase-2 inhibitor, on N-nitrosomethyl-benzylamine-induced rat esophageal tumorigenesis. Carcinogenesis. 2005;26:1590-1595. [PubMed] |