修回日期: 2007-09-27
接受日期: 2007-09-28
在线出版日期: 2007-10-18
目的: 观察下调X连锁凋亡抑制蛋白(XIAP)基因表达对胃癌细胞化疗敏感性的影响.
方法: 构建XIAP基因反义真核表达载体, 稳定转染胃癌细胞株MKN-45, RT-PCR和Western blot法检测癌细胞XIAP基因表达. 选用顺铂、丝裂霉素分别处理转染前后的胃癌细胞, 采用MTT比色法、克隆形成抑制实验检测癌细胞体外生长活性; 透射电镜、流式细胞术、TUNEL检测癌细胞凋亡及比率; Western blot和比色法检测细胞内caspase-3蛋白表达和活性水平.
结果: RT-PCR和Western blot证实, 稳定转染反义XIAP基因的胃癌细胞MKN-45的XIAP mRNA和蛋白表达水平分别降低84.75%(P<0.01)和89.75%(P<0.01). 各浓度顺铂、丝裂霉素处理24 h后, 转染反义XIAP基因的MKN-45细胞生长抑制率分别增加7.3%-25.3%(P<0.01), 12.3%-16.3%(P<0.01). 透射电镜下可见部分细胞发生典型的凋亡形态学改变, 凋亡率分别为34.12%和32.5%, 显著高于未转染对照组MKN-45细胞的凋亡率(14.2%, P<0.05). 与MKN-45细胞比较, 稳定转染反义XIAP基因的MKN-45细胞内caspase-3表达水平增高2.45倍(P<0.01), 活性水平提高3.68倍(P<0.01).
结论: 通过反义RNA技术下调XIAP基因表达, 能提高癌细胞中caspase-3的表达和活性, 增强化疗药物对癌细胞的诱导凋亡作用.
引文著录: 郑丽端, 童强松, 汪良, 董继华, 侯晓华. 下调XIAP表达增强化疗药物诱导胃癌细胞凋亡的作用. 世界华人消化杂志 2007; 15(29): 3067-3073
Revised: September 27, 2007
Accepted: September 28, 2007
Published online: October 18, 2007
AIM: To observe the effects of down-regulating expression of X-linked inhibitor of apoptosis (XIAP) on the chemotherapeutic sensitivity of gastric cancer cells.
METHODS: The antisense eukaryotic vector for XIAP was constructed and stably transfected into gastric cancer cell line MKN-45. Reverse transcription polymerase chain reaction (RT-PCR) and Western blotting were applied to detect XIAP gene expression. Cisplatin and mitomycin C were administrated to untransfected and transfected gastric cancer cells. MTT colorimetry and clone formation were performed to measure in vitro cell viability. Apoptosis and its rates were detected by electron microscopy, acridine orange-ethidium bromide fluorescent staining and in situ terminally labeled transferase technique (TUNEL). Cellular caspase 3 protein expression and its activity were assayed by Western blotting and colorimetry.
RESULTS: RT-PCR and Western blotting indicated that the mRNA and protein levels within gastric cancer MKN-45 cells stably transfected with antisense XIAP vector were significantly decreased by 84.75% (P < 0.01) and 89.75% (P < 0.01), respectively. After treatment with various concentrations of cisplatin and mitomycin C for 24 h, growth inhibition of MKN-45 cells stably transfected with antisense XIAP was enhanced by 7.3%-25.3% (P < 0.01) and 12.3%-16.3% (P < 0.01), respectively. Partial cancer cells presented characteristic changes of apoptosis under electron microscopey, with apoptosis rates of 34.12% and 32.5%, respectively, which were significantly higher than those in untransfected control MKN-45 cells (14.2%, P < 0.05). Compared with MKN-45 cells, caspase 3 expression in cells stably transfected with antisense XIAP was significantly increased by 2.45 times (P < 0.01), while the activity of caspase 3 was enhanced by 3.68 times (P < 0.01).
CONCLUSION: Down-regulation of XIAP expression via antisense RNA may increase the expression and activity of caspase 3 and enhance chemotherapy-induced apoptosis of cancer cells.
- Citation: Zheng LD, Tong QS, Wang L, Dong JH, Hou XH. Effects of down-regulating expression of X-linked inhibitor of apoptosis on chemotherapy-induced apoptosis of gastric cancer cells. Shijie Huaren Xiaohua Zazhi 2007; 15(29): 3067-3073
- URL: https://www.wjgnet.com/1009-3079/full/v15/i29/3067.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i29.3067
胃癌是最常见的消化道恶性肿瘤, 全世界胃癌的发病患者数每年达60多万, 其中35%发生在中国. 目前化疗仍是胃癌术后和晚期胃癌的一种主要辅助治疗手段, 但由于肿瘤细胞对化疗药物易产生耐受性, 使得胃癌化疗疗效有待进一步提高. 现已明确, 化疗药物、放射治疗、温热治疗均以不同程度的诱导凋亡途径发挥抗肿瘤作用[1-3]. 研究发现, 哺乳动物细胞内存在抑制凋亡蛋白(inhibitor of apoptosis proteins, IAPs), 能通过阻止前caspases的激活和成熟caspases的催化活性而阻抑凋亡[4-5]. 其中已经发现的IAPs包括X连锁凋亡抑制蛋白(X-linked inhibitor of apoptosis, XIAP), c-IAP1, c-IAP2和survivin. XIAP能直接和caspases家族成员中介导蛋白酶级联下游效应分子caspase-3, caspase-7, caspase-9结合并抑制他们的活性, 并且能抑制化疗药物、紫外线照射、Bax诱导的凋亡[6-8]. 有研究显示, 肿瘤细胞中XIAP水平的升高是肿瘤细胞逃避抗癌药物杀伤作用的原因之一[9]. 我们观察反义RNA技术下调XIAP基因表达对化疗药物诱导胃癌细胞凋亡效应的影响, 旨在探索改善胃癌化疗的新途径.
真核表达载体pEF-XIAP由澳大利亚Walter & Eliza Hall研究所David Vaux博士惠赠, 在其限制性酶切位点XbaⅠ和BamHⅠ间含有人类XIAP基因全长cDNA(1494 bp); 空白载体pcDNA3.1由本院中心实验室常规保存; XIAP多克隆抗体由加拿大Eastern Ontario儿童医院Robert G. Korneluk教授惠赠; Caspase-3 mAb和活性测定试剂盒分别购自Santa Cruz和Clontech公司; 脂质体GeneSHUTTLE-40购自Q-bio基因有限公司; 新生牛血清、RPMI 1640、TRIzolTM Reagent Kit、G418购自Gibco公司; 二甲基亚砜(DMSO)、四甲基偶氮唑盐(MTT)及碘化丙锭(PI)均购自Clontech公司; 顺铂(Cis Diammine Dichloroplatinum, DDP)为山东齐鲁制药厂产品, 丝裂霉素(Mitomycin C, MMC)为日本协和发酵工业株式会社产品, 按参考文献[3]配成1g/L储存液备用; 高表达XIAP基因的人胃癌细胞株MKN-45购自美国典型培养物保藏中心(ATCC), 在含有100 mL/L新生牛血清、100 kU/L青霉素及100 mg/L链霉素的 RPMI 1640培养基中, 37℃, 50 mL/L CO2条件下培养, 每3 d传代1次.
XIAP反义表达载体的构建采用限制性内切酶XbaⅠ和BamHⅠ双切质粒pEF-XIAP和pcDNA3.1, 分别回收1.5 kb的基因片段和5.4 kb的线性化载体片段, T4 DNA酶进行连接反应, 将XIAP基因片段反向插入到载体pcDNA3.1中, 将重组体命名为pcDNA3.1-AS-XIAP(6.9 kb). 根据基因和载体的物理图谱, 选取BamHⅠ和XbaⅠ单酶切和BamHⅠ和XbaⅠ双酶切该重组体, 酶切产物经10 g/L琼脂糖凝胶电泳, 进行鉴定. 取对数生长期的MKN-45细胞接种于24孔板, 设置未转染对照组、空载体pcDNA3.1转染组、pcDNA3.1-AS-XIAP转染组. 基因转染步骤参照GeneSHUTTLE-40试剂盒说明书进行. 转染72 h后, 加入G418(终浓度600 mg/L), 筛选2 wk. 当未转染对照组细胞大部分死亡时, 将G418浓度降至300 mg/L维持筛选2 wk, 克隆细胞形成后, 随机挑选克隆, 大量扩增抗性细胞, 将稳定表达反义XIAP基因、新霉素抗性(neo)基因的亚克隆细胞株分别命名为MKN-45/AS-XIAP, MKN-45/neo.
1.2.1 癌细胞XIAP表达的检测: 收集上述3种细胞各1×106, 按TRIzolTM试剂盒说明书提取总RNA. 逆转录反应体系: 模板RNA 2 μg, 10 mmol/L dNTP 1 μL, RNA酶抑制剂(RNasin)335 nKat, Oligo dT18 1 μL, AMV逆转录酶3334 nKat, 5×逆转录酶缓冲液5 μL, 终体积为25 μL, 42℃水浴30 min. 采用Primer premier 5.0软件设计XIAP基因PCR引物(扩增产物大小650 bp), 序列如下: 上游引物5'-GGACATGGATATACTCAGTT-3', 下游引物5'-AGTAATGACTGTGTAGCACA-3', 由上海捷倍思基因技术有限公司合成. 同时以α-tubulin(310 bp)作为内参照, 梯度PCR扩增. 产物经20 g/L琼脂糖凝胶电泳, MGIAS-1000凝胶成像系统扫描定量并拍照. XIAP蛋白表达采用Western blot法. 参照《分子克隆实验指南》进行上述3种细胞蛋白质的提取、定量和分离. 转膜后先后与10 g/L脱脂奶粉、鼠抗人XIAP、过氧化物酶偶联的二抗孵育, 运用ECL Western blot kit显色. 北航CMIASWIN计算机图像分析系统测定XIAP蛋白条带密度.
1.2.2 细胞生长的检测: 将MKN-45, MKN-45/neo, MKN-45/AS-XIAP细胞以4×103/孔的密度接种于96孔培养板, 参照药物体内有效浓度或其IC50, 分别加入DDP(1, 5, 10 mg/L), MMC(0.1, 1, 10 mg/L), 并设置未加药空白对照组. 常规条件下培养24 h后, 每孔加入5 g/L MTT 20 μL, 继续培养4 h, 弃去上清. 每孔加入1 L/L DMSO 100 μL, 振摇15 min, 酶标仪波长490 nm处检测各孔吸光度(A)值. 重复实验3次. 根据公式计算化疗药物对癌细胞的生长抑制率(%) = (1-化疗药物实验组平均A值/空白对照组平均A值)×100%. 取上述各组癌细胞, 以300个细胞/平皿的密度接种于35 mm2塑料培养皿, 37℃, 50 mL/L CO2条件下培养14 d, 甲醇固定10 min, 2 g/L结晶紫染色20 min, 倒置显微镜下计数大于50个细胞的克隆. 集落形成率(%) = (集落数/300)×100%. 5 mg/L DDP、10 mg/L MMC作用24 h后, 收集上述3种癌细胞, PBS洗涤1次, 25 g/L戊二醛固定30 min, PBS洗涤并悬浮细胞, 常规包埋、切片, 透射电镜观察.
1.2.3 细胞凋亡的检测: 采用3 mL/L H2O2封闭30 min, 20 mg/L蛋白酶K消化液作用20 min, TUNEL反应液37℃孵育60 min, 滴加过氧化物酶连接的抗体, 37℃孵育30 min, DAB显色, 苏木素复染. 以PBS替代TUNEL反应液作阴性对照. 结果判断: 光学显微镜下, 凋亡细胞体积缩小, 核固缩, 染色质呈特异的棕黄色. 细胞凋亡率(%) = (500个细胞中凋亡细胞数/500)×100%. 收集2×106各组癌细胞, 0.01 mol/L PBS(pH 7.4)洗涤2次, 700 mL/L乙醇固定过夜, 4℃保存, 检测前用PBS洗涤1次, 加入1 g/L RNase 200 μL, 37℃水浴30 min, 用50 mg/L碘化丙啶400 μL进行染色, 室温避光30 min, 用流式细胞仪(Becton Dickson公司)进行DNA含量分析, 所用软件为CellQest. 另分别收集2×105上述3种细胞, 采用Western blot法检测上述细胞内caspase-3蛋白表达水平(方法同前), 加入细胞裂解缓冲液50 μL, 冰上孵育10 min, 12 000 r/min, 4℃离心细胞裂解液3 min, 回收上清, 每管依次加入50 μL的2×反应缓冲液, 1.0 mmol/L caspase-3底物DEVD-pNA 5 μL, 37℃孵育1 h, 转移至96孔板中, 用酶标仪测定波长405 nm的吸光度(A405nm)值, 表示caspase-3的相对活性.
统计学处理 采用t检验或方差分析, 运用SPSS10.0统计学软件进行数据分析.
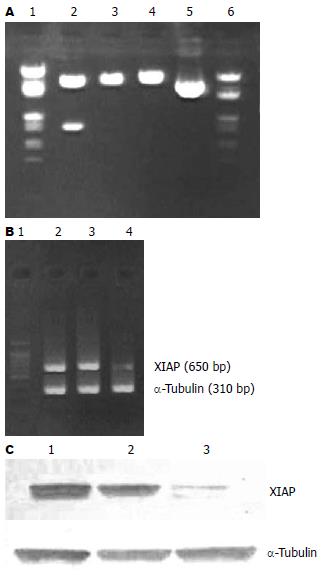
酶切鉴定证实XIAP cDNA已反向插入到重组体pcDNA3.1-AS-XIAP中(图1A). G418(600 mg/L)筛选1 wk后, 对照组MKN-45细胞完全死亡. pcDNA3.1, pcDNA3.1-AS-XIAP转染组经G418持续筛选4 wk, 可见明显的阳性克隆, 继续扩增培养, 分别获得稳定表达neo基因、反义XIAP基因的亚克隆细胞MKN-45/neo和MKN-45/AS-XIAP.
MKN-45和MKN-45/neo细胞经RT-PCR检测癌细胞XIAP mRNA表达均可见650 bp的XIAP扩增条带, MKN-45/AS-XIAP细胞以同量的RNA模板扩增后仅有微弱的显影(图1B). 经MGIAS-1000凝胶成像分析系统证实, MKN-45/AS-XIAP细胞株的XIAP条带灰度值分别为MKN-45和MKN-45/neo细胞的15.25%(P<0.01)、14.12%(P<0.01); 而MKN-45和MKN-45/neo细胞XIAP条带灰度值之间无显著性差异(P>0.05). MKN-45和MKN-45/neo细胞株经Western blot检测均可见分子量为27 kDa的蛋白条带(图1C). 图像分析系统证实, MKN-45/AS-XIAP细胞的XIAP蛋白条带灰度值为MKN-45和MKN-45/neo细胞的10.25%(P<0.01)和9.68%(P<0.01); MKN-45和MKN-45/neo细胞XIAP蛋白条带灰度值之间无显著性差异(P>0.05).
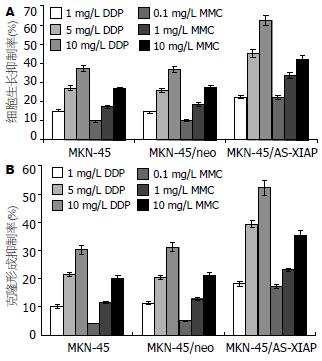
各浓度DDP和MMC作用24 h后, MKN-45/AS-XIAP细胞的生长抑制率较对照组MKN-45分别增加7.3%-25.3%(P<0.01)和12.3%-16.3%(P<0.01). 其中, 1 mg/L DDP作用24 h后, 对照组MKN-45细胞生长抑制率为15.16%, 而MKN-45/AS-XIAP的抑制率达到22.46%(P<0.05); 10 mg/L MMC作用24 h后, 未转染对照组MKN-45细胞生长抑制率为26.65%, 而MKN-45/AS-XIAP细胞的抑制率达到42.16% (P<0.01). 各浓度化疗药物对未转染对照组、空载体转染组细胞生长抑制率间的差异无显著性(P>0.05, 图2A). 另未加药对照组MKN-45, MKN-45/neo和MKN-45/AS-XIAP细胞克隆形成率分别为86.25%±10.12%, 84.56%±11.16%和80.12%±12.24%. 各浓度DDP和MMC作用24 h后, MKN-45/AS-XIAP细胞克隆形成率均较MKN-45和MKN-45/neo细胞明显降低(图2B).
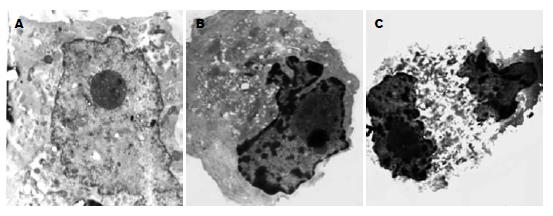
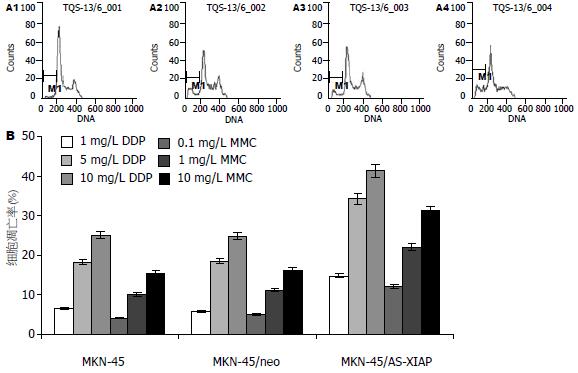
5 mg/L DDP和10 mg/L MMC作用24 h后, 电镜下可见部分MKN-45/AS-XIAP细胞呈现较为典型的凋亡超微形态学改变: 胞体缩小、核固缩、染色质浓缩成块部分核膜下聚集、但胞膜完整(图3). 而MKN-45/AS-XIAP凋亡细胞明显增多, 细胞凋亡率分别达到34.12%和32.5%, 显著高于MKN-45和MKN-45/neo细胞凋亡率(P<0.05). MKN-45、MKN-45/neo细胞凋亡率间比较差异无显著性意义(P>0.05). 各浓度DDP和MMC作用24 h后, DNA直方图上均可见亚G1期凋亡峰, 其中MKN-45/AS-XIAP细胞较MKN-45和MKN-45/neo细胞更为明显(图4). 10 mg/L MMC作用24 h后, MKN-45, MKN-45/neo和MKN-45/AS-XIAP细胞均出现了caspase-3的分解产物-有活性的17 kDa(p17)亚单位. 图像分析系统证实: MKN-45/AS-XIAP的p17表达水平较MKN-45和MKN-45/neo分别增高2.45倍(P<0.01)和2.52倍(P<0.01, 图5). 未加药MKN-45, MKN-45/neo和MKN-45/AS-XIAP细胞A405nm值分别为0.058±0.010, 0.054±0.011和0.062±0.012, 各组间比较差异无显著性意义(P>0.05). 10 mg/L MMC作用24 h后, MKN-45/AS-XIAP A405nm值达到0.386±0.012, 较MKN-45细胞(0.105±0.010)升高3.68倍(P<0.01).
胃癌是我国常见的恶性肿瘤, 且近年来发病率有上升趋势, 其晚期治疗效果仍不理想[10-11]. 越来越多的研究表明, 诱导细胞凋亡是化疗药物杀伤肿瘤细胞的重要作用机制. 许多化疗药物 (如表鬼臼毒吡喃葡糖甙)可通过活化相关蛋白酶而引发最终的凋亡执行阶段[12-13]. 有些凋亡调节剂也与化疗诱导的细胞凋亡相互作用, 如破坏p53基因可以保护乳腺癌细胞免遭顺铂诱导的凋亡[14]; Epstein-Barr病毒蛋白BHRF-1(与Bcl-2在结构和功能上都类似)可以使癌细胞逃避表鬼臼毒吡喃葡糖甙和顺铂诱导的凋亡[15]; Safingol(一种蛋白激酶C抑制剂)可以增加丝裂霉素C杀伤结肠癌细胞的能力[16].
目前, 许多化疗耐药机制已在体外肿瘤细胞中得到证实, 包括MDR编码的p-糖蛋白、肺耐药相关蛋白、p450活性增加、多种药物靶目标-拓扑异构酶Ⅱ突变等[17-20]. 近年来人们发现, 肿瘤细胞对凋亡的抗性是化疗耐药性产生的主要机制之一[21-22]. Bcl-2基因的过度表达可以对抗一些化疗药物(如表鬼臼毒吡喃葡糖甙、喜树碱、放线菌素D)引起的细胞凋亡[23-25]. 对细胞凋亡的抗性是耐药性产生的新机制, 可以解释相当一部分化疗失败的原因[26-27]. 凋亡抑制蛋白(inhibitors of apoptosis, IAPs)是细胞内一类独特的抗凋亡蛋白家族, 包括XIAP, cIAP1, cIAP2, 神经元凋亡抑制蛋白(NIAP), livin和survivin等[28]. IAPs通过在体外或体内抑制不同的caspases而拮抗细胞凋亡. 与其他的可抑制上游caspases的蛋白不同, IAPs是唯一的内源性caspases抑制物[29]. 1996年, XIAP和cIAP1, cIAP2同时被鉴定出来, 并在体外研究中发现其对Fas和caspases诱发的内源、外源细胞凋亡过程有明显的抑制效应[30-31]. XIAP基因定位于Xq25, N端的3个BIR结构域可以抑制启动性的caspase-9和效应性的caspase-3和caspase-7[32]. 体外动力学研究表明, XIAP是IAP家族里最强有力的caspase抑制物. XIAP的BIR2及BIR2前的连接区参与抑制caspase-3和caspase-7的活性, 单独的BIR3结构域也足以能够抑制caspase-9, 这表明XIAP的不同BIR是以不同的方式抑制caspase-3和caspase-9的[33-34]. 研究证实, 过表达XIAP可抑制凋亡, 提示XIAP可作为肿瘤等疾病基因治疗的新靶点. 一些药物亦可通过下调XIAP和活化caspase-3而诱导白血病细胞的凋亡[35]. 在对78名急性髓系白血病(AML)患者的研究中发现, XIAP和患者存活率有直接相关性, XIAP低水平的患者比XIAP高水平者的存活时间明显延长[36].
我们通过反义RNA技术下调了胃癌细胞中XIAP的表达, 发现能显著增强化疗药物(顺铂、丝裂霉素)的诱导凋亡作用, 说明XIAP的高表达是化疗耐药的一个重要因素, 与Mansouri et al[37]的结论相符. Ekedahl et al[38]发现, 在非小细胞肺癌中, 低剂量γ照射可导致XIAP表达上调, 同时伴随对放射线诱发的细胞凋亡的抵抗. 携带有反义XIAP的腺病毒感染非小细胞肺癌细胞系H661后, 降低了XIAP蛋白水平, 增加了细胞对低剂量γ照射的敏感性[39]. 另有研究表明, 转导反义XIAP不仅使卵巢癌细胞对顺铂诱导的细胞凋亡的敏感性增加, 而且还激活了caspase介导的MDM2活化和p53的积聚[40]. 我们通过比色法检测细胞内caspase-3活性水平, 发现下调XIAP基因能显著提高化疗药物作用后细胞内活性caspase-3的水平, 与文献报道的XIAP作用机制相符合. 这一结果为提高胃癌患者化疗敏感性提供新的思路, 也为进一步研究XIAP基因在胃癌凋亡调控途径中的作用奠定了基础.
目前化疗是胃癌术后和晚期胃癌的一种主要辅助治疗手段, 但由于肿瘤细胞对化疗药物易产生耐受性, 使得胃癌化疗疗效有待进一步提高, 有研究显示, 肿瘤细胞中X连锁凋亡抑制蛋白水平的升高是肿瘤细胞逃避抗癌药物杀伤作用的原因之一.
本文观察反义RNA技术下调XIAP基因表达对化疗药物诱导胃癌细胞凋亡效应的影响, 旨在探索改善胃癌化疗的新途径.
研究证实, 肿瘤组织中过表达的XIAP可抑制化疗药物、紫外线照射、Bax诱导的凋亡, 提示XIAP可作为肿瘤治疗的新靶点, 一些药物亦可通过下调XIAP和活化caspase-3而诱导白血病细胞的凋亡.
本文通过反义RNA技术下调胃癌细胞中XIAP的表达, 发现能显著增强化疗药物(顺铂、丝裂霉素)诱导胃癌细胞凋亡的作用, 说明XIAP的高表达是化疗耐药的一个重要因素. 此外, 本文发现下调XIAP能显著提高化疗药物作用后细胞内活性caspase-3的水平, 与文献报道的XIAP作用机制相符合.
本文结果为提高胃癌患者化疗敏感性提供了新思路, 也为进一步研究XIAP基因在胃癌凋亡调控途径中的作用奠定了基础.
凋亡抑制蛋白(IAPs): 是细胞内一类独特的抗凋亡蛋白家族, 包括XIAP, cIAP1, cIAP2, 神经元凋亡抑制蛋白(NIAP), livin和survivin等. IAPs通过在体外或体内抑制不同的caspases而拮抗细胞凋亡.
本文有一定的创新性, 研究方法合适, 结果结论可信, 图表清楚, 有一定的理论意义.
编辑: 程剑侠 电编:何基才
| 1. | Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153-164. [PubMed] |
| 2. | Szostak MJ, Kyprianou N. Radiation-induced apoptosis: predictive and therapeutic significance in radiotherapy of prostate cancer (review). Oncol Rep. 2000;7:699-706. [PubMed] |
| 3. | Schulze PC, Adams V, Busert C, Bettag M, Kahn T, Schober R. Effects of laser-induced thermotherapy (LITT) on proliferation and apoptosis of glioma cells in rat brain transplantation tumors. Lasers Surg Med. 2002;30:227-232. [PubMed] |
| 4. | Martin SJ. Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell. 2002;109:793-796. [PubMed] |
| 5. | Deveraux QL, Reed JC. IAP family proteins--suppressors of apoptosis. Genes Dev. 1999;13:239-252. [PubMed] |
| 6. | Li J, Sasaki H, Sheng YL, Schneiderman D, Xiao CW, Kotsuji F, Tsang BK. Apoptosis and chemoresistance in human ovarian cancer: is Xiap a determinant? Biol Signals Recept. 2000;9:122-130. [PubMed] |
| 7. | Vaziri SA, Grabowski DR, Tabata M, Holmes KA, Sterk J, Takigawa N, Bukowski RM, Ganapathi MK, Ganapathi R. c-IAP1 is overexpressed in HL-60 cells selected for doxorubicin resistance: effects on etoposide-induced apoptosis. Anticancer Res. 2003;23:3657-3661. [PubMed] |
| 8. | Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-8589. [PubMed] |
| 9. | Notarbartolo M, Cervello M, Dusonchet L, Cusimano A, D'Alessandro N. Resistance to diverse apoptotic triggers in multidrug resistant HL60 cells and its possible relationship to the expression of P-glycoprotein, Fas and of the novel anti-apoptosis factors IAP (inhibitory of apoptosis proteins). Cancer Lett. 2002;180:91-101. [PubMed] |
| 10. | Gulmann C, Hegarty H, Grace A, Leader M, Patchett S, Kay E. Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via the retinoblastoma pathway. World J Gastroenterol. 2004;10:17-21. [PubMed] |
| 11. | Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L, Liu YX. Correlation of tumor-positive ratio and number of perigastric lymph nodes with prognosis of patients with surgically-removed gastric carcinoma. World J Gastroenterol. 2004;10:182-185. [PubMed] |
| 12. | Dubrez L, Savoy I, Hamman A, Solary E. Pivotal role of a DEVD-sensitive step in etoposide-induced and Fas-mediated apoptotic pathways. EMBO J. 1996;15:5504-5512. [PubMed] |
| 13. | Yoshida A, Takauji R, Inuzuka M, Ueda T, Nakamura T. Role of serine and ICE-like proteases in induction of apoptosis by etoposide in human leukemia HL-60 cells. Leukemia. 1996;10:821-824. [PubMed] |
| 14. | Wosikowski K, Regis JT, Robey RW, Alvarez M, Buters JT, Gudas JM, Bates SE. Normal p53 status and function despite the development of drug resistance in human breast cancer cells. Cell Growth Differ. 1995;6:1395-1403. [PubMed] |
| 15. | McCarthy NJ, Hazlewood SA, Huen DS, Rickinson AB, Williams GT. The Epstein-Barr virus gene BHRF1, a homologue of the cellular oncogene Bcl-2, inhibits apoptosis induced by gamma radiation and chemotherapeutic drugs. Adv Exp Med Biol. 1996;406:83-97. [PubMed] |
| 16. | Caponigro F, French RC, Kaye SB. Protein kinase C: a worthwhile target for anticancer drugs? Anticancer Drugs. 1997;8:26-33. [PubMed] |
| 17. | Campone M, Vavasseur F, Le Cabellec MT, Meflah K, Vallette FM, Oliver L. Induction of chemoresistance in HL-60 cells concomitantly causes a resistance to apoptosis and the synthesis of P-glycoprotein. Leukemia. 2001;15:1377-1387. [PubMed] |
| 18. | Brockdorff BL, Skouv J, Reiter BE, Lykkesfeldt AE. Increased expression of cytochrome p450 1A1 and 1B1 genes in anti-estrogen-resistant human breast cancer cell lines. Int J Cancer. 2000;88:902-906. [PubMed] |
| 19. | Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22:7537-7552. [PubMed] |
| 20. | Zhou R, Frostvik Stolt M, Kronenwett U, Gruber A, Liliemark J, Liliemark E. Real-time RT-PCR for the determination of topoisomerase II mRNA level in leukaemic cells. Leuk Res. 2002;26:487-494. [PubMed] |
| 21. | Soengas MS, Lowe SW. Apoptosis and melanoma chemoresistance. Oncogene. 2003;22:3138-3151. [PubMed] |
| 22. | Schmitt CA, Lowe SW. Apoptosis and chemo-resistance in transgenic cancer models. J Mol Med. 2002;80:137-146. [PubMed] |
| 23. | Fennell DA. Bcl-2 as a target for overcoming chemoresistance in small-cell lung cancer. Clin Lung Cancer. 2003;4:307-313. [PubMed] |
| 24. | Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265-7279. [PubMed] |
| 25. | Pommier Y, Sordet O, Antony S, Hayward RL, Kohn KW. Apoptosis defects and chemotherapy resistance: molecular interaction maps and networks. Oncogene. 2004;23:2934-2949. [PubMed] |
| 26. | Zheng LD, Tong QS, Tao KX, Wang L, Zhang B. Effects of Smac gene overexpression on chemotherapeutic sensitivity of gastric cancer cell line MKN-45. Ai Zheng. 2004;23:361-366. [PubMed] |
| 27. | Blagosklonny MV. Prospective strategies to enforce selectively cell death in cancer cells. Oncogene. 2004;23:2967-2975. [PubMed] |
| 28. | Liston P, Fong WG, Korneluk RG. The inhibitors of apoptosis: there is more to life than Bcl2. Oncogene. 2003;22:8568-8580. [PubMed] |
| 29. | LaCasse EC, Baird S, Korneluk RG, MacKenzie AE. The inhibitors of apoptosis (IAPs) and their emerging role in cancer. Oncogene. 1998;17:3247-3259. [PubMed] |
| 30. | Rajcan-Separovic E, Liston P, Lefebvre C, Korneluk RG. Assignment of human inhibitor of apoptosis protein (IAP) genes xiap, hiap-1, and hiap-2 to chromosomes Xq25 and 11q22-q23 by fluorescence in situ hybridization. Genomics. 1996;37:404-406. [PubMed] |
| 31. | Suzuki Y, Nakabayashi Y, Takahashi R. Ubiquitin-protein ligase activity of X-linked inhibitor of apoptosis protein promotes proteasomal degradation of caspase-3 and enhances its anti-apoptotic effect in Fas-induced cell death. Proc Natl Acad Sci U S A. 2001;98:8662-8667. [PubMed] |
| 32. | Takahashi R, Deveraux Q, Tamm I, Welsh K, Assa-Munt N, Salvesen GS, Reed JC. A single BIR domain of XIAP sufficient for inhibiting caspases. J Biol Chem. 1998;273:7787-7790. [PubMed] |
| 33. | Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242-5251. [PubMed] |
| 34. | Riedl SJ, Renatus M, Schwarzenbacher R, Zhou Q, Sun C, Fesik SW, Liddington RC, Salvesen GS. Structural basis for the inhibition of caspase-3 by XIAP. Cell. 2001;104:791-800. [PubMed] |
| 35. | Messmer UK, Pereda-Fernandez C, Manderscheid M, Pfeilschifter J. Dexamethasone inhibits TNF-alpha-induced apoptosis and IAP protein downregulation in MCF-7 cells. Br J Pharmacol. 2001;133:467-476. [PubMed] |
| 36. | Carter BZ, Milella M, Tsao T, McQueen T, Schober WD, Hu W, Dean NM, Steelman L, McCubrey JA, Andreeff M. Regulation and targeting of antiapoptotic XIAP in acute myeloid leukemia. Leukemia. 2003;17:2081-2089. [PubMed] |
| 37. | Mansouri A, Zhang Q, Ridgway LD, Tian L, Claret FX. Cisplatin resistance in an ovarian carcinoma is associated with a defect in programmed cell death control through XIAP regulation. Oncol Res. 2003;13:399-404. [PubMed] |
| 38. | Ekedahl J, Joseph B, Grigoriev MY, Muller M, Magnusson C, Lewensohn R, Zhivotovsky B. Expression of inhibitor of apoptosis proteins in small- and non-small-cell lung carcinoma cells. Exp Cell Res. 2002;279:277-290. [PubMed] |
| 39. | Holcik M, Yeh C, Korneluk RG, Chow T. Translational upregulation of X-linked inhibitor of apoptosis (XIAP) increases resistance to radiation induced cell death. Oncogene. 2000;19:4174-4177. [PubMed] |
| 40. | Sasaki H, Sheng Y, Kotsuji F, Tsang BK. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Res. 2000;60:5659-5666. [PubMed] |