修回日期: 2007-02-04
接受日期: 2007-02-08
在线出版日期: 2007-05-08
目的: 研究大鼠肝卵圆细胞(HOC)增殖模型肝脏缝隙连接蛋白32, 43(CX)的表达及缝隙连接细胞间通讯(GJIC)的功能, 探讨HOC增殖的可能机制.
方法: 健康♂Wistar大鼠, 随机分成正常对照组(n = 6), 模型组. 模型组大鼠按每天20 mg/kg剂量灌喂2-AFF, 连续4 d, 第5天不灌喂行2/3肝切除, 术后次日按每天20 mg/kg剂量继续灌喂5 d (2-AFF/PH). 模型组在术后4 h, 4, 8, 12和16 d随机取6只大鼠检测. 采用组织病理技术观察肝组织的形态学变化; 免疫组化和细胞形态学方法计数HOC; 切开标记/染料示踪技术(incision loading/dye transfer, IL/DT)技术确定GJIC; 免疫组化及RT-PCR技术检测CX32蛋白及mRNA表达; 免疫组化、Western blot及RT-PCR技术分析CX43蛋白及mRNA水平.
结果: 对照组及模型组4 h未见HOC增殖. 模型组4 d汇管区有HOC增殖反应, 8 d HOC增殖达峰值, 12 d HOC从汇管区向肝实质内浸润, 16 d HOC增殖减少较12 d减少; IL/DT检测结果显示, 模型组各时点(4 h, 4, 8, 12和16 d)染料扩散距离低于对照组(84.5±3.4, 60.6±3.3, 108.6±4.2, 150.6±2.6, 199.6±3.7 μm vs 250.0±5.0 μm, P<0.01), GJIC功能降低. 模型组各时点CX32表达均低于对照组(P<0.05), 在4 h下调, 4 d达低峰(2.85±0.39), 8 d后逐渐恢复; RT-PCR显示模型组CX32 mRNA在4 h开始下降, 4 d达低峰(0.33±0.11), 8 d后逐渐恢复, 16 d高于对照组, 但无显著差异(P>0.05). Western blot结果显示模型组CX43蛋白表达在4 h上调(P>0.05)、4-16 d明显升高; RT-PCR显示模型组4 h CX43 mRNA上调(P>0.05), 4 d表达明显升高, 12 d达高峰(5.46±0.58), 16 d低于对照组, 但无显著差异(P>0.05).
结论: 采用Solt-Farber方法成功建立了HOC增殖动物模型; 大鼠2-AAF/PH肝脏CX表达呈时空特异性变化, 使肝脏GJIC功能抑制. 肝脏的GJIC抑制可能启动了HOC增殖.
引文著录: 李学东, 傅华群, 李少华, 尚西亮, 邢宏松, 胡鹏. 大鼠肝卵圆细胞增殖模型肝脏的GJIC功能和意义. 世界华人消化杂志 2007; 15(13): 1475-1481
Revised: February 4, 2007
Accepted: February 8, 2007
Published online: May 8, 2007
AIM: To study the expression of connexin 32 (CX32) and connexin 43 (CX43) and function of gap junction intercellular communication (GJIC) in rat liver during 2-acetylaminofluorene/partial hepatectomy (2AAF/PH) for hepatic oval cell (HOC) proliferation, and explore the potential mechanism of HOC proliferation in vivo.
METHODS: Male Wistar rats were randomized into normal control group (n = 6) and model group. Rats in model group were used to induce HOC proliferation: 9 days of treatment with 2-AAF, 20 mg/kg per day by gavage, interrupted on day 5 to perform a 70% hepatectomy (2-AAF/PH). At the 4th hour, 4th, 8th, 12th and 16th day, 6 rats of model group were sacrificed respectively. The morphological changes of liver tissues were observed by pathological examination and the proliferation of HOC was counted using immunohistochemistry and morphological recognition. GJIC was confirmed by incision loading/dye transfer (IL/DT), and the levels of CX32 protein and mRNA were detected by immunohistochemistry and reverse transcription-polymerase chain reaction (RT-PCR), respectively. The expression of CX43 protein and mRNA were determined by immunohistochemistry, Western blot and RT-PCR, respectively.
RESULTS: No HOC proliferation was seen in the rat liver of control and 4-hour model group. Pathologic examination revealed that HOC appeared at portal area in model group on day 4, increased to the peak on day 8, intensely proliferated from the portal spaces and invaded the liver parenchyma on day 12, and decreased on day 16 as compared with day 12. In comparison with that in control group, the distance of dye transfer in model groups (4 h, 4, 8, 12, 16 d) was significantly reduced (84.5 ± 3.4, 60.6 ± 3.3, 108.6 ± 4.2, 150.6 ± 2.6, 199.6 ± 3.7 μm vs 250.0 ± 5.0 μm, P < 0.01). The signal number of CX32 in the rat liver of model groups began to decrease at the 4th hour, reached to the minimum (2.85 ± 0.39) on day 4, and recovered starting from day 8, and it was markedly reduced as compared with that in control group (P < 0.05). CX32 mRNA in model groups was decreased at the 4th hour, reached the lowest level (0.33 ± 0.11) on day 4 and started to recover on day 8. On day 16, CX32 mRNA expression was also higher than that in control group, but the difference was not significant (P > 0.05). Western blot analysis showed an increased CX43 protein expression at the 4th hour (P > 0.05), on day 4, 8, 12 and 16 (P < 0.01). In comparison with that in control group, the level of CX43 mRNA in model group had a slight increase at the 4th hour (P > 0.05), an obvious increase on day 4, reached the peak on day 12 (5.46 ± 0.58), and started to decrease on day 16 (P > 0.05).
CONCLUSION: Satisfactory rat model of HOC proliferation is successfully obtained using AAF/PH, and this method is convenient, stable and repeatable. Inhibition of GJIC function, which may activate the proliferation of HOC, is regulated by CX expression patterns.
- Citation: Li XD, Fu HQ, Li SH, Shang XL, Xing HS, Hu P. Function and significance of gap junction intercellular communication in rat liver for hepatic oval cell proliferation in vivo. Shijie Huaren Xiaohua Zazhi 2007; 15(13): 1475-1481
- URL: https://www.wjgnet.com/1009-3079/full/v15/i13/1475.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i13.1475
肝卵圆细胞(hepatic oval cells, HOC)是肝干细胞的子代细胞, 自HOC被确认以来, 人们对肝损伤后修复的认识进入了一个新的阶段. 在肝脏受到严重损伤或肝细胞增殖能力受损时, HOC的增殖、分化显得格外重要, 成为再生肝脏的重要细胞源泉[1-6]. 到目前为止, HOC增殖、分化的机制远未阐明. 由于缝隙连接细胞间通讯(gap junction intercellular communication, GJIC)是细胞间重要的信息交流形式, 可调节组织细胞的生长、增殖与分化[7-9]. 本实验采用大鼠Solt-Farber(2-乙酰氨基芴喂食+2/3肝切除术2-acetylaminofluorene/two-thirds partial hepatectomy, 2-AAF/PH)模型, 观察肝脏GJIC与体内HOC动员的关系, 并研究GJIC变化的机制, 为进一步探讨GJIC对HOC的调控打下基础.
成年健康♂Wistar大鼠, 体质量150 g左右, 由南昌大学医学院动物科学部提供; 2-乙酰氨基芴(2-acetylaminofluorene, 2-AAF; Sigma公司); 聚乙二醇(PEG400, 上海试剂一厂); 小鼠抗大鼠OV-6 mAb(美国S Sell实验室); Mouse anti-human CX43抗体、Mouse anti-human CX32抗体(Santa Cruz公司); 抗小鼠SP试剂盒(北京中杉金桥生物技术有限公司); Lucifer yellow(LY)、Rhodamine D(Sigma公司); TRIzol(Invitrogen公司); Oligo(dT)15(北京天为时代公司); dNTPs(BM公司); M-MLV逆转录酶(Promega公司); 大鼠CX43, CX32, GADPH引物(上海生工公司).
1.2.1 动物处理及标本采集: 大鼠首先随机分成对照组、模型组. 2-AFF用分子量为400的聚乙二醇溶解成浓度为4 g/L的溶液, 模型组大鼠每天按20 mg/kg剂量灌喂, 连续4 d, 第5天不灌喂, 在乙醚吸入麻醉下行2/3肝切除, 术后次日按每天20 mg/kg剂量继续灌喂5 d(2-AFF/PH). 模型组在术后4 h, 4, 8, 12和16 d随机取6只大鼠检测. 对照组大鼠未预特殊处理, 作正常对照(n = 6). 大鼠在乙醚吸入麻醉后剖腹, 取0.5 cm×0.5 cm×0.3 cm的右肝中叶, 置中性缓冲福尔马林液固定, 备行HE染色及免疫组化; 取1.0 cm×1.0 cm×1.0 cm的右肝中叶, 行切开标记/染料示踪技术(incision loading/dye transfer, IL/DT)分析; 剪右肝中叶成50-100 mg的小块若干, 分置液氮保存, 提取总蛋白及RNA.
1.2.2 免疫组织化学染色: 石蜡切片经常规脱蜡水化后, 抗原热修复15-20 min; 按SP免疫组化试剂盒说明书操作, 一抗工作液浓度分别为: OV-6 1:80, CX32 1:250, CX43 1:300. 磷酸盐(PBS)缓冲液代替一抗做空白对照.
1.2.3 IL/DT: 方法参考文献[10-13], 在新鲜肝组织表面上滴少许(约200 μL)含有LY (5 g/L) 和RhD (5 g/L)染料的PBS缓冲液, 用刀片作3-4个深1 mm, 长7-8 mm的切口, 再于切口内注入少许染料, 室温下染料扩散3 min, 后用PBS洗3次, 每次1 min, 再将组织放入40 g/L福尔马林液内避光过夜, 石蜡包埋, 垂直切口作5 μm厚的石蜡切片, 于免疫荧光显微镜下测量LY的净染色传输距离.
1.2.4 图像分析: 每张OV-6免疫组织化学切片选取5个互不重叠且肝卵圆细胞增殖最明显的视野, 在400倍显微镜下对符合肝卵圆细胞特征(体积较小约为肝细胞直径的1/3-1/5, 细胞核呈卵圆形, 胞质较少, 略嗜碱性)且OV-6染色阳性的细胞进行计数, 其平均值作为此标本卵圆细胞增殖的数量; 每张CX32免疫组织化学切片于400倍镜下随机取5个视野, 计算CX32阳性信号数与视野内的肝细胞数之比, 作为该标本CX32的信号数; 每张IL/DT切片随机在免疫荧光显微镜下取5处测量LY和RhD的染色区域. LY的净染色区分析GJIC. 其均值作为GJIC结果.
1.2.5 CX43蛋白Western blot分析: 取100 mg肝组织在冰冻下研成粉末, 加入1.0 mL裂解缓冲液匀浆, 12 000 g 4 ℃, 离心20 min, 取上清液Folin-酚法进行蛋白质的定量-70 ℃冷冻备用. 取50 μg总蛋白加入上样缓冲液煮沸5 min变性, 经SDS-PAGE凝胶电泳, 电泳后将蛋白转至硝酸纤维素膜上; 脱脂奶封闭150 min, 与1:1000稀释的CX43抗体4 ℃孵育过夜, 再与1:500稀释的二抗孵育2 h, 与化学发光试剂ECL温浴1 min后曝光、显影和定影. X光胶片扫描后, 在医学图形分析系统上进行分析. 计算方法为: 所得CX43蛋白带的综合灰度除以对照组样本的综合灰度值即为表达量.
1.2.6 RT-PCR检测肝组织CX32, CX43 mRNA表达: 取肝组织50-100 mg, 总RNA提取用TRIzol试剂按说明操作进行. cDNA采用M-MLV RT kit(Promaga, USA)试剂盒进行. CX32, CX43和GADPH引物设计利用PrimerPremier5.0软件(PremierBiosoft, CA)设计完成. 引物序列及长度见表1. PCR反应条件: 94 ℃预变性5 min, 94 ℃变性40 s, 58 ℃退火45 s(CX32, GADPH)、63 ℃退火45 s(CX43), 72 ℃延伸1 min, 30个循环后, 72 ℃延伸10 min.
| 基因 | 正义(5'-3') | 反以(5'-3') | 产物长度(bp) |
| GADPH | TGAAGGTCGGTGTCAACGGATTTGGC | CATGTAGGCCATGAGGTCCACCAC | 983 |
| CX32 | CTGCTCTACCCGGGCTATGC | CAGGCTGAGCATCGGTCGCTCTT | 386 |
| CX43 | TGGGGGAAAGGCGTGAG | CTGCTGGCTCTGCTGGAAGGT | 1275 |
统计学处理 数据以mean±SD表示, 组间比较用方差分析. P<0.05被认为差异具有统计学意义.
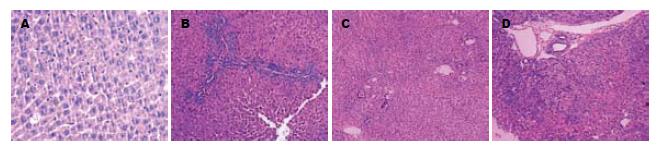
对照组肝小叶、肝窦结构清晰, 肝细胞索排列正常, 未见增殖反应. 模型组4 h肝组织结构无明显破坏及增殖反应. 4, 8 d肝索结构紊乱, 肝细胞水肿、变性坏死, 第4天汇管区有增殖反应, 增殖细胞体积较小、约为肝细胞直径的1/3-1/5, 细胞核呈卵圆形, 胞质较少, 略嗜碱性、大小分布不均匀、呈簇状分布, 从汇管区向肝小叶中央浸润. 第8天增殖反应明显, 并向小叶中央区穿插生长; 12 d肝细胞水肿、坏死减轻多由增殖细胞替代, 增殖细胞已连成片, 增殖反应减少; 16 d仍可见增殖反应(图1).
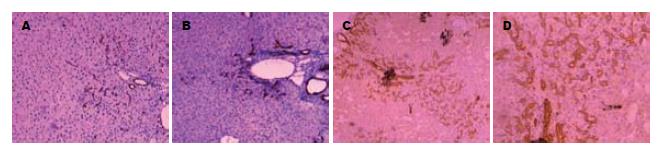
依据OV-6阳性染色和细胞形态学计数HOC. 对照组未见HOC; 模型组在肝切后4 h无明显HOC增殖, 第4天肝汇管区和小叶周边可见增殖的HOC, 但数量较少为8.20±1.50个, 第8天HOC增殖达到高峰为26.53±1.56个; 第12天向肝实质内穿插生长的HOC已连接成片为16.60±2.30个, 与第8天相比数量减少(P<0.01); 第16天与第12天比较HOC明显减少, HOC为11.10±3.20个(P<0.05, 图2).
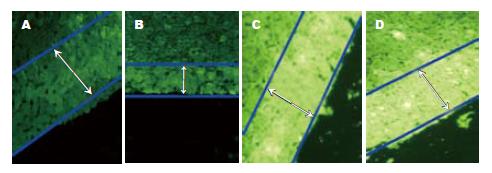
IL/DT检测结果显示对照组GJIC功能良好, 染料扩散距离大约250.0±5.0 μm, 约相当于10个肝细胞的直径. 模型组4 h-16 d各时点染料扩散距离低于对照组(P<0.01), 分别为: 84.5±3.4, 60.6±3.3, 108.6±4.2, 150.6±2.6, 199.6±3.7 μm, 4 h明显降低, 4 d至低峰, 8 d后逐渐恢复(图3).
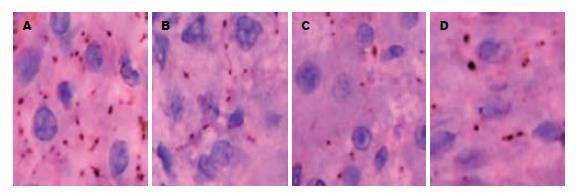
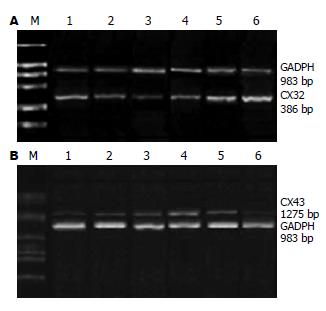
免疫组化显示CX32表达于肝细胞膜上, 对照组每个肝细胞CX32阳性染色信号数为9.8±1.3个; 模型组各时点CX32的表达低于对照组(P<0.05), 分别为: 5.10±0.70, 2.85±0.39, 3.19±0.20, 4.34±0.80, 7.20±0.68个, 在4 h表达下调, 4 d最低峰, 8 d后逐渐恢复(图4).
免疫组化显示CX43表达于汇管区, 伴随HOC数量增加而染色增强. Western blot结果显示对照组大鼠肝组织CX43蛋白水平很低, 模型组CX43蛋白表达在4 h上调、4 d表达明显、8 d达高峰、12 d后下降, 呈逐渐升高而后逐渐恢复趋势, 4 h-16 d各时点分别为对照组的: 1.14±0.17, 3.87±0.35, 5.28±0.48, 2.96±0.33, 2.12±0.19倍, 与对照组比较4 h CX43蛋白的表达上调, 无显著差异(P>0.05), 余时间点明显高于对照组(P<0.01). 模型组各时点CX43蛋白印迹呈现程度不同的三条带, 代表了CX43的不同磷酸化状态(P1, P2是磷酸化CX43, NP为非磷酸化CX43, 图5)[14].
模型组4 h-16 d各时点CX32 mRNA水平分别为对照组的0.82±0.13, 0.33±0.11, 0.51±0.13, 0.68±0.14, 1.12±0.18倍. 在4 h开始下降, 4 d达低峰, 8 d后逐渐恢复, 4 h, 4, 8,12 d时点明显低于对照组(P<0.01), 16 d时点高于对照组, 但无显著差异(P>0.05, 图6).
对照组CX43 mRNA表达很低. 模型组4 h-16 d各时点CX43 mRNA分别为对照组的1.09±0.16, 2.82±0.23, 5.46±0.58, 3.34±0.64, 0.91±0.11倍. 即在建模后4 h上调(P>0.05), 4 d表达明显升高, 12 d达高峰, 4, 8, 12 d时点明显高于对照组(P<0.01), 16 d低于对照组, 但无显著差异(P>0.05, 图6).
GJ是相邻细胞间的通道结构, 每一个细胞提供一个半通道(connexon), 由6个连接蛋白(connexin, CX)围绕中央孔相互锚锭而成[15]. GJ的主要功能是细胞通讯(cell communication), 又称GJIC. GJIC是细胞间唯一、直接的信息交流形式, 许多与生长、分化密切相关的小分子物质(<1000 Da, 如单糖、氨基酸、核苷酸、维生素、激素等), 可以通过GJ从一个细胞至另一个细胞, 从而影响组织细胞的生长、增殖与分化, 是组织保持内稳态的核心[7]. 抑制GJIC可减少细胞凋亡、分化和生长抑制, 增加细胞增殖[16]. GJIC的表达变化对于正常细胞周期是必需的. 例如, GJIC表达在G1/S期适度, 在S期增加, 而在G2/M期则降低[17]. GJIC对一系列生理刺激, 如生长因子PDGF和EGF等刺激反应的关闭, 与增加细胞分裂相关, 从而调节组织细胞分化与增殖状态[18-20]. 研究表明GJIC与多种干细胞的增殖、分化密切关联[21-24]. 但是体内HOC增殖过程中, 肝脏GJIC的功能如何? 目前尚不明确.
大鼠Solt-Farber(2-AAF/PH)模型是研究HOC应用最多的动物模型之一[25]. 其基本原理是: 肝脏毒性药物抑制肝细胞增殖后, 再采用肝切除刺激肝再生时, 可激活HOC并向肝细胞演变. HOC细胞形态学特点是体积较小约为肝细胞直径的1/3-1/5, 细胞核呈卵圆形, 胞质较少, 略嗜碱性. 鉴定HOC多采用细胞形态学结合OV-6阳性染色方法. 目前, 对HOC调控机制的研究多集中于细胞因子、细胞外机制的作用. 为明确大鼠HOC增殖模型肝脏GJIC的变化, 我们采用大鼠Solt-Farber模型观察肝脏GJIC与HOC动员的关系.
本实验中, 对照组大鼠肝组织结构完整, 未见增生反应及HOC, 且GJIC功能良好; 模型组大鼠肝索结构紊乱, 肝细胞水肿、变性坏死, 4 d可见HOC增殖, 8 d增殖达到高峰, 12 d后数量逐渐减少. IL/DT结果显示肝脏GJIC 4 h明显降低, 4 d至低峰, 8 d后逐渐恢复. GJIC功能与HOC的增殖趋势相反, 且变化时点早于HOC的增殖. 结果提示肝脏GJIC的抑制可能启动了HOC的增殖.
由于肝脏主要表达CX32和CX43, 是完成GJIC功能必需的蛋白分子. 为了解CX32, CX43介导的GJIC对体内HOC的调节, 免疫组化结果显示模型组大鼠肝脏CX32蛋白的表达与GJIC趋势相同, Western blot结果显示CX43蛋白的表达与GJIC趋势相反. 结果提示GJIC下调是CX32蛋白表达抑制所致. 在模型组大鼠肝脏CX32的表达下调减少了肝细胞与偶联细胞间的GJIC, 使肝细胞对生长刺激反应迟钝; 由于星型细胞能生成促进HOC增殖的生长因子并表达CX43[26], 因而HOC CX43表达的上调增加了其与星型细胞间的GJIC, 使HOC迅速对外界的生长刺激作出反应. 由于CX对交流物质的选择转运特性[27-31], 在2-AAF/PH肝脏CX的表达重塑, 影响了肝脏的对生长刺激信息的整合, 抑制肝细胞增殖、动员HOC重建损伤肝脏. 从理论上说肝脏的GJIC是其所有的CX功能的总和, 研究发现GJIC与CX32表达趋势一致, 与CX43趋势相反, 这与CX32表达于肝细胞、CX43表达于胆管上皮细胞和HOC相关. 肝脏大多数细胞是肝细胞(约为肝小叶细胞数的79.3%), 即使CX32表达的轻微改变, 也会影响肝脏的GJIC, 胆管上皮细胞及HOC在肝脏比例低, 所以CX43变化对的GJIC影响不大.
CX蛋白的稳定表达是GJIC的功能基础, 控制GJIC过程中mRNA的稳定对于CX蛋白的表达是关键的. 为了透析模型组大鼠肝脏GJIC的下调环节, 我们进一步从转录水平探讨了CX32、CX43 mRNA的表达, 结果显示CX32、CX43 mRNA的水平与其蛋白表达趋势一致, 提示模型组大鼠肝脏CX32、CX43蛋白表达变化极有可能在转录水平被调节.
总之, 肝脏GJIC与HOC密切关联, 肝脏GJIC的抑制可能启动了HOC增殖. 通过调节肝脏CX的时空表达模式, 改变肝脏GJIC可能会影响体内HOC的增殖、分化过程.
肝干细胞增殖、分化的调控是当今研究热点之一, 目前相关研究多集中于细胞因子、细胞外机制的作用. 缝隙连接细胞间通讯(GJIC)参与多种干细胞的增殖、分化的调节. 为了探讨GJIC对肝干细胞的调控, 本文观察了体内肝卵圆细胞增殖与肝脏GJIC功能及CX32, CX43表达的关系.
本文发现肝脏GJIC与肝卵圆细胞增殖密切相关, 为进一步探讨CX/GJIC对肝卵圆细胞的调控打下基础.
1 肝卵圆细胞: 是肝干细胞的子代细胞. 细胞体积较小, 细胞核呈卵圆形, 胞质较少, 略嗜碱性, 具有高增殖、双向分化潜能.
2 缝隙连接(GJ): 是相邻细胞间的通道结构, 由2个半通道相互锚锭组成. 每个半通道由6个连接蛋白围绕形成一个直径约1.5-2 nm的中空通道, 允许与细胞生长、分化密切相关的小分子物质(分子量<1000 Da)通过, 从而发挥其生物学效应, 这种"看家功能"被称为GJIC, 不同连接蛋白构成的GJ对交流物质具有通道选择性.
本文从CX转录、翻译及功能多水平观察了肝脏CX32, CX43表达与体内肝卵圆细胞增殖的关系, 实验方法可靠, 结果可信, 有一定的学术价值.
电编: 张敏 编辑:张焕兰
| 1. | Theise ND, Saxena R, Portmann BC, Thung SN, Yee H, Chiriboga L, Kumar A, Crawford JM. The canals of Hering and hepatic stem cells in humans. Hepatology. 1999;30:1425-1433. [PubMed] [DOI] |
| 2. | Stieger B, Peters R, Sidler MA, Meier PJ. Hepatocyte transplantation: potential of hepatocyte progenitor cells and bone marrow derived stem cells. Swiss Med Wkly. 2006;136:552-556. [PubMed] |
| 3. | Conzelmann LO, Hines IN, Kremer M, Perry AW, Lemasters JJ, Wheeler MD. Extrahepatic cells contribute to the progenitor/stem cell response following reduced-size liver transplantation in mice. Exp Biol Med (Maywood). 2007;232:571-580. [PubMed] |
| 4. | Dorrell C, Grompe M. Liver repair by intra- and extrahepatic progenitors. Stem Cell Rev. 2005;1:61-64. [PubMed] [DOI] |
| 5. | Fausto N, Campbell JS. The role of hepatocytes and oval cells in liver regeneration and repopulation. Mech Dev. 2003;120:117-130. [PubMed] [DOI] |
| 6. | Oh SH, Hatch HM, Petersen BE. Hepatic oval 'stem' cell in liver regeneration. Semin Cell Dev Biol. 2002;13:405-409. [PubMed] [DOI] |
| 7. | Trosko JE, Madhukar BV, Chang CC. Endogenous and exogenous modulation of gap junctional intercellular communication: toxicological and pharmacological implications. Life Sci. 1993;53:1-19. [PubMed] [DOI] |
| 8. | Yanagiya T, Tanabe A, Hotta K. Gap-junctional communication is required for mitotic clonal expansion during adipogenesis. Obesity (Silver Spring). 2007;15:572-582. [PubMed] [DOI] |
| 9. | Lu F, Gao J, Ogawa R, Hyakusoku H. Variations in gap junctional intercellular communication and connexin expression in fibroblasts derived from keloid and hypertrophic scars. Plast Reconstr Surg. 2007;119:844-851. [PubMed] [DOI] |
| 10. | Sai K, Kanno J, Hasegawa R, Trosko JE, Inoue T. Prevention of the down-regulation of gap junctional intercellular communication by green tea in the liver of mice fed pentachlorophenol. Carcinogenesis. 2000;21:1671-1676. [PubMed] [DOI] |
| 11. | Jeong SH, Habeebu SS, Klaassen CD. Cadmium decreases gap junctional intercellular communica-tion in mouse liver. Toxicol Sci. 2000;57:156-166. [PubMed] [DOI] |
| 12. | Dagli ML, Yamasaki H, Krutovskikh V, Omori Y. Delayed liver regeneration and increased suscepti-bility to chemical hepatocarcinogenesis in trans-genic mice expressing a dominant-negative mutant of connexin32 only in the liver. Carcinogenesis. 2004;25:483-492. [PubMed] [DOI] |
| 13. | Asamoto M, Hokaiwado N, Murasaki T, Shirai T. Connexin 32 dominant-negative mutant transgenic rats are resistant to hepatic damage by chemicals. Hepatology. 2004;40:205-210. [PubMed] [DOI] |
| 14. | Musil LS, Goodenough DA. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991;115:1357-1374. [PubMed] [DOI] |
| 15. | Gaietta G, Deerinck TJ, Adams SR, Bouwer J, Tour O, Laird DW, Sosinsky GE, Tsien RY, Ellisman MH. Multicolor and electron microscopic imaging of connexin trafficking. Science. 2002;296:503-507. [PubMed] [DOI] |
| 16. | Trosko JE, Ruch RJ. Cell-cell communication in carcinogenesis. Front Biosci. 1998;3:d208-236. [PubMed] [DOI] |
| 17. | Solan JL, Fry MD, TenBroek EM, Lampe PD. Connexin43 phosphorylation at S368 is acute during S and G2/M and in response to protein kinase C activation. J Cell Sci. 2003;116:2203-2211. [PubMed] [DOI] |
| 18. | Nicholson BJ. Gap junctions - from cell to molecule. J Cell Sci. 2003;116:4479-4481. [PubMed] [DOI] |
| 19. | Anderson C, Catoe H, Werner R. MIR-206 regulates connexin43 expression during skeletal muscle development. Nucleic Acids Res. 2006;34:5863-5871. [PubMed] [DOI] |
| 20. | Sridharan S, Simon L, Meling DD, Cyr DG, Gutstein DE, Fishman GI, Guillou F, Cooke PS. Proliferation of adult sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol Reprod. 2007;76:804-812. [PubMed] [DOI] |
| 21. | Trosko JE, Chang CC, Upham BL, Tai MH. Ignored hallmarks of carcinogenesis: stem cells and cell-cell communication. Ann N Y Acad Sci. 2004;1028:192-201. [PubMed] [DOI] |
| 22. | Trosko JE, Chang CC, Wilson MR, Upham B, Haya-shi T, Wade M. Gap junctions and the regulation of cellular functions of stem cells during development and differentiation. Methods. 2000;20:245-264. [PubMed] [DOI] |
| 23. | Yang SR, Cho SD, Ahn NS, Jung JW, Park JS, Jo EH, Hwang JW, Jung JY, Kim TY, Yoon BS. Role of gap junctional intercellular communication (GJIC) through p38 and ERK1/2 pathway in the differentiation of rat neuronal stem cells. J Vet Med Sci. 2005;67:291-294. [PubMed] [DOI] |
| 24. | Trosko JE. From adult stem cells to cancer stem cells: Oct-4 Gene, cell-cell communication, and hormones during tumor promotion. Ann N Y Acad Sci. 2006;1089:36-58. [PubMed] [DOI] |
| 25. | Paku S, Nagy P, Kopper L, Thorgeirsson SS. 2-acetylaminofluorene dose-dependent differentia-tion of rat oval cells into hepatocytes: confocal and electron microscopic studies. Hepatology. 2004;39:1353-1361. [PubMed] [DOI] |
| 26. | Bustos M, Sangro B, Alzuguren P, Gil AG, Ruiz J, Beraza N, Qian C, Garcia-Pardo A, Prieto J. Liver damage using suicide genes. A model for oval cell activation. Am J Pathol. 2000;157:549-559. [PubMed] [DOI] |
| 27. | Nicholson BJ, Weber PA, Cao F, Chang H, Lampe P, Goldberg G. The molecular basis of selective permeability of connexins is complex and includes both size and charge. Braz J Med Biol Res. 2000;33:369-378. [PubMed] [DOI] |
| 28. | Ek-Vitorin JF, Burt JM. Quantification of gap junction selectivity. Am J Physiol Cell Physiol. 2005;289:C1535-1546. [PubMed] [DOI] |
| 29. | Bedner P, Niessen H, Odermatt B, Kretz M, Willecke K, Harz H. Selective permeability of different connexin channels to the second messenger cyclic AMP. J Biol Chem. 2006;281:6673-6681. [PubMed] [DOI] |
| 30. | Gong XQ, Nicholson BJ. Size selectivity between gap junction channels composed of different connexins. Cell Commun Adhes. 2001;8:187-192. [PubMed] [DOI] |