修回日期: 2007-02-01
接受日期: 2007-02-08
在线出版日期: 2007-04-28
目的: 观察丙酮酸乙酯(EP)对急性坏死性胰腺炎(ANP)肺损伤大鼠血清肿瘤坏死因子-α (TNF-α)、白细胞介素-1b(IL-1β)和肺组织高迁移率族蛋白B1(HMGB1) mRNA表达的影响, 探讨丙酮酸乙酯治疗急性坏死性胰腺炎肺损伤的机制.
方法: 逆行性胰胆管注射50 g/L牛磺胆酸钠制作ANP模型. 随机分成3组, 对照组、ANP组和EP治疗组(40 mg/kg, 每隔6 h静脉注射一次). ELISA法检测血清TNF-α和IL-1β水平; 半定量逆转录聚合酶链反应(RT-PCR)法检测肺组织HMGB1 mRNA表达, 并观察血氧变化及肺组织的病理变化.
结果: ANP组血清TNF-α和IL-1β水平在建模后6 h达高峰, 12 h下降, 在此两时点治疗组血清TNF-α和IL-1β水平明显低于ANP组(TNF-α: 131.6±29.6 ng/L vs 196.3±16.3 ng/L, 65.0±16.6 ng/L vs 90.2±20.1 ng/L, P<0.05; IL-1β: 194.9±26.8 ng/L vs 223.0±34.8 ng/L, 105.2±24.0 ng/L vs 130.4±23.0 ng/L, P<0.05). ANP组大鼠肺组织HMGB1 mRNA表达水平在ANP后12 h明显升高, 至24 h仍维持在高水平. 治疗组肺组织HMGB1 mRNA表达水平在各时间点均明显低于ANP组(0.68±0.11 vs 0.88±0.11, 0.81±0.11 vs 1.04±0.10, 1.08±0.08 vs 1.33±0.15, P<0.05), 且同期肺损伤比ANP组轻. 治疗组PaO2均明显高于ANP组.
结论: 丙酮酸乙酯能显著抑制TNF-α、IL-1β和HMGB1等早晚期炎症因子, 改善低氧血症, 对ANP肺损伤有明显保护作用.
引文著录: 朱峰, 葛春林, 郭仁宣. 丙酮酸乙酯对急性坏死性胰腺炎大鼠肺损伤的保护作用. 世界华人消化杂志 2007; 15(12): 1364-1369
Revised: February 1, 2007
Accepted: February 8, 2007
Published online: April 28, 2007
AIM: To investigate the effects of ethyl pyruvate (EP) on the levels of tumor necrosis factor-α (TNF-α), interleukin-1b (IL-1β) in serum and mRNA expression of high mobility group box 1 (HMGB1) in lung tissues, and explore the mechanisms of ethyl pyruvate in protecting the lungs against acute injury induced by acute necrotizing pancreatitis (ANP).
METHODS: ANP model was induced by retrograde injection of 50 g/L sodium taurocholate into pancreatic duct. Animals were divided randomly into 3 groups: control group, ANP group, and EP treatment group. EP solution was administered intravenously every 6 hours (40 mg/kg once). The serum levels of TNF-α and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA). The mRNA expression of HMGB1 in lung tissues was detected by reverse transcription-polymerase chain reaction (RT-PCR). The partial pressure of blood oxygen (PaO2) was measured, and histological examination of the lungs was also performed.
RESULTS: The serum levels of TNF-α and IL-6 were rapidly increased after ANP model was induced, and reached peak at the 6th hour, but they were decreased at the 12th hour. In comparison with those in ANP group, the serum levels of TNF-α and IL-1β were markedly lowered in EP group 6 and 12 hours after ANP model was induced (TNF-α: 131.6 ± 29.6 ng/L vs 196.3 ± 16.3 ng/L, 65.0 ± 16.6 ng/L vs 90.2 ± 20.1 ng/L, P < 0.05; IL-1β: 194.9 ± 26.8 ng/L vs 223.0 ± 34.8 ng/L, 105.2 ± 24.0 ng/L vs 130.4 ± 23.0 ng/L, P < 0.05). The mRNA expression of HMGB1 in lung tissues was increased significantly at the 12th hour and maintained to the 24th hour after ANP model was induced, whereas in EP group, HMGB1 mRNA expression was significantly lower than that in ANP group at each time point (0.68 ± 0.11 vs 0.88 ± 0.11, 0.81 ± 0.11 vs 1.04 ± 0.10, 1.08 ± 0.08 vs 1.33 ± 0.15, all P < 0.05). The injury of lung tissues in EP group was milder than that in ANP group, and the value of PaO2 was also obviously higher in EP group than that in ANP group (P < 0.05).
CONCLUSION: EP can inhibit the release of TNF-α and IL-1β, down-regulate HMGB1 mRNA expression in lung tissues of ANP rats, and protect lungs from acute injury induced by ANP.
- Citation: Zhu F, Ge CL, Guo RX. Protective effects of ethyl pyruvate against acute lung injury induced by acute necrotizing pancreatitis in rats. Shijie Huaren Xiaohua Zazhi 2007; 15(12): 1364-1369
- URL: https://www.wjgnet.com/1009-3079/full/v15/i12/1364.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i12.1364
重症急性胰腺炎(severe acute pancreatitis, SAP)是普外科常见的、危重的急腹症, 除有局部的病理损伤表现外, 常伴有明显的全身炎症反应及并发多器官损伤, 其病死率可达10%-25%[1-3]. 其中急性肺损伤(acute lung injury, ALI)/急性呼吸窘迫综合征(acute respiratory distress syndrome, ARDS)是其常见并发症之一, SAP发病1 wk内死亡的患者, 60%死于ALI/ARDS或伴有ALI/ARDS的多器官功能障碍综合征(multiple organ dysfunction syndrome, MODS)[4], 防治肺损伤成为降低SAP死亡率的重要措施. 促炎因子拮抗剂或抑炎因子协同剂的作用是近年来急性胰腺炎并发肺损伤的防治研究的热点. 最近研究表明, 高迁移率族蛋白-1(high mobility group box1, HMGB1)是相对于肿瘤坏死因子-α(TNF-α)、白细胞介素-1b(IL-1β)新的"晚期"炎症因子, 能给脓毒症带来更广的"治疗窗"[5-6]. 丙酮酸乙酯(ethyl pyruvate, EP)是一种稳定的亲脂性丙酮酸衍生物, 许多研究表明他具有抗炎和免疫调节作用[7-8]. 我们通过50 g/L牛磺胆酸钠逆行胰胆管内注射诱发大鼠急性坏死性胰腺炎(acute necrotizing pancreatitis, ANP)合并肺损伤模型, 观察丙酮酸乙酯对相关炎症因子的调节, 探讨丙酮酸乙酯对急性坏死性胰腺炎肺损伤的保护作用及其机制, 为临床治疗提供理论依据.
丙酮酸乙酯和牛磺胆酸钠, Sigma公司; TNF-α, IL-1β的ELISA试剂盒, 深圳晶美公司; 总RNA提取及逆转录试剂盒, Promega公司; PCR仪及电泳仪; 凝胶扫描分析系统, 美国Kodak. ♂清洁级健康Wistar大鼠72只, 体质量250-300 g(由中国医科大学实验动物中心提供). 随机分为3组, 每组24只, 分别为假手术组、ANP组及EP治疗组.
1.2.1 动物模型制备: 大鼠于术前12 h禁食, 自由饮水, 100 g/L水合氯醛(4 mL/kg体质量)腹腔内注射麻醉, 固定. 参照Aho et al介绍的方法逆行胰胆管内注射牛磺胆酸钠制作急性坏死性胰腺炎模型. 行上腹部正中切口, 除假手术组仅开腹暴露胰腺并轻轻翻动外, 余2组动物在胆管近肝门端用小动脉夹暂时阻断胆汁流, 4.5号头皮针经十二指肠壁插入胰管开口处, 逆行加压注入50 g/L牛磺胆酸钠(1 mL/kg体质量, 推注速度为0.2 mL/s), 拔出穿刺针后动脉夹夹闭穿刺点约5 min, 见到部分胰腺明显水肿、出血后, 去除小动脉夹, 将各器官纳入正常解剖位置. 分层关闭腹腔. 各组按模型制备后6, 12, 24 h分成3个亚组, 每组8只. 在上述时点分别采集动静脉血及切取双侧肺叶.
1.2.2 给药剂量及方式: EP治疗组分别于造模成功后2, 8, 14和20 h经阴茎静脉或股静脉注射EP溶液(EP溶于乳酸钠林格液中, 28 mmol/L), 6 h组给药1次, 12 h组给药2次, 24 h组给药4次; 每次剂量为EP 40 mg/kg. ANP组同时点注射等量的乳酸钠林格液.
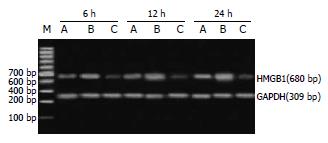
1.2.3 主要检测指标及方法: (1)动脉血气分析: 于上述时点取抗凝股动脉血0.5 mL作血气分析, 采用全自动进口血气分析机测定动脉血氧分压; (2)血清TNF-α, IL-1β的检测: 于上述时点大鼠100 g/L水合氯醛(4 mL/kg体质量)腹腔内注射麻醉开腹, 下腔静脉采血, 室温下静置2 h, 待析出血清后, 将血清分装冻存于-80 ℃待检测. 采用ELISA法检测各时点血清TNF-α和IL-1β水平, 具体操作步骤按说明书进行; (3)肺组织HMGB1 mRNA表达水平: 采用逆转录聚合酶链反应技术(RT-PCR)检测肺组织中HMGB1 mRNA的表达, 以三磷酸甘油醛脱氢酶(GAPDH)作为内对照. 大鼠HMGB1序列(扩增片段为680 bp): 5'-ATGGGCAAAGGAGATCCTA-3'(上游); 5'-AATTCATCATCATCATCTTCT-3'(下游); GAPDH序列(扩增片段为309 bp): 5'-TCCCTCAAGATTGTCAGCAA-3'(上游); 5'-AGATCCACAACGGATACATT-3'(下游). PCR反应条件如下: 94 ℃预变性3 min, 94 ℃ 45 s, 61 ℃ 40 s, 72 ℃ 1 min, 扩增35个循环, 72 ℃延伸10 min, 4 ℃终止反应. 取10 μL扩增产物经15 g/L琼脂糖凝胶电泳(120 V, 45 min), 溴化乙锭染色后, 于凝胶成像分析系统(GDS-8000)中进行条带密度扫描分析, 以HMGB1与GAPDH条带密度比值表示HMGB1 mRNA的表达水平.
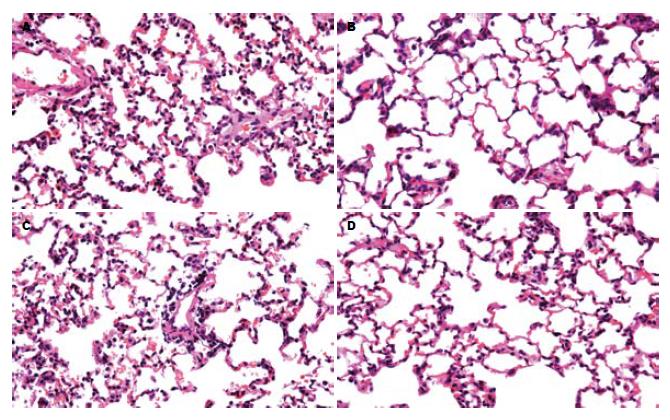
1.2.4 肺组织病理形态观察: 肺组织40 g/L中性福尔马林固定, 石蜡包埋, 切片, HE染色. 光镜下专人阅片行组织学观察. 肺脏组织病理学评分参照雷文章[9]和Osman et al[10]标准.
统计学处理 数据均以mean±SD表示. 统计采用SPSS10.0软件, 单因素方差分析(One-way ANOVA). P<0.05认为差异有显著性.
ANP组和EP治疗组的血氧分压随制模后时间的延长而降低. 同一时间点的血氧分压水平, ANP组和EP治疗组均明显低于假手术组; 而EP治疗组均较ANP组为高, 且在12及24 h组有显著性差异(表1).
ANP组大鼠肺组织HMGB1 mRNA水平在ANP后12 h明显升高, 至24 h仍维持在高水平. 经丙酮酸乙酯延迟治疗, 治疗组肺组织HMGB1 mRNA水平均明显低于ANP组(P<0.05)(表1, 图1).
ANP组大鼠血清TNF-α和IL-1β水平在ANP后迅速升高, 约在6 h达高峰, 之后迅速下降, 在ANP后12 h即大幅下降, 然后一直维持至24 h. 经丙酮酸乙酯延迟治疗, 血清TNF-α和IL-1β水平均低于ANP组, 且在6, 12 h差异显著(P>0.05, 表2).
假手术组各时点肺脏光镜下未见明显的形态学改变. ANP组及EP治疗组病理改变均随制模后时间延长而加重. ANP组肺脏光镜下见早期肺泡大小不一, 肺泡壁局灶性增厚, 肺静脉及毛细血管充血, 间质充血水肿及炎性细胞浸润, 肺泡内可见炎性细胞及红细胞. 24 h可见肺组织结构紊乱, 局部可见肺泡壁完全性破坏, 断裂、融合成肺大泡或形成局灶性肺不张, 部分标本有间质及肺泡腔出血; EP治疗组与APN组相比, 肺组织损伤明显减轻(图2).
有研究结果显示, ANP早期大量炎症因子过度释放导致严重的全身炎症反应综合征(SIRS), 是造成全身毛细血管渗漏综合征、多器官功能不全综合征(MODS)、多器官功能衰竭(MOF)的重要原因. 这些炎症因子包括TNF-α, IL-1β和HMGB1等. 急性肺损伤是ANP最常见的胰外器官早期并发症. 过度增加的促炎细胞因子对肺脏等器官是有害的, 因此, 在ANP时探索合理的免疫调节治疗策略是必要的.
丙酮酸乙酯是一种稳定的亲脂性的丙酮酸衍生物, 是有效的氧自由基清除剂[11-12], 在医学领域的应用很广泛. 近期研究提出, 丙酮酸乙酯是一种有效的抗炎药物[13-14], 对脓毒症引起的SIRS组织损伤有显著保护作用. 他可抑制内毒素诱导的巨噬细胞内TNF-α和HMGB1的表达, 并且可能与调节NF-kB和p38-MAPK细胞信号通路有关[15]. 既往普遍认为, TNF-α和IL-1β等在全身性炎症反应的早期迅速合成释放, 是引起机体失控性炎症反应和组织损害的关键介质, 故称早期细胞因子[16-17]. 本实验结果显示, ANP组大鼠血清TNF-α和IL-1β水平在ANP后迅速升高, 约在6 h达高峰, 之后迅速下降, 在ANP后12 h即大幅下降, 然后一直维持至24 h. 经丙酮酸乙酯延迟治疗, 血清TNF-α和IL-1β水平均低于ANP组, 且在6, 12 h差异显著. 既往研究表明外源TNF-α和IL-1β可引起明显的组织损伤, 早期应用相应抗体可减轻肺损伤的程度[18-19], 但临床治疗难以做到早期或预防性干预, 治疗的时机很难把握.
高迁移率族蛋白(HMG)是一大类高度保守的蛋白质, 分子量较低(30 kDa), 带电荷氨基酸含量丰富, 因其在聚丙烯酰胺凝胶电泳中迁移迅速而得名[20]. HMGB1是HMG家族成员之一, 既往的研究表明, HMGB1是细胞核内含量最丰富的非组蛋白染色质蛋白, 他可参与DNA复制、细胞分化及基因表达等多种细胞生命活动[21]. 最近有研究发现, 细胞经内毒素刺激后, 可将HMGB1分泌到细胞外, 介导了内毒素的致死效应. 由于内毒素攻击后HMGB1的产生明显晚于其他介质并持续时间较长, 故被称为脓毒症的"晚期"介质[22]. HMGB1通过两种不同的途径释放至细胞外: 活化的单核/巨噬细胞的主动分泌和坏死细胞的被动释放[5,23], 同时存在内源危险信号的两种不同释放机制说明了HMGB1作为内源性炎症介质的重要性. 与TNF-α和IL-1β等早期细胞因子相比, HMGB1出现较晚且持续时间更长, 因而可能成为脓毒症防治切实可行的潜在干预目标[24-25], 能给脓毒症和其他SIRS提供更广的治疗窗. 本实验结果显示, ANP组大鼠肺组织HMGB1 mRNA水平在ANP后12 h明显升高, 至24 h仍维持在高水平, 这可能与炎症时中性粒细胞在肺组织募集有关[26]. 经丙酮酸乙酯延迟治疗, 治疗组肺组织HMGB1水平12和24 h明显低于ANP组(P<0.05). 同时同一时间点的血氧分压水平, EP治疗组均较ANP组为高, 且在12及24 h组有显著性差异, 且EP治疗组与ANP组相比, 肺组织损伤明显减轻.
本研究表明, 丙酮酸乙酯能明显抑制ANP肺损伤早期和晚期炎症细胞因子的释放或表达, 减轻肺组织病理损伤程度, 与专门调控单一细胞因子的重组蛋白相比, 丙酮酸乙酯在ANP肺损伤发病后有较长的"治疗窗", 无潜在的免疫反应, 无细胞毒性, 这些优点充分说明, 丙酮酸乙酯对ANP肺损伤的防治具有良好应用前景. 鉴于目前所有的治疗经验都来自动物实验, 所以对丙酮酸乙酯的抗炎作用机制和安全性仍需作进一步研究.
急性肺损伤是急性坏死性胰腺炎最常见的胰外器官早期并发症. 目前对其早期防治尚缺乏有效的手段, 本研究通过建立大鼠急性坏死性胰腺炎合并肺损伤模型, 观察丙酮酸乙酯延迟应用对急性坏死性胰腺炎肺损伤的作用并探讨其可能机制.
促炎因子拮抗剂或抑炎因子协同剂的作用是近年来急性胰腺炎并发肺损伤防治研究的热点, 但这些药物多针对单一靶点, 其应用可致炎性因子失衡进一步加重; 且这些药物的药代动力学和毒性效应也限制了其应用. 近来在脓毒症中研究颇多且疗效明显的丙酮酸乙酯在急性胰腺炎及其器官功能损伤中的作用尚鲜有报道.
本文的创新之处在于较系统地探讨了丙酮酸乙酯对急性坏死性胰腺炎大鼠肺损伤的保护作用及其可能的作用机制, 并表明丙酮酸乙酯在急性坏死性胰腺炎肺损伤发病后有较长的"治疗窗".
本研究结果表明丙酮酸乙酯延迟应用能够减轻急性坏死性胰腺炎大鼠肺损伤, 为其在急性坏死性胰腺炎肺损伤治疗中的应用提供理论基础和实验依据, 也为急性坏死性胰腺炎肺损伤的防治提供了新的思路.
HMGB1: 是细胞核内含量丰富的非组蛋白染色质蛋白, 近来研究表明HMGB1是脓毒症发病时重要的迟发炎症介质, 他 可能成为脓毒症防治切实可行的干预目标, 能给脓毒症和其他SIRS提供更广的治疗窗.
本文研究了丙酮酸乙酯对急性坏死性胰腺炎大鼠肺损伤的保护作用, 论文书写规范, 逻辑清楚, 参考文献引用也比较新颖, 有着实际的指导意义.
电编: 张敏 编辑:王晓瑜
| 1. | Pitchumoni CS, Patel NM, Shah P. Factors influencing mortality in acute pancreatitis: can we alter them? J Clin Gastroenterol. 2005;39:798-814. [PubMed] [DOI] |
| 2. | Bank S, Singh P, Pooran N, Stark B. Evaluation of factors that have reduced mortality from acute pancreatitis over the past 20 years. J Clin Gastroenterol. 2002;35:50-60. [PubMed] [DOI] |
| 3. | Mayerle J, Hlouschek V, Lerch MM. Current management of acute pancreatitis. Nat Clin Pract Gastroenterol Hepatol. 2005;2:473-483. [PubMed] [DOI] |
| 4. | Steer ML. Relationship between pancreatitis and lung diseases. Respir Physiol. 2001;128:13-16. [PubMed] [DOI] |
| 5. | Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248-251. [PubMed] [DOI] |
| 6. | Yang H, Wang H, Tracey KJ. HMG-1 rediscovered as a cytokine. Shock. 2001;15:247-253. [PubMed] [DOI] |
| 7. | Yang R, Han X, Delude RL, Fink MP. Ethyl pyruvate ameliorates acute alcohol-induced liver injury and inflammation in mice. J Lab Clin Med. 2003;142:322-331. [PubMed] [DOI] |
| 8. | Miyaji T, Hu X, Yuen PS, Muramatsu Y, Iyer S, Hewitt SM, Star RA. Ethyl pyruvate decreases sepsis-induced acute renal failure and multiple organ damage in aged mice. Kidney Int. 2003;64:1620-1631. [PubMed] [DOI] |
| 10. | Osman MO, Kristensen JU, Jacobsen NO, Lausten SB, Deleuran B, Deleuran M, Gesser B, Matsushima K, Larsen CG, Jensen SL. A monoclonal anti-interleukin 8 antibody (WS-4) inhibits cytokine response and acute lung injury in experimental severe acute necrotising pancreatitis in rabbits. Gut. 1998;43:232-239. [PubMed] [DOI] |
| 11. | Dobsak P, Courderot-Masuyer C, Zeller M, Vergely C, Laubriet A, Assem M, Eicher JC, Teyssier JR, Wolf JE, Rochette L. Antioxidative properties of pyruvate and protection of the ischemic rat heart during cardioplegia. J Cardiovasc Pharmacol. 1999;34:651-659. [PubMed] [DOI] |
| 12. | Song M, Kellum JA, Kaldas H, Fink MP. Evidence that glutathione depletion is a mechanism responsible for the anti-inflammatory effects of ethyl pyruvate in cultured lipopolysaccharide-stimulated RAW 264.7 cells. J Pharmacol Exp Ther. 2004;308:307-316. [PubMed] [DOI] |
| 13. | Fink MP. Ethyl pyruvate: a novel anti-inflammatory agent. Crit Care Med. 2003;31:S51-56. [PubMed] [DOI] |
| 14. | Venkataraman R, Kellum JA, Song M, Fink MP. Resuscitation with Ringer's ethyl pyruvate solution prolongs survival and modulates plasma cytokine and nitrite/nitrate concentrations in a rat model of lipopolysaccharide-induced shock. Shock. 2002;18:507-512. [PubMed] [DOI] |
| 15. | Ulloa L, Ochani M, Yang H, Tanovic M, Halperin D, Yang R, Czura CJ, Fink MP, Tracey KJ. Ethyl pyruvate prevents lethality in mice with established lethal sepsis and systemic inflammation. Proc Natl Acad Sci USA. 2002;99:12351-12356. [PubMed] [DOI] |
| 16. | Kingsnorth A. Role of cytokines and their inhibitors in acute pancreatitis. Gut. 1997;40:1-4. [PubMed] [DOI] |
| 17. | Mayer J, Rau B, Gansauge F, Beger HG. Inflamma-tory mediators in human acute pancreatitis: clinical and pathophysiological implications. Gut. 2000;47:546-552. [PubMed] [DOI] |
| 18. | Koh Y, Hybertson BM, Jepson EK, Repine JE. Tumor necrosis factor induced acute lung leak in rats: less than with interleukin-1. Inflammation. 1996;20:461-469. [PubMed] [DOI] |
| 19. | Yoshinari D, Takeyoshi I, Koibuchi Y, Matsumoto K, Kawashima Y, Koyama T, Ohwada S, Morishita Y. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2001;29:628-634. [PubMed] [DOI] |
| 20. | Ulloa L, Messmer D. High-mobility group box 1 (HMGB1) protein: friend and foe. Cytokine Growth Factor Rev. 2006;17:189-201. [PubMed] [DOI] |
| 21. | Bustin M. Revised nomenclature for high mobility group (HMG) chromosomal proteins. Trends Biochem Sci. 2001;26:152-153. [PubMed] [DOI] |
| 22. | Sunden-Cullberg J, Norrby-Teglund A, Treutiger CJ. The role of high mobility group box-1 protein in severe sepsis. Curr Opin Infect Dis. 2006;19:231-236. [PubMed] [DOI] |
| 23. | Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191-195. [PubMed] [DOI] |
| 24. | Czura CJ, Tracey KJ. Targeting high mobility group box 1 as a late-acting mediator of inflammation. Crit Care Med. 2003;31:S46-50. [PubMed] [DOI] |
| 25. | Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol. 2000;165:2950-2954. [PubMed] [DOI] |
| 26. | Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005;288:L958-965. [PubMed] [DOI] |