修回日期: 2007-01-05
接受日期: 2007-01-20
在线出版日期: 2007-04-18
目的: 快速建立一种非酒精性脂肪肝大鼠模型.
方法: 24只♂SD大鼠随机平均分为正常组、常规组和改良组, 分别喂普通基础饲料、常规用高脂饲料及常规高脂饲料+蔗糖+丙基硫氧嘧啶+胆酸钠. 5 wk内动态观察三组大鼠的一般情况、体质量变化, 均5 wk处死, 观察大鼠肝脏病理形态变化, 苏丹Ⅳ染色和电镜检测了解大鼠肝细胞胞质内脂滴存在情况, 并比较三组大鼠血清甘油三酯(TG)、胆固醇(TC)、谷丙转氨酶(ALT)、谷草转氨酶(AST)水平、丙二醛(MDA)含量和超氧化物歧化酶(SOD)活力及肝组织甘油三酯(TG)、胆固醇(TC)含量的变化.
结果: 第4周开始正常组和改良组两组大鼠与常规组体质量之间有显著性差异(249.63±34.25, 241.88±20.75 vs 275.38±6.59, P<0.05), 改良组与正常组之间体质量无显著性差异(P>0.05). 第5周在光镜下见改良组大鼠肝细胞内弥漫大量的脂肪空泡, 电镜和苏丹Ⅳ染色证实肝细胞内有大量脂滴. 肝脂肪变性程度为+~+++ (H = 13.36, P = 0.0003), 正常组和常规组大鼠肝细胞内未见明显脂肪变性. 改良组大鼠血清TG, TC, ALT水平和MDA含量均高于正常组和常规组(TG: 1.28±0.61 mmol/L vs 0.72±0.12, 0.76±0.04 mmol/L; TC: 12.78±1.47 mmol/L vs 1.71±0.03, 2.31±0.49 mmol/L; ALT: 1518.64±186.04 nkat/L vs 1181.57±37.84, 1262.92±159.20 nkat/L; MDA: 13.40±4.24 μmol/L vs 5.89±1.05, 7.23±1.15 μmol/L; 均P<0.05), 常规组与正常组比无显著差异(P>0.05); 而改良组血清SOD活力显著低于正常组和常规组(5.21±0.81 nkat/mL vs 11.91±2.69, 11.19±0.78 nkat/mL, P<0.05). 肝组织TG, TC含量常规组和模型组均比正常组有增高(TG: 2.14±0.26, 5.83±1.42 mmol/L vs 1.20±0.16 mmol/L, P<0.05; TC: 3.19±0.23, 9.63±1.12 mmol/L vs 2.13±0.16 mmol/L, P<0.05), 常规组与改良组也有显著差异(P<0.05).
结论: 通过与利用常规高脂饲料建模的方法比较, 该改良方法5 wk成功地构建了非酒精性脂肪肝大鼠模型, 缩短了建模时间, 降低了实验成本且模型基本符合人类发病的自然状态, 是一种理想的建模方式.
引文著录: 王倩, 管小琴. 大鼠非酒精性脂肪肝造模方法的改进. 世界华人消化杂志 2007; 15(11): 1219-1224
Revised: January 5, 2007
Accepted: January 20, 2007
Published online: April 18, 2007
AIM: To establish a rapid rat model of non-alcoholic fatty liver.
METHODS: Twenty-four male Sprague Dawley rats were averagely and randomly divide into group A, B and C, fed with normal diet, routine high-fat diet and routine high-fat diet plus sucrose, propylthiouracil, and sodium cholate, respectively. The general conditions and weight changes were dynamically observed for 5 wk, and then all the rats were killed. The pathological changes of liver tissues were observed by HE staining, and Sudan IV staining and electron microscopy were used to investigate the presence status of cytoplasmic lipid droplets in liver cells. The following indexes were compared between the three groups, including serum levels of triglyceride (TG), total cholesterol (TC), alanine aminotransferase (ALT), aspartate aminotransferase (AST), malondialdehyde (MDA), and superoxide dismutase (SOD), and tissue contents of TG and TC.
RESULTS: Starting from the fourth week, the weights of rats were significantly decreased in group A and C as compared with those in group B (249.63 ± 34.25, 241.88 ± 20.75 vs 275.38 ± 6.59, P < 0.05), but there was no marked difference between group A and C (P > 0.05). In the 5th week, light microscopy showed a great number of fatty vacuoles in liver cells, and electron microscopy confirmed the presence of abundant lipid droplets. Different degrees of hepatic fatty degeneration (+~+++) was observed in group C (H = 13.36, P = 0.0003), but not in group A and B. The serum levels of TG, TC, ALT and MDA were markedly higher in group C than those in group A and B (TG: 1.28 ± 0.61 mmol/L vs 0.72 ± 0.12, 0.76 ± 0.04 mmol/L; TC: 12.78 ± 1.47 mmol/L vs 1.71 ± 0.03, 2.31 ± 0.49 mmol/L; ALT: 1518.64 ± 186.04 nkat/L vs 1181.57 ± 37.84, 1262.92 ± 159.20 nkat/L; MDA: 13.40 ± 4.24 μmol/L vs 5.89 ± 1.05, 7.23 ± 1.15 μmol/L; all P < 0.05), but the activity of SOD was lower in group C (5.21 ± 0.81 nkat/mL vs 11.91 ± 2.69, 11.19 ± 0.78 nkat/mL, P < 0.05). There were no notable differences between group A and B (P > 0.05). The tissue contents of TG and TC were dramatically higher both in group B and C than those in group A (TG: 2.14 ± 0.26, 5.83 ± 1.42 mmol/L vs 1.20 ± 0.16 mmol/L, P < 0.05; TC: 3.19 ± 0.23, 9.63 ± 1.12 mmol/L vs 2.13 ± 0.16 mmol/L, P < 0.05), and there was also statistical difference between group B and C (P < 0.05).
CONCLUSION: The rat model of non-alcoholic fatty liver can be successfully established within 5 wk by the improved method, which needs less time and cost during the construction, and basically simulating the occurrence and progression of non-alcoholic fatty liver in human beings.
- Citation: Wang Q, Guan XQ. Improvement of induction method of non-alcoholic fatty liver model in rats. Shijie Huaren Xiaohua Zazhi 2007; 15(11): 1219-1224
- URL: https://www.wjgnet.com/1009-3079/full/v15/i11/1219.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i11.1219
非酒精性脂肪肝大鼠模型现有的建模方法可分为两类, 一类是利用转基因技术建模, 另一类是利用药物和饮食诱导建模[1]. 目前, 常用的建模方法还是选用超营养饮食方法, 这类方法重复性强, 较稳定且简单易行, 但建模耗时较长, 成本高. 我们参考国内外建造动物脂肪肝模型的文献[2]及预实验的结果, 对大鼠饲料配方进行了改进, 采用在常规高脂饲料中加入蔗糖[3-4]、丙基硫氧嘧啶[5]和胆酸钠[6]的方法建立大鼠非酒精性脂肪肝模型, 这种方法能缩短建模时间, 降低实验成本, 基本符合自然疾病发病规律, 有利于进行科学实验研究.
1.1.1 实验动物: ♂7周龄SD大鼠共24只, 体质量200-280 g, 由重庆医科大学实验动物中心提供.
1.1.2 主要药物与试剂: 胆固醇, 广州天马精细化工厂生产(批号: 050531), 猪胆酸钠, 北京奥博星生物技术有限责任公司(批号: 20050428), 丙硫氧嘧啶片, 上海复星朝晖药业有限公司(生产批号: 050606), 猪油、蔗糖购自重客隆超市. 丙二醛(MDA)含量和超氧化物歧化酶(SOD)活力测定试剂盒, 南京建成生物工程研究所(批号分别为20051123和20051212), 苏丹Ⅳ染色试剂盒, 福州迈新生物技术有限公司(批号: 611088023).
1.1.3 主要仪器: 小磅秤, 四川大竹衡器厂; RM 2135切片机, 德国莱卡公司; 752紫外分光光度计, 上海棱光技术有限公司; 康宁560全自动生化分析仪, 美国康宁公司; Hitachi-7500透视电镜, 日本日立公司.
1.2.1 饲料的制备: (1)常规高脂饲料的制备: 胆固醇2%, 猪油10%, 基础饲料88%; (2)改良高脂饲料的制备: 胆固醇2%, 胆酸钠0.5%, 丙硫氧嘧啶0.2%, 蔗糖5%, 猪油10%, 基础饲料82.3%.
1.2.2 动物模型的制备: SD大鼠按完全随机分组原则分为3组(正常组、常规组和改良组), 每组各8只. 正常组用基础饲料喂养, 常规组给予上述常规高脂饲料喂养, 改良组给予改良高脂饲料喂养, 自由取食, 实验5 wk, 处死3组全部大鼠, 摘取眼球取血, 并迅速取出肝脏, 每份大鼠肝脏一部分放置-70 ℃低温冰箱保存备用, 一部分用40 g/L甲醛固定以作病理组织检查用, 一部分加生理盐水制成100 g/L的肝组织匀浆以作检测肝组织TG, TC含量用.
1.2.3 观察指标和测定方法: (1)一般情况. 实验期间每周称大鼠体质量1次, 并观察其毛色、食欲行动、粪便、对外界刺激的反应情况; (2)病理组织学检查. 肉眼观察大鼠肝脏大体变化, 并作病理切片HE染色, 光镜下观察大鼠肝脏脂肪变性情况, 其判断标准详见《非酒精性脂肪肝诊断标准》(2002-10中华肝病学会脂肪肝和酒精性肝病学组制定); 并做苏丹Ⅳ染色和电镜检查了解肝细胞内脂滴的存在情况; (3)血清生化指标. 测定各组大鼠血清甘油三酯(TG)、胆固醇(TC)、谷草转氨酶(AST)、谷丙转氨酶(ALT)、丙二醛(MDA)含量和超氧化物化酶(SOD)活力. (4)肝组织甘油三酯(TG)、胆固醇(TC)含量的测定.
统计学处理 用SPSS11.5统计学软件进行数据分析. 体质量的变化分析采用重复测定资料方差分析. 其他指标属多样本均数比较采用单因素方差分析(正态分布)和Kruskal-wallis秩和检验(非正态分布), 等级资料采用Kruskal-wallis秩和检验.
实验过程中, 各组大鼠均无死亡, 皮毛光泽度好, 进食饮水尚可, 活动自如, 对外界刺激反应迅速.
每周称3组大鼠体质量, 动态观察其体质量的改变情况(表1), 不同时间点的体质量间的差别具有统计学意义(F = 62.84, P<0.0001); 不同饲料喂养与时间之间有明显的交互作用(F = 13.68, P<0.0001), 说明3组大鼠体质量的变化趋势不同. 采用LST法进行多样本均数的两两比较, 结果表示前2 wk改良组、常规组和正常组大鼠体质量之间均无显著性差异(P>0.05); 第4周开始常规组与正常组和改良组两组大鼠体质量之间均有显著性差异(P<0.05), 改良组与正常组之间体质量无显著性差异(P>0.05). 提示改良高脂饲料喂养大鼠在实验过程中体质量无明显的变化, 常规高脂饲料喂养大鼠从第4周开始体质量就有增加.
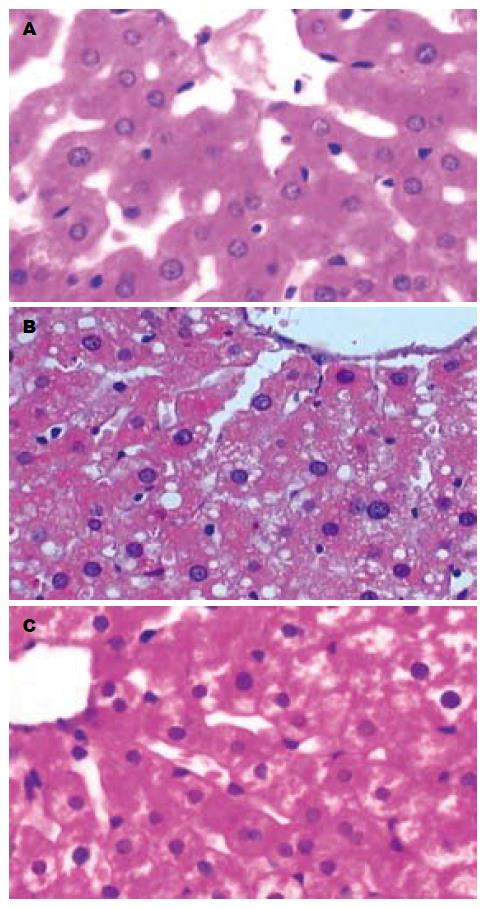
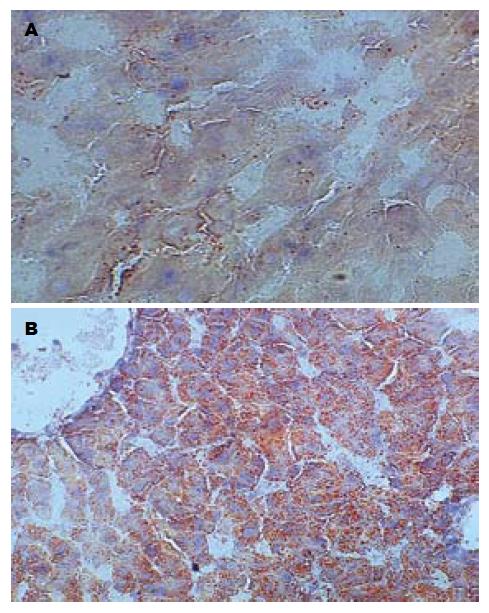
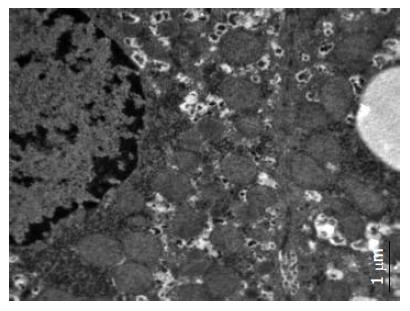
肉眼观察, 5 wk处死的正常组大鼠肝脏颜色呈暗红; 常规组大鼠肝脏颜色仍呈暗红. 体积稍增大; 改良组大鼠肝脏体积增大, 包膜稍紧张, 边缘圆钝, 呈奶黄色, 有油腻感; 光镜下, 正常组大鼠肝脏基本无异常情况, 常规组大鼠肝细胞以浊肿为主, 少量肝细胞可见空泡变性, 改良组均可见以肝腺泡Ⅲ区为主的弥漫性肝细胞空泡变性, 未见炎性变、坏死灶及肝纤维化, 变性肝细胞体积较正常明显增加, 胞质内充满大量脂肪空泡(图1). 做苏丹Ⅳ染色见肝细胞内的脂滴染成桔红色, 电镜下发现肝细胞胞质内存在大小不等、电子密度中等的均质性无界膜的脂滴(图2-3). 根据《非酒精性脂肪肝诊断标准》对脂肪变性进行分级评价(表2), 可知改良高脂饲料喂养建模的方法比常规高脂饲料喂养建模的方法更快速有效(H = 13.36, P = 0.0003).
常规组与正常组间血清生化指标无显著差异(P>0.05); 与正常组和常规组比较, 改良组大鼠血清TG, TC和ALT水平均有明显升高(P<0.05, 表3).
常规组与正常组比较血清SOD活力和MDA含量无显著性差异(P>0.05); 改良组与正常组和常规组比, 血清SOD活力有明显降低, MDA含量有明显升高(P<0.05, 表4).
近年来, 我国由高脂血症[7]、高血压[8]、肥胖[9-10]、糖尿病[11]、药物损害[12]等引起的非酒精性脂肪肝的发病率呈上升趋势, 为深入研究脂肪肝的发病机制, 筛选预防和治疗脂肪肝的有效药物, 探讨药物的作用机制, 选择一个符合人类自然发病规律的动物模型极为重要. 目前, 常规的用高脂饲料建立大鼠非酒精性脂肪肝模型的方法[13]虽然有简便, 重复性好的优点, 但也存在造模时间长, 病变轻, 实验成本较高的缺点. 现已有了一些改良方法, 如Zou et al[14]用高脂乳剂给♂SD大鼠灌胃, 6 wk后建模成功, 这种方法建模时间较短, 但每天灌胃较为繁琐且操作不当可引起动物窒息死亡. Leclercq et al[15]给♂Wistar大鼠高糖饲料2 d后肝细胞脂肪有变性, 但需先禁食, 不符合人类自然发病情况. Dela et al[16] Ota et al[17], Uto et al[18]给予小鼠缺乏胆氨酸-胆碱的饲料喂养4 wk后出现脂肪变性, 8 wk肝组织内出现炎症改变, 12 wk后可出现肝纤维化, 这方法虽耗时不多, 但其不符合人类膳食情况, 且动物对胆碱缺乏的敏感性取决于胆碱氧化酶的含量, 但人类肝脏不含胆碱氧化酶, 故此类模型与人的自然发病情况大相径庭, 不宜用于发病机制的研究, 而仅能用于药物筛选[18]. Cheung et al[19]给予Wistar♂大鼠皮下注射CCL4来建模, Natarajan et al[20]连续5 d给予大鼠腹腔内注射丙戊酸钠可出现肝细胞脂肪变性, 这两种方法虽具有简便、快速的优点, 但从发病机制, 病程演变过程, 及最终组织学形态改变来看都与人类脂肪肝有较大差异, 且动物易中毒死亡. Ikejima et al[21], Fulop et al[22], Rhee et al[23]采用fa/fa大鼠, ob/ob小鼠, 及BHE大鼠等特殊种系均可形成糖、脂肪代谢相关性的脂肪肝. 但这些特殊种系对品系有一定的要求, 且造模动物死亡较多, 代价较昂贵, 实用性有限.
本建模方法以HE染色、电镜、苏丹Ⅳ染色、血清生化指标、血清SOD和MDA、肝组织TG和TC含量检测作为大鼠建模成功的观测指标; 与常规建模方法相比, 该法成功建立大鼠非酒精性脂肪肝模型仅需5 wk. 建模期间大鼠体质量无明显变化; 改良组肝组织和血清生化指标中TC, TG含量高于正常组和常规组; 改良组血清SOD活力比正常组和常规组有明显降低、MDA含量比正常组和常规组有明显增高. 本建模方法遵循了建立一个理想的动物模型的4条原则: (1)与人类疾病特征相似; (2)病变有一定发展过程; (3)形成率高, 死亡率低, 重复性好; (4)简便易行. 本法建模的机制是: 饲料中加入蔗糖, 可增加长链脂肪酸的合成而使TG的合成增加, 并且当血糖升高时, NADPH供应充分, 使乙酰辅酶A转变成的胆固醇也增加[24-25]; 饲料中加入少量胆酸盐后, 可增加外源性胆固醇的吸收[26-27]; 加入甲状腺抑制药丙基硫氧嘧啶(该药物在安全剂量中使用, 不导致肝毒性)[28], 可诱发高脂血症[29-31], 其具体作用机制为: (1)抑制过氧化物酶, 使摄入甲状腺内的碘不能变成活性碘; (2)抑制MIT和DIT缩合成T3, T4和抑制T4转变为T3. 而甲状腺素可促进胆固醇在肝转变为胆汁酸, 因此在加入丙基硫氧嘧啶后, 甲状腺素这一作用减弱, 从而使肝内胆固醇增加. 因此, 与常规高脂饲料比, 加入蔗糖、丙基硫氧嘧啶和胆酸盐后可加快建模进程, 使肝内胆固醇堆积形成脂肪肝. 本实验中, 改良组大鼠肝组织和血清生化指标中TC, TG含量均显著高于正常组, 并发现TC含量升高极为显著, 这一现象也许与改良饲料中加入丙基硫氧嘧啶、胆酸钠促进胆固醇的吸收有关.
用此种方法建立非酒精性单纯性脂肪肝大鼠模型, 用时短, 死亡率低, 成本低, 基本符合人类疾病自然发病情况, 避免了其他改良方法的不足, 值得推广.
近年来, 由于生活水平的提高, 饮食结构的变化, 我国由高脂血症、高血压、肥胖、糖尿病和药物损害等引起的非酒精性脂肪肝发病率呈上升趋势, 现已成为危害人类健康的三大肝病之一, 并与失代偿期肝硬化、肝功能衰竭、原发性肝癌的发生密切相关. 非酒精性脂肪肝病是遗传-环境-代谢应激相关性疾病, 与胰岛素抵抗及其相关的代谢综合征和遗传易感性有关.
许多学者对利用实验动物复制非酒精性脂肪肝的模型进行了大量的研究, 非酒精性脂肪肝大鼠模型现有的建模方法可分为两类, 一类是利用转基因技术建模, 另一类是利用药物和饮食诱导建模. 选择一种建模时间短、实验成本低、符合人类自然发病规律的模型, 能更有利于科学实验研究, 因此寻求建立这种模型的方法是现在亟待研究的问题.
在现有的利用药物和饮食诱导方式建立大鼠非酒精性脂肪肝模型的方法中, 利用常规的用高脂饲料建模的方法虽具简便, 重复性好的优点, 但却存在造模时间长, 病变轻, 成本较高的缺点. 对于已有的一些改良方法, 如用缺乏胆氨酸-胆碱的饲料喂养小鼠建模的方法、注射四氯化碳等药物诱导建模等, 虽然具有简便、快速的特点, 但从发病机制、病程演变过程、以及最终组织学形态改变来看都与人类脂肪肝的发生有较大差异. 本实验的建模方法用时短、死亡率低、成本率低, 建模过程基本符合人类自然发病情况, 较好的避免了其他改良方法的不足.
本文改进了非酒精性脂肪肝的建模方法, 其研究结果为我们深入研究脂肪肝的发病机制, 筛选预防和治疗脂肪肝的有效药物, 探讨药物的作用机制奠定了基础.
非酒精性脂肪肝病(non-alcoholicfatty liver disease): 是一种无过量饮酒史肝实质细胞脂肪变性和脂质贮积为特征的临床病理综合征. 疾病谱随病程的进展表现不一, 包括单纯性脂肪肝、脂肪性肝炎、脂肪性肝纤维化和肝硬化.
本文改进了大鼠非酒精性脂肪肝的造模方法, 具有一定的创新及科研应用价值.
编辑: 王晓瑜 电编:张敏
| 1. | Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1-16. [PubMed] [DOI] |
| 2. | Ai J, Wang N, Yang M, Du ZM, Zhang YC, Yang BF. Development of Wistar rat model of insulin resistance. World J Gastroenterol. 2005;11:3675-3679. [PubMed] [DOI] |
| 3. | Feldstein AE, Canbay A, Guicciardi ME, Higuchi H, Bronk SF, Gores GJ. Diet associated hepatic steatosis sensitizes to Fas mediated liver injury in mice. J Hepatol. 2003;39:978-983. [PubMed] [DOI] |
| 4. | Li Z, Oben JA, Yang S, Lin H, Stafford EA, Soloski MJ, Thomas SA, Diehl AM. Norepinephrine regulates hepatic innate immune system in leptin-deficient mice with nonalcoholic steatohepatitis. Hepatology. 2004;40:434-441. [PubMed] [DOI] |
| 5. | Lanni A, Moreno M, Lombardi A, de Lange P, Silvestri E, Ragni M, Farina P, Baccari GC, Fallahi P, Antonelli A. 3,5-diiodo-L-thyronine powerfully reduces adiposity in rats by increasing the burning of fats. FASEB J. 2005;19:1552-1554. [PubMed] [DOI] |
| 6. | Jeong WI, Jeong DH, Do SH, Kim YK, Park HY, Kwon OD, Kim TH, Jeong KS. Mild hepatic fibrosis in cholesterol and sodium cholate diet-fed rats. J Vet Med Sci. 2005;67:235-242. [PubMed] [DOI] |
| 7. | Ahmed MH, Saad RA, Osman MM. Ezetimibe: effective and safe treatment for dyslipidaemia associated with nonalcoholic fatty liver disease. Response to: Toth PP, Davidson MH: simvastatin and ezetimibe: combination therapy for the management of dyslipidaemia. Expert Opin. Pharmacother. (2005) 6(1): 131-139. Expert Opin Drug Saf. 2006;5:487-488. [PubMed] [DOI] |
| 8. | Brookes MJ, Cooper BT. Hypertension and fatty liver: guilty by association? J Hum Hypertens. 2007;21:264-270. [PubMed] [DOI] |
| 9. | Benjaminov O, Beglaibter N, Gindy L, Spivak H, Singer P, Wienberg M, Stark A, Rubin M. The effect of a low-carbohydrate diet on the nonalcoholic fatty liver in morbidly obese patients before bariatric surgery. Surg Endosc. 2007;21:1423-1427. [PubMed] [DOI] |
| 10. | Cavanilles Walker E, Solar Boga A, García Alonso L, Lorenzo Patiño MJ. [Effectiveness of weight loss in the treatment of non-alcoholic steatohepatitis in an obese adolescent]. An Pediatr (Barc). 2007;66:184-187. [PubMed] [DOI] |
| 11. | Yoneda M, Iwasaki T, Fujita K, Kirikoshi H, Inamori M, Nozaki Y, Maeyama S, Wada K, Saito S, Terauchi Y. Hypoadiponectinemia plays a crucial role in the development of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus independent of visceral adipose tissue. Alcohol Clin Exp Res. 2007;31:S15-S21. [PubMed] [DOI] |
| 12. | Grieco A, Forgione A, Miele L, Vero V, Greco AV, Gasbarrini A, Gasbarrini G. Fatty liver and drugs. Eur Rev Med Pharmacol Sci. 2005;9:261-263. [PubMed] |
| 13. | Fan JG, Xu ZJ, Wang GL. Effect of lactulose on establishment of a rat non-alcoholic steatohepatitis model. World J Gastroenterol. 2005;11:5053-5056. [PubMed] [DOI] |
| 14. | Zou Y, Li J, Lu C, Wang J, Ge J, Huang Y, Zhang L, Wang Y. High-fat emulsion-induced rat model of nonalcoholic steatohepatitis. Life Sci. 2006;79:1100-1107. [PubMed] [DOI] |
| 15. | Leclercq I, Horsmans Y, Desager JP, Delzenne N, Geubel AP. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. J Hepatol. 1998;28:410-416. [PubMed] [DOI] |
| 16. | Dela Peña A, Leclercq I, Field J, George J, Jones B, Farrell G. NF-kappaB activation, rather than TNF, mediates hepatic inflammation in a murine dietary model of steatohepatitis. Gastroenterology. 2005;129:1663-1674. [PubMed] [DOI] |
| 17. | Ota T, Takamura T, Kurita S, Matsuzawa N, Kita Y, Uno M, Akahori H, Misu H, Sakurai M, Zen Y. Insulin resistance accelerates a dietary rat model of nonalcoholic steatohepatitis. Gastroenterology. 2007;132:282-293. [PubMed] [DOI] |
| 18. | Uto H, Nakanishi C, Ido A, Hasuike S, Kusumoto K, Abe H, Numata M, Nagata K, Hayashi K, Tsubouchi H. The peroxisome proliferator-activated receptor-gamma agonist, pioglitazone, inhibits fat accumulation and fibrosis in the livers of rats fed a choline-deficient, l-amino acid-defined diet. Hepatol Res. 2005;32:235-242. [PubMed] |
| 19. | Cheung PY, Zhang Q, Zhang YO, Bai GR, Lin MC, Chan B, Fong CC, Shi L, Shi YF, Chun J. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol. 2006;12:1912-1917. [PubMed] [DOI] |
| 20. | Natarajan SK, Eapen CE, Pullimood AB, Balasubramanian KA. Oxidative stress in experimental liver microvesicular steatosis: role of mitochondria and peroxisomes. J Gastroenterol Hepatol. 2006;21:1240-1249. [PubMed] [DOI] |
| 21. | Ikejima K, Okumura K, Lang T, Honda H, Abe W, Yamashina S, Enomoto N, Takei Y, Sato N. The role of leptin in progression of non-alcoholic fatty liver disease. Hepatol Res. 2005;33:151-154. [PubMed] [DOI] |
| 22. | Fülöp P, Derdák Z, Sheets A, Sabo E, Berthiaume EP, Resnick MB, Wands JR, Paragh G, Baffy G. Lack of UCP2 reduces Fas-mediated liver injury in ob/ob mice and reveals importance of cell-specific UCP2 expression. Hepatology. 2006;44:592-601. [PubMed] [DOI] |
| 23. | Rhee YS, Burnham K, Stoecker BJ, Lucas E. Effects of chromium and copper depletion on lymphocyte reactivity to mitogens in diabetes-prone BHE/cdb rats. Nutrition. 2004;20:274-279. [PubMed] [DOI] |
| 24. | Li Z, Soloski MJ, Diehl AM. Dietary factors alter hepatic innate immune system in mice with nonalcoholic fatty liver disease. Hepatology. 2005;42:880-885. [PubMed] [DOI] |
| 25. | Dentin R, Girard J, Postic C. Carbohydrate responsive element binding protein (ChREBP) and sterol regulatory element binding protein-1c (SREBP-1c): two key regulators of glucose metabolism and lipid synthesis in liver. Biochimie. 2005;87:81-86. [PubMed] [DOI] |
| 26. | Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774-42784. [PubMed] [DOI] |
| 27. | Kokkona M, Kallinteri P, Fatouros D, Antimisiaris SG. Stability of SUV liposomes in the presence of cholate salts and pancreatic lipases: effect of lipid composition. Eur J Pharm Sci. 2000;9:245-252. [PubMed] [DOI] |
| 28. | Rambaldi A, Gluud C. Propylthiouracil for alcoholic liver disease. Cochrane Database Syst Rev. 2005;CD002800. [PubMed] [DOI] |
| 29. | Varghese S, Shameena B, Sudhakaran PR, Oommen OV. Short-term effects of thyroid hormones on lipogenic enzymes and 14C-acetate incorporation into various lipid classes: in vivo and in vitro studies. Indian J Exp Biol. 2001;39:431-435. [PubMed] |
| 30. | Hapon MB, Varas SM, Jahn GA, Giménez MS. Effects of hypothyroidism on mammary and liver lipid metabolism in virgin and late-pregnant rats. J Lipid Res. 2005;46:1320-1330. [PubMed] [DOI] |
| 31. | Berti JA, Amaral ME, Boschero AC, Nunes VS, Harada LM, Castilho LN, Oliveira HC. Thyroid hormone increases plasma cholesteryl ester transfer protein activity and plasma high-density lipoprotein removal rate in transgenic mice. Metabolism. 2001;50:530-536. [PubMed] [DOI] |