修回日期: 2006-10-25
接受日期: 2006-11-02
在线出版日期: 2007-01-08
目的: 探讨血红素氧合酶(heme oxygenase, HO)的干预对糖尿病(diabetes mellitus, DM)大鼠结肠动力障碍的影响.
方法: 链脲佐菌素ip建立DM模型, 饲养6 wk时以碳沫推进实验证实DM大鼠存在胃肠慢传输运动后, 所有大鼠分为正常对照组、DM未干预组、DM+Hemin组[予HO诱导剂正铁血红素(Hemin)]及DM+ZnPP组[予HO阻滞剂锌原卟啉(zinc protoporphyrin Ⅸ, ZnPP Ⅸ)]; 监测体质量、血糖. 饲养9 wk时再测胃肠推进率, 离体肌条实验记录结肠平滑肌条自发收缩反应及对Ach的反应性, Western blot及免疫组化检测近、远端结肠HO的表达.
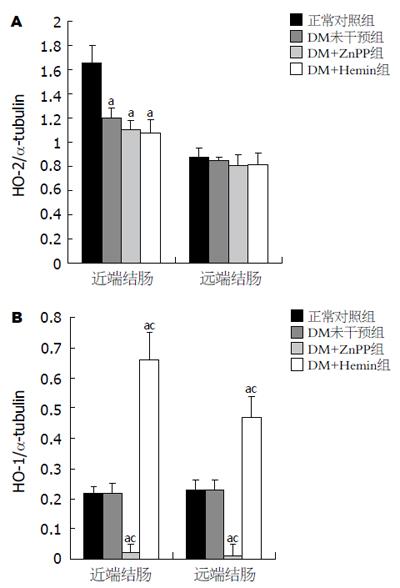
结果: 6 wk时DM大鼠胃肠慢传输运动模型建立. HO干预对DM大鼠体质量、血糖无影响(P>0.05). 9 wk时Western blot示DM未干预组(1.20±0.09)、DM+Hemin组(1.08±0.11)及DM+ZnPP组(1.10±0.08)近端结肠HO-2表达无显著差异(P>0.05), 但均较正常对照组(1.66±0.14)显著减少(P<0.05); 各实验组远端结肠HO-2表达无差异. 正常对照组与DM未干预组近、远端结肠HO-1的表达无差异(Western blot示HO-1/α-tubulin: 近端结肠0.22±0.02 vs 0.22±0.03; 远端结肠0.23±0.03 vs 0.23±0.03, P>0.05); DM+Hemin组结肠HO-1的表达(近端结肠0.66±0.09; 远端结肠0.47±0.07)较前两组显著增多(P<0.05); DM+ZnPP组结肠HO-1基本无表达. DM+Hemin组胃肠推进指数(54.4%±2.9% vs 63.0%±1.2%, P<0.05)、结肠平滑肌条自发收缩频率、波幅和对Ach的反应性较DM未干预组显著下降(P<0.05), 而DM+ZnPP组胃肠推进指数(72.5%±2.6% vs 63.0%±1.2%, P<0.05)、结肠平滑肌条自发收缩频率、波幅和对Ach的反应性较DM未干预组明显改善(P<0.05).
结论: HO干预(诱导或阻断), 对DM大鼠体质量、血糖无影响. 诱导HO-1使DM大鼠慢传输型结肠动力障碍加重, 而阻断HO-1可能改善DM大鼠慢传输型结肠动力障碍.
引文著录: 吴高珏, 林琳, 张红杰, 李学良, 罗云, 王美峰. 血红素氧合酶的干预对糖尿病大鼠结肠动力障碍的影响. 世界华人消化杂志 2007; 15(1): 14-21
Revised: October 25, 2006
Accepted: November 2, 2006
Published online: January 8, 2007
AIM: To investigate the effect of heme oxygenase (HO) interference on the colonic dysfunction in rats with diabetes mellitus (DM).
METHODS: DM model was established by intraperitoneal injection of streptozotocin (STZ) in Sprague and Dawley rats. Six weeks later, the diabetic rats were validated to be suffered with gastrointestinal dysfunction using charcoal (Indian ink) propulsion experiment. Then the rest rats were randomly divided into 4 groups, named group A (normal control), B (diabetic rats without interference), C (diabetic rats administrated with Hemin, the inducer of HO) and D [diabetic rats administrated with zinc protoporphyrin Ⅸ (ZnPP Ⅸ), the inhibitor of HO]. The weight and blood glucose of the rats were tested. Three more weeks later, the motilities of the strips isolated from the proximal and distal colon were recorded. The level of HO in the colon was also detected by immunohistochemistry and Western blot.
RESULTS: The model of diabetic rats suffered with gastrointestinal dysfunction was successfully duplicated. Administration of Hemin or ZnPP Ⅸ had no effect on the weight or blood glucose of diabetic rats (P > 0.05). There was no significant difference in HO-2 expression of the distal colon between the diabetic rats with or/and without interference (P > 0.05). But in comparison with the controls, HO-2 expression of the proximal colon in group B, C or D was significantly declined (Western blot: 1.20 ± 0.09, 1.08 ± 0.11, 1.10 ± 0.08 vs 1.66 ± 0.14, P < 0.05). The colonic expression of HO-1 was not significantly different between group A and B (Western blot: proximal 0.22 ± 0.02 vs 0.22 ± 0.03; distal 0.23 ± 0.03 vs 0.23 ± 0.03; both P > 0.05), but HO-1 expression was markedly higher in group C (proximal 0.66 ± 0.09; distal 0.47 ± 0.07) than that in the former two groups (P < 0.05); the expression of HO-1 was hardly found in group D. In comparison with those in group B, the gastrointestinal propulsion rate (54.4% ± 2.9% vs 63.0% ± 1.2%, P < 0.05), spontaneous contraction frequencies, amplitudes, and reaction to acetylcholine of colonic smooth muscles were dramatically declined in group C (P < 0.05), while those (gastrointestinal propulsion rate: 72.5% ± 2.6% vs 63.0% ± 1.2%, P < 0.05) in group D were markedly improved (all P < 0.05).
CONCLUSION: HO interference has no effect on the body weight or blood glucose of diabetic rats. The induction of HO-1 may aggravate the decline of colonic motility in diabetic rats, while HO-1 blockage may improve the declined colonic motility.
- Citation: Wu GJ, Lin L, Zhang HJ, Li XL, Luo Y, Wang MF. Effect of interference in heme oxygenase on colonic dysfunction of diabetic rats. Shijie Huaren Xiaohua Zazhi 2007; 15(1): 14-21
- URL: https://www.wjgnet.com/1009-3079/full/v15/i1/14.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v15.i1.14
糖尿病(diabetes mellitus, DM)胃肠动力障碍是糖尿病极为常见的并发症, 发病率达70%[1-2]. 病理生理特点是胃肠张力和收缩力下降、蠕动减慢、排空延迟[3]. 便秘可见于25%左右的DM患者[4], 成为糖尿病胃肠动力障碍突出症状之一. 有关DM胃肠动力障碍的机制尚不完全清楚. 近年来, 气体信使一氧化碳(carbon monoxide, CO)作为一种胃肠道抑制性神经递质[5], 在胃肠动力障碍性疾病中的作用受到重视. 内源性CO主要由血红素氧合酶(heme oxygenase, HO)催化血红素分解产生, 人体内HO有3种同工酶, 即: HO-1, HO-2和HO-3. 本研究借助糖尿病大鼠胃肠动力障碍模型, 对胃肠道HO进行干预, 探讨HO在糖尿病结肠动力障碍中的作用及其病理生理意义.
链脲佐菌素(streptozotocin, STZ), 正铁血红素(Hemin), 锌原卟啉(zinc protoporphyrin Ⅸ, ZnPP Ⅸ), 乙酰胆碱(acetylcholine, Ach)购自美国Sigma公司; c-kit兔抗鼠多克隆抗体(sc-168), HO-1兔抗鼠多克隆抗体(sc-10789), HO-2兔抗鼠多克隆抗体(sc-11361)购自美国Santa Cruz Biotechnology公司; SP免疫组化试剂盒购自福州迈新生物公司; 多道水平高感度离体器官实验系统(日本国立医学生理研究所), RM6240生物信号采集处理系统(成都仪器厂), Indian墨水购自南京晚晴化玻仪器有限公司.
1.2.1 糖尿病模型的建立和实验分组: ♂ SD大鼠(上海斯莱克公司), 体质量200-250 g, SPF环境饲养. DM模型建立: 大鼠按60 mg/kg体质量一次性ip STZ[6-7], 3 d后尾静脉采血测血糖, 血糖≥16.7 mmol/L, 并能维持1 wk以上, 确定为DM模型建立成功. 血糖<16.7 mmol/L者剔除实验. 正常对照组一次性ip等量的柠檬酸缓冲液. 所有进入实验的大鼠均每周监测记录血糖和体质量变化. 实验分组: 第一阶段: 饲养6 wk时, 随机选取DM大鼠和正常大鼠各8只, 行碳末推进实验, 确认有无胃肠动力障碍. 第二阶段: 剩余大鼠分为正常对照组(n = 8), DM未干预组(n = 8), DM+Hemin组(n = 8)和DM+ZnPP组(n = 8). 自饲养6 wk起予以干预: (1)DM+Hemin组: 6 wk起隔日ip Hemin 30 μmol/kg 3 wk后处死; (2)DM+ZnPP组: 6 wk起隔日ip ZnPP Ⅸ 10 μmol/kg 3 wk后处死(Hemin和ZnPP Ⅸ均以0.1 mol/L NaOH溶液溶解, 再以0.1 mol/L磷酸盐缓冲液调pH至7.4); (3)正常对照组和DM未干预组: 均6 wk起隔日ip 0.1 mol/L磷酸盐缓冲液(pH 7.4) 3 wk后处死. 分别采集各组大鼠近端、远端结肠各长0.5 cm.
1.2.2 碳末推进实验(胃肠推进指数): 所有大鼠监测血糖、体质量. 处死前行碳末推进实验: 禁食12 h后, 给予Indian墨水(10 mL/kg)灌胃, 30 min后将大鼠颈椎脱臼处死, 剖腹取出全部胃肠道, 测量并计算无张力下胃肠推进指数(墨水前端至幽门括约肌距离/幽门括约肌至肛门距离×100%)[8].
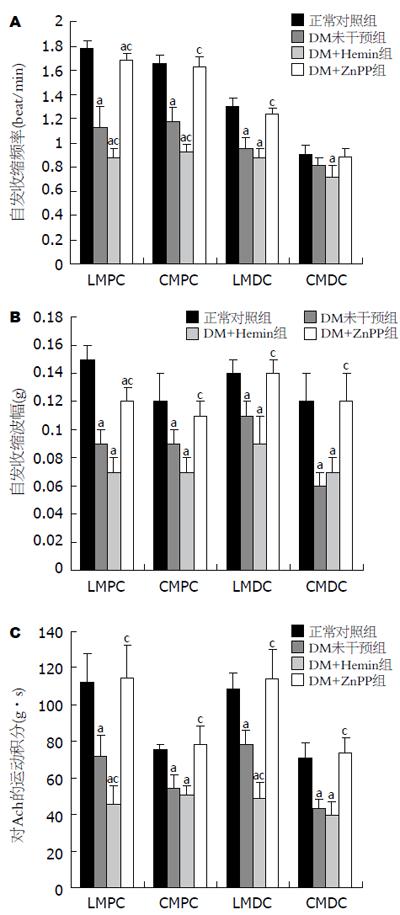
1.2.3 离体平滑肌条实验: 处死大鼠后, 按常规分别取近端结肠纵行肌肌条(longitudinal muscle of proximal colon, LMPC)、近端结肠环行肌肌条(circular muscle of proximal colon, CMPC)、远端结肠纵行肌肌条(longitudinal muscle of distal colon, LMDC)和远端结肠环行肌肌条(circular muscle of distal colon, CMDC), 各长7 mm、宽1 mm[9]; 肌条固定于浴槽中, 与张力换能器相连, 给予0.5 g的预张力, 持续灌流含950 mL/L O2+50 mL/L CO2的37℃恒温Kreb液, 以"RM6240生物信号采集系统"记录平滑肌条自发性等长收缩活动, 测定近、远端结肠纵、环行肌条平均波幅、收缩频率; 在平滑肌条自发收缩稳定后, 在浴槽中加入10-2 mol/L Ach, 记录肌条反应情况, 测定运动指数(即加入Ach后反应曲线的曲线下面积).
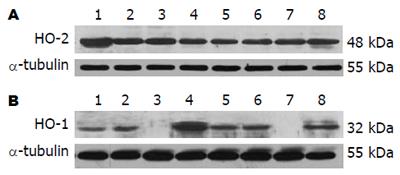
1.2.4 Western blot测定结肠组织HO-1和HO-2表达: HO-1: 结肠组织提取的蛋白样品80 μg, SDS-PAGE凝胶电泳分离后, 90 V电转膜1.5 h, 将蛋白转印至PVDF膜. 50 g/L脱脂奶粉于室温封闭2 h, 加入1:150稀释的HO-1兔抗鼠多克隆抗体, 4℃孵育过夜. TBST洗膜后, 加1:10000 HRP标记羊抗兔单克隆二抗(Vector公司), 室温孵育1 h, TBST洗膜, 加入化学发光剂至膜上1 min, 最后曝光、显影. HO-2: 方法同上, 上样量为30 μg, 一抗为HO-2兔抗鼠多克隆抗体, 以1:500稀释. 所得图片扫描入计算机, GIS凝胶图像处理系统软件对目标条带及内参(α-tubulin)条带进行灰度扫描分析.
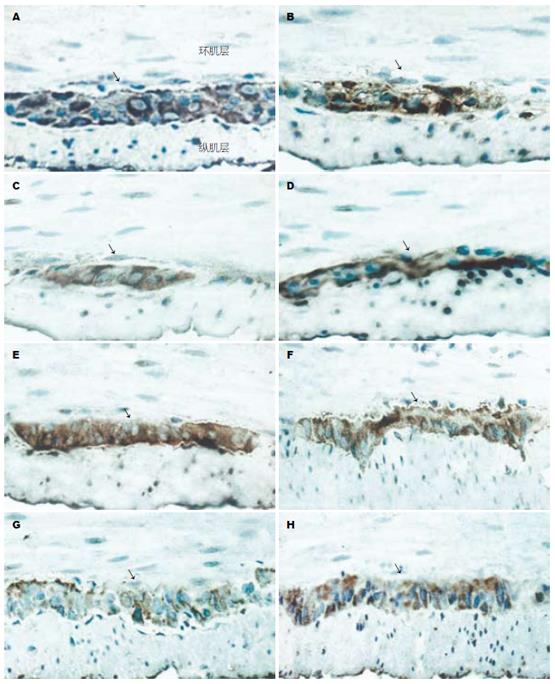
1.2.5 免疫组化(SP法)及结果判断: 常规固定、包埋、切片、梯度脱蜡、染色. HO-1一抗浓度为1:30, HO-2一抗浓度为1:50, 苏木素衬染胞核, 阳性结果判断: 显微镜下胞质出现棕黄色片状或颗粒状物为阳性反应. 每张切片选择5个高倍视野(×400倍), 应用Leica RX250型图像分析系统进行定量灰度扫描, Qwin软件分析并自动计算HO-1和HO-2阳性细胞面积.
统计学处理 所有数据录入SPSS 11.0统计软件包, 以均数±标准差(mean±SD)表示, 进行单因素方差分析, 组间比较采用q检验, P<0.05为有显著性差异.
第一阶段(6 wk): DM建模6 wk后. (1)体质量(g): DM大鼠(258.38±52.35)较对照组(436.38±19.87)显著下降(P<0.05); (2)血糖(mmol/L): DM大鼠(25.95±2.71)较对照组(4.73±0.42)显著升高(P<0.05); (3)胃肠推进指数: DM大鼠(61.4%±3.7%)较对照组(71.9%±4.3%)显著减低(P<0.05), 证实糖尿病胃肠慢传输型动力障碍模型建立[10].
第二阶段(9 wk): DM建模6 wk并干预3 wk后: (1)DM未干预组大鼠较正常对照组体质量显著下降, 血糖显著升高, 胃肠推进指数显著减低(P<0.05); (2)DM+Hemin组及DM+ZnPP组大鼠体质量、血糖较DM未干预组无显著差异(P>0.05); 胃肠推进指数: DM+Hemin组较DM未干预组显著减低(P<0.05); 而DM+ZnPP组较DM未干预组明显改善(P<0.05), 且与正常对照组无显著差异(P>0.05, 表1).
DM未干预组大鼠较正常对照组显著降低(P<0.05); DM+Hemin组较DM未干预组更加降低, 尤以近端结肠为显著(P<0.05); 而DM+ZnPP组则较DM未干预组有明显改善(P<0.05), 部分指标已达到正常对照组水平(P>0.05, 图1).
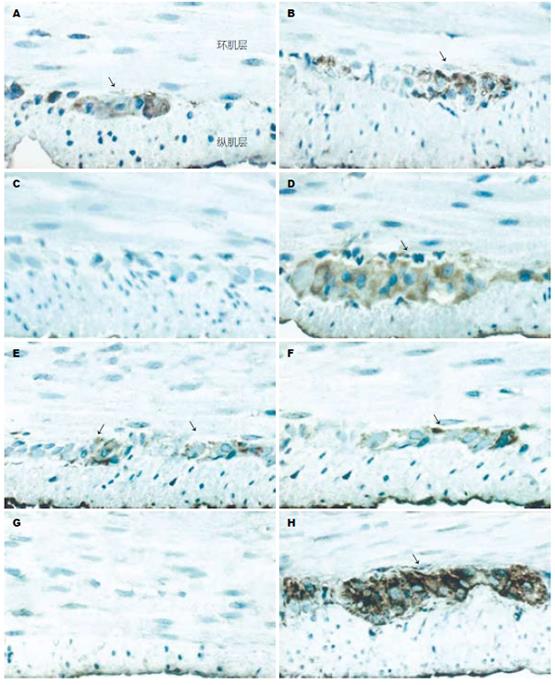
HO-2: 近端结肠: DM未干预组、DM+Hemin组及DM+ZnPP组HO-2的表达无显著差异(P>0.05), 但均较对照组显著减少(P<0.05). 远端结肠: 各实验组HO-2的表达无显著差异(P>0.05, 图2A, 图3A); HO-1: 正常对照组与DM未干预组近、远端结肠HO-1的表达无显著差异(P>0.05), DM+Hemin组近、远端结肠HO-1的表达较前两组显著增多(P<0.05), 且尤以近端结肠显著; DM+ZnPP组近、远端结肠HO-1基本均无表达(图2B, 图3B).
HO-1和HO-2阳性细胞主要分布于结肠肌间神经丛和黏膜下神经丛, 尤以肌间神经丛为著, 环肌和纵肌内亦有少量分布. 免疫组化显示, 各实验组大鼠(9 wk)近、远端结肠HO-1和HO-2的表达情况与Western blot的结果相似(图4-5).
糖尿病结肠动力障碍是糖尿病常见的并发症之一, 严重影响人们的生活质量. 该病以腹胀和便秘为主要临床表现, 以结肠张力和收缩力低下、蠕动减慢、排空延迟[11]为病理生理特点. 目前, DM胃肠动力障碍的机制尚不完全清楚, 可能与自主神经、Cajal间质细胞及胃肠平滑肌病变、高血糖和激素分泌异常等多种因素有关[12-14], 临床缺乏有效的治疗手段. HO有3个同工酶, 为诱导型HO-1、结构型HO-2和HO-3. 生理情况下, HO-2在胃肠道分布较丰富[15], HO-1表达较少[16], 但在疾病、损伤等情况下(包括血红素、内毒素、紫外线、重金属和NO等)可诱导HO-1的表达升高, 这些诱导剂的共同特征是可以产生氧化应激. HO-3不能分解血红素, 其作用尚不清楚[17]. 在胃肠道, HO主要产生于神经元、神经纤维、血管内皮细胞、血管平滑肌细胞、Cajal间质细胞中[18-20]. 目前研究认为, 在DM较长的病程中, 上述细胞逐步发生不同程度的病变[14,21]. 本实验中所观察到的DM未干预组近端结肠HO-2的表达较正常对照组减少, 与上述产生HO的细胞随DM病程进展而逐步病变的过程相符, 但这一改变在DM未干预组远端结肠却不甚明显. 而对于HO-1, 本实验发现, DM未干预组HO-1表达并未下降, 可能是DM病程中(或者某一阶段)发生了氧化应激[22], 诱导HO-1生成, 使病变轻或残余的HO表达细胞代偿性的产生一定的HO-1; 但如果DM病程继续延长, HO-1将发生怎样的变化, 有待进一步研究.
我们应用Hemin和ZnPP Ⅸ分别作为HO的诱导剂和阻滞剂[23-25], Western blot和免疫组化结果显示, DM+Hemin(HO的诱导剂)组结肠HO-1的表达较正常对照组和DM未干预组显著增多, 而DM+ZnPP(HO的阻滞剂)组近、远端结肠HO-1基本无表达, 证实了本实验对HO的干预是成功的. 但HO-2作为结构型HO, 表达不易受到干预的影响, 文献认为仅肾上腺皮质激素能诱导其生成, 本实验以小剂量Hemin对HO-2干预作用不明显, 与文献结果相符; 本实验中, 无论DM+Hemin组或DM+ZnPP组, HO-2的表达较DM未干预组均无显著差异, 以小剂量ZnPP Ⅸ对HO-2进行干预, 作用不明显, 其机制不详. 以往我们的实验提示, DM大鼠建模6 wk后, 开始出现胃肠推进率下降[26], 本实验再次证实糖尿病大鼠建模6 wk后, 胃肠慢传输型动力障碍已经出现. 在DM大鼠已产生胃肠慢传输运动的基础上, 我们以Hemin和ZnPP Ⅸ对DM大鼠进行干预. 在DM+Hemin组, 结肠HO-1的表达显著增多, 尤以近端结肠更为显著; 碳末推进实验及离体平滑肌条实验均证实结肠慢传输型动力障碍较DM未干预组进一步加重, 同样以近端结肠更为显著. 这与Kadinov et al[27]在体外以Hemin对豚鼠HO-1进行诱导, 使胃平滑肌条兴奋性下降的结果一致, 且他们发现, HO的阻滞剂锡原卟啉Ⅸ(Sn-protoporphyrin Ⅸ, SnPP Ⅸ)可阻断这一现象. 同时, 本实验发现DM+ZnPP组, 近、远端结肠HO-1的表达被阻滞, 而其结肠慢传输型动力障碍较DM未干预组明显改善, 胃肠推进率及离体平滑肌条实验均提示其结肠动力已恢复到与正常对照组相似的水平.
研究认为, 血红素在HO的作用下降解产生内源性CO. 在胃肠道, 内源性CO作为一种非胆碱能、非肾上腺素能抑制性神经递质, 主要通过cGMP途径介导胃肠道平滑肌舒张, 即CO与鸟苷酸环化酶中血红素基团上的铁原子结合, 激活该酶, 增加细胞内cGMP的生成, 降低胞质中的Ca2+, 介导平滑肌舒张[28-30], 胃肠运动减慢. 本实验中, 干预HO-1, 使内源性CO这一抑制性神经递质的生成发生变化, 进而影响DM结肠运动的改变, 提示CO可能是DM大鼠结肠慢传输动力障碍的重要机制之一.
总之, HO的干预可以影响糖尿病结肠动力, 阻滞HO-1可能改善糖尿病结肠动力障碍, 而诱导HO-1则加重结肠动力障碍. 至于HO的干预是否还通过其他机制影响糖尿病结肠动力仍有待进一步研究.
糖尿病胃肠功能紊乱的发病机制目前仍不清楚, 临床也缺乏有效治疗手段. 因此研究糖尿病胃肠动力障碍的发生机制, 为寻找该病新的治疗方法提供理论基础, 具有十分重要的社会和经济意义.
糖尿病结肠动力障碍是糖尿病极为常见的并发症, 其发病机制目前仍不清楚. 近来CO/HO体系在胃肠动力障碍性疾病中的病理生理作用已成为研究热点.
CO/HO体系在神经、心血管、呼吸、泌尿等系统已有较多的报道, 已有对以上系统HO干预的研究, 且关于其在胃肠道的表达及与相关疾病的关联也有较多报道.
本研究首次对结肠HO进行干预, 并综合运用分子生物学及机能学的实验方法, 验证了干预的有效性及其对糖尿病大鼠结肠动力产生的显著影响.
本研究对HO的干预显著影响了糖尿病大鼠的结肠动力, 这一发现有望为糖尿病胃肠功能障碍开拓新的治疗靶点.
血红素氧合酶(HO): 在体内分布广泛, 血红素在其作用下生成胆绿素、内源性CO和Fe2+. CO是继NO后发现的又一具有重要生理作用的气体分子. 作为胃肠道抑制性的神经递质, CO通过活化鸟苷酸环化酶介导平滑肌舒张, 且大多数学者认为CO在胃肠道肌层是个超极化因子.
本文用血红素氧合酶干预观察对糖尿病大鼠结肠动力障碍的影响, 发现HO的干预可以影响糖尿病结肠动力改变, 阻滞HO-1能改善糖尿病结肠动力障碍, 而诱导HO-1则加重结肠动力障碍, 有较高的学术价值.
电编: 张敏 编辑: 王晓瑜
| 1. | Troncon LE, Lopes RP, Simão MN, Iquegami M, Rosa-e-Silva L, Nobre-e-Souza MA, Foss MC. [Frequency of digestive symptoms in Brazilian patients with Diabetes Mellitus]. Rev Assoc Med Bras. 2001;47:157-164. [PubMed] [DOI] |
| 2. | Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670-674. [PubMed] [DOI] |
| 3. | Iida M, Ikeda M, Kishimoto M, Tsujino T, Kaneto H, Matsuhisa M, Kajimoto Y, Watarai T, Yamasaki Y, Hori M. Evaluation of gut motility in type II diabetes by the radiopaque marker method. J Gastroenterol Hepatol. 2000;15:381-385. [PubMed] [DOI] |
| 4. | Talley NJ, Young L, Bytzer P, Hammer J, Leemon M, Jones M, Horowitz M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71-76. [PubMed] [DOI] |
| 5. | Johnson RA, Johnson FK. The effects of carbon monoxide as a neurotransmitter. Curr Opin Neurol. 2000;13:709-713. [PubMed] [DOI] |
| 6. | Yi CR, Wei ZQ, Deng XL, Sun ZY, Li XR, Tian CG. Effects of coffee and caffeine on bladder dysfunction in streptozotocin-induced diabetic rats. Acta Pharmacol Sin. 2006;27:1037-1043. [PubMed] [DOI] |
| 7. | Nicolau J, Souza DN, Nogueira FN. Activity, distribution and regulation of phosphofructokinase in salivary gland of rats with streptozotocin-induced diabetes. Pesqui Odontol Bras. 2006;20:108-113. [DOI] |
| 8. | 乔 娴, 刘 劲松, 吴 汉妮, 侯 晓华. 糖尿病大鼠胃肠运动障碍的实验研究. 胃肠病学和肝病学杂志. 1998;9:242-244. |
| 9. | 周 吕, 柯 美云. 胃肠动力学基础与临床. 北京: 科学出版社 1999; 294-295. |
| 10. | Zhang Y, Zhang K, Luo J, Qi H. [Changes of ultrastructure characteritics of Cajal interstitial cell in intestinal tract of diabetic rats]. Zhonghua Nei Ke Za Zhi. 2002;41:310-312. [PubMed] |
| 11. | Jung HK, Kim DY, Moon IH, Hong YS. Colonic transit time in diabetic patients--comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med J. 2003;44:265-272. [PubMed] [DOI] |
| 12. | El-Salhy M. Neuroendocrine peptides of the gastrointestinal tract of an animal model of human type 2 diabetes mellitus. Acta Diabetol. 1998;35:194-198. [PubMed] [DOI] |
| 13. | Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061-1075. [PubMed] [DOI] |
| 14. | Nakahara M, Isozaki K, Hirota S, Vanderwinden JM, Takakura R, Kinoshita K, Miyagawa J, Chen H, Miyazaki Y, Kiyohara T. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666-670. [PubMed] [DOI] |
| 15. | Chen Y, Lui VC, Sham MH, Tam PK. Distribution of carbon monoxide-producing neurons in human colon and in Hirschsprung's disease patients. Hum Pathol. 2002;33:1030-1036. [PubMed] [DOI] |
| 16. | Barton SG, Rampton DS, Winrow VR, Domizio P, Feakins RM. Expression of heat shock protein 32 (hemoxygenase-1) in the normal and inflamed human stomach and colon: an immunohistochemical study. Cell Stress Chaperones. 2003;8:329-334. [PubMed] [DOI] |
| 17. | McCoubrey WK, Huang TJ, Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725-732. [PubMed] [DOI] |
| 18. | Miller SM, Reed D, Sarr MG, Farrugia G, Szurszewski JH. Haem oxygenase in enteric nervous system of human stomach and jejunum and co-localization with nitric oxide synthase. Neurogastroenterol Motil. 2001;13:121-131. [PubMed] [DOI] |
| 19. | Piotrowska AP, Solari V, de Caluwé D, Puri P. Immunocolocalization of the heme oxygenase-2 and interstitial cells of Cajal in normal and aganglionic colon. J Pediatr Surg. 2003;38:73-77. [PubMed] [DOI] |
| 20. | Oates PS, West AR. Heme in intestinal epithelial cell turnover, differentiation, detoxification, inflammation, carcinogenesis, absorption and motility. World J Gastroenterol. 2006;12:4281-4295. [PubMed] [DOI] |
| 21. | Guo C, Quobatari A, Shangguan Y, Hong S, Wiley JW. Diabetic autonomic neuropathy: evidence for apoptosis in situ in the rat. Neurogastroenterol Motil. 2004;16:335-345. [PubMed] [DOI] |
| 22. | Shin CS, Moon BS, Park KS, Kim SY, Park SJ, Chung MH, Lee HK. Serum 8-hydroxy-guanine levels are increased in diabetic patients. Diabetes Care. 2001;24:733-737. [PubMed] [DOI] |
| 23. | Huang TY, Tsai PS, Wang TY, Huang CL, Huang CJ. Hyperbaric oxygen attenuation of lipopolysaccharide-induced acute lung injury involves heme oxygenase-1. Acta Anaesthesiol Scand. 2005;49:1293-1301. [PubMed] [DOI] |
| 24. | Huang XL, Ling YL, Ling YQ, Zhou JL, Liu Y, Wang QH. Heme oxygenase-1 in cholecystokinin-octapeptipe attenuated injury of pulmonary artery smooth muscle cells induced by lipopolysaccharide and its signal transduction mechanism. World J Gastroenterol. 2004;10:1789-1794. [PubMed] [DOI] |
| 25. | Iwasashi H, Suzuki M, Unno M, Utiyama T, Oikawa M, Kondo N, Matsuno S. Inhibition of heme oxygenase ameliorates sepsis-induced liver dysfunction in rats. Surg Today. 2003;33:30-38. [PubMed] [DOI] |
| 26. | 陈 娟, 林 琳, 许 新芳, 张 红杰, 林 征. 血红素氧合酶-2在糖尿病大鼠结肠组织中的表达及意义. 南京医科大学学报(自然科学版). 2006;26:14-16. |
| 27. | Kadinov B, Itzev D, Gagov H, Christova T, Bolton TB, Duridanova D. Induction of heme oxygenase in guinea-pig stomach: roles in contraction and in single muscle cell ionic currents. Acta Physiol Scand. 2002;175:297-313. [PubMed] [DOI] |
| 28. | Rattan S, Chakder S. Influence of heme oxygenase inhibitors on the basal tissue enzymatic activity and smooth muscle relaxation of internal anal sphincter. J Pharmacol Exp Ther. 2000;294:1009-1016. [PubMed] |
| 29. | Kwon S, Chung S, Ahn D, Yeon D, Nam T. Mechanism of carbon monoxide-induced relaxation in the guinea pig ileal smooth muscle. J Vet Med Sci. 2001;63:389-393. [PubMed] [DOI] |