修回日期: 2006-04-15
接受日期: 2006-05-08
在线出版日期: 2006-06-18
目的: 探讨大肠癌发生与演进中p16, Rb, cyclin D1甲基化状态与蛋白表达的关系及意义.
方法: 提取正常黏膜、腺瘤、癌旁组织及癌组织基因组DNA, 应用MSP法检测不同病变阶段组织中各基因的甲基化状态, 并对其与蛋白表达及临床病理参数的关系进行分析.
结果: 在大肠癌发生与演进过程中, p16, Rb基因甲基化率呈增高趋势, cyclin D1基因甲基化率呈下降趋势, p16(切缘: r = -0.185, P = 0.173; 腺瘤: r = -0.381, P = 0.013; 癌旁: r = -0.419, P = 0.001; 癌: r = -0.516, P = 0.000)、cyclin D1(切缘: r = -0.282, P = 0.035; 腺瘤: r = -0.329, P = 0.033; 癌旁: r = -0.298, P = 0.026; 癌: r = -0.618, P = 0.000)基因甲基化程度分别与其蛋白表达呈明显负相关, 且在癌组织分化程度(p16: χ2 = 11.232, P = 0.002, cyclin D1: χ2 = 9.144, P = 0.015)、浸润深度(p16: χ2 = 6.229, P = 0.013; cyclin D1: χ2 = 8.023, P = 0.006)和淋巴结转移(p16: χ2 = 5.707, P = 0.016; cyclin D1: χ2 = 7.794, P = 0.005)上有显著性差异. Rb基因的甲基化状态在Rb表达抑制上不起主要作用.
结论: 大肠癌p16高甲基化和cyclin D1低甲基化可能是p16失活和cyclin D1过表达的主要机制, 在大肠癌的发生、发展中发挥重要作用, 对于大肠癌的早期诊断、恶性程度及预后判断有重要意义.
引文著录: 李庚, 千新来, 崔静, 王中群, 冶亚平, 杨晓煜, 李永真. DNA甲基化对大肠癌相关基因表达的调控意义. 世界华人消化杂志 2006; 14(17): 1699-1703
Revised: April 15, 2006
Accepted: May 8, 2006
Published online: June 18, 2006
AIM: To investigate the relationship between DNA methylation and expression of p16, Rb, and cyclin D1 in the carcinogenesis of colorectal carcinoma.
METHODS: Methylation-specific polymerase chain reaction (MS-PCR) was used to detect the methylation status of p16, Rb, and cyclin D1 in the specimens from colorectal carcinoma, cancer-adjacent tissues of carcinoma and adenoma, and normal colorectal mucosa, respectively. The correlations of methylation with protein expression and the clinicopathological indexes were analyzed.
RESULTS: For the levels of p16 and Rb methylation, there were increased tendencies in the carcinogenesis of colorectal cancer, while for the level of cyclin D1 methylation, there was a decreased tendency. The expression of p16 and cyclin D1 protein were inversely correlated with the methylation status of p16 (normal mucosa: r = -0.185, P = 0.173; adenoma: r = -0.381, P = 0.013; cancer-adjacent tissues: r = -0.419, P = 0.001; cancer tissues: r = -0.516, P = 0.000) and cyclin D1 gene (normal mucosa: r = -0.282, P = 0.035; adenoma: r = -0.329, P = 0.033; cancer-adjacent tissues: r = -0.298, P = 0.026; cancer tissues: r = -0.618, P = 0.000). The levels of p16 and cyclin D1 methylation were significantly correlated with the degrees of differentiation (p16: χ2 = 11.232, P = 0.002, cyclin D1: χ2 = 9.144, P = 0.015), the depth of invasion (p16: χ2 = 6.229, P = 0.013; cyclin D1: χ2 = 8.023, P = 0.006) and the metastasis of lymph node (p16: χ2 = 5.707, P = 0.016; cyclin D1: χ2 = 7.794, P = 0.005). The methylation of Rb gene didn't play a main role in the inhibition of Rb protein expression during colorectal carcinogenesis.
CONCLUSION: Aberrant methylations of p16 and cyclin D1 are the main mechanisms of p16 inactivation and cyclin D1 over-expression, which play important roles in colorectal carcinogenesis. They are valuble in the early diagnosis and prognosis of colorectal carcinoma.
- Citation: Li G, Qian XL, Cui J, Wang ZQ, Ye YP, Yang XY, Li YZ. Role of DNA methylation in control of tumor suppressor gene and oncogene expression in colorectal carcinoma. Shijie Huaren Xiaohua Zazhi 2006; 14(17): 1699-1703
- URL: https://www.wjgnet.com/1009-3079/full/v14/i17/1699.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i17.1699
大肠癌的发生与演进涉及多种癌基因的过表达和/或抑癌基因的失活[1-6]. 另外, 在基因表达的调控中, DNA甲基化起着重要作用[7], 多种肿瘤的发生过程中伴有癌基因的低甲基化或抑癌基因的高甲基化[8-11]. 我们曾报道了p27基因甲基化在大肠癌发生中的变化[12], 并报道了p16, Rb, cyclin D1表达与大肠癌的关系[13], 发现p27基因异常甲基化可能是p27失活的主要机制. 为了进一步从细胞周期的角度探讨大肠癌发生的分子机制, 我们检测了大肠癌相关基因p16, Rb, cyclin D1的甲基化状态, 探讨基因甲基化改变与蛋白表达的关系及其对大肠癌发生、发展的影响.
新乡医学院三附院、新乡市中心医院、第二人民医院2001-01/12手术切除的大肠癌标本56例(包括癌、癌旁组织、手术切缘上皮)、腺瘤42例, 年龄37-76(中位55)岁, 标本经病理学证实. DNA分子质量Marker, TaqDNA聚合酶、脱氧核苷三磷酸、DNA purify system等为Promega公司产品; 亚硫酸氢钠、氢醌等为国产分析纯. 酚-氯仿法提取组织DNA并定量, 按参考文献[14]方法略作修改进行亚硫酸氢盐处理. 即: 氢氧化钠变性DNA后, 亚硫酸氢钠、氢醌处理, DNA purify system纯化、回收DNA.
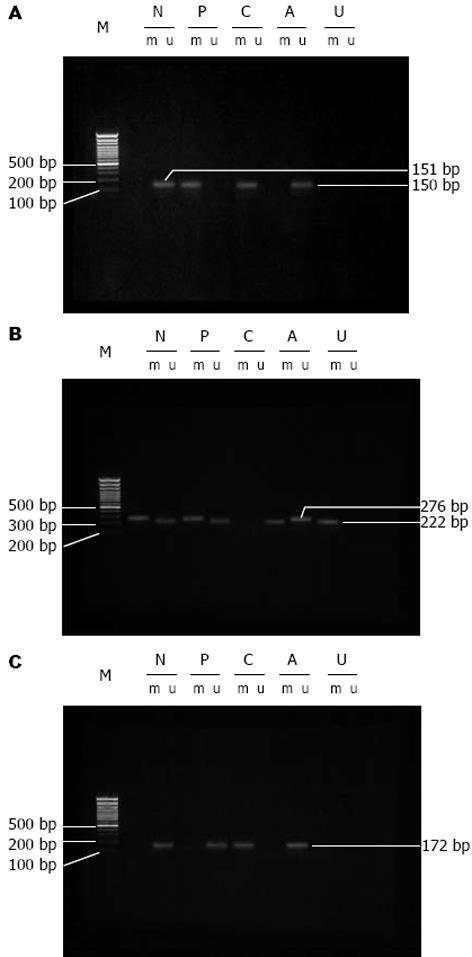
MSP检测基因甲基化状态. PCR反应条件: 95℃预变性10 min后接由94℃变性45 s, 退火(温度见表1)45 s, 72℃延伸45 s三步组成的35个循环, 最后72℃延伸5 min. 引物序列、退火温度及产物长度见表1.
| 引物 | 序列(5'→3') | 退火温度 | 产物长度 |
| p16M | TTATTAGAGGGTGGGGCGGATCGC | 65℃ | 150 bp |
| GACCCCGAACCGCGACCGTAA | |||
| p16U | TTATTAGAGGGTGGGGTGGATTGT | 60℃ | 151 bp |
| CAACCCCAAACCACAACCATAA | |||
| RbM | GGGAGTTTCGCGGACGTGAC | 55℃ | 172 bp |
| ACGTCGAAACACGCCCCG | |||
| RbU | GGGAGTTTTGTGGATGTGAT | 55℃ | 172 bp |
| ACATCAAAACACACCCCA | |||
| Cyclin D1M | TACGTGTTAGGGTCGATCG | 55℃ | 276 bp |
| CGAAATATCTACGCTAAACG | |||
| Cyclin D1U | GTTATGTTATGTTTGTTGTATG | 55℃ | 222 bp |
| TAAAATCCACCAACACAATCA |
统计学处理 采用χ2检验或确切概率法及相关分析, 应用SPSS 10.0统计软件处理.
MSP分析各基因的甲基化状态(图1)结果显示, 在大肠癌的发生与演进过程中, p16和Rb甲基化率呈增高趋势, 其在手术切缘上皮、癌旁、腺瘤、癌组织中的甲基化率分别为28.6%和3.6%, 44.6%和10.7%, 0.0%和11.9%, 64.3%和14.3%; cyclin D1甲基化率呈下降趋势, 其在切缘、癌旁、腺瘤、癌组织中的甲基化率为64.3%, 37.5%, 31.0%和23.2%.
各病变阶段, p16(切缘: r = -0.185, P = 0.173>0.005; 腺瘤: r = -0.381, P = 0.013<0.05; 癌旁: r = -0.419, P = 0.001<0.001; 癌: r = -0.516, P = 0.000<0.01)、cyclin D1(切缘: r = -0.282, P = 0.035<0.005; 腺瘤: r = -0.329, P = 0.033<0.05; 癌旁: r = -0.298, P = 0.026<0.005; 癌: r = -0.618, P = 0.000<0.01)甲基化率与其蛋白表达呈明显负相关, 而Rb甲基化率与蛋白表达无相关性(表2).
p16, cyclin D1甲基化率在癌组织分化程度(p16: χ2 = 11.232, P = 0.002<0.01, cyclin D1: χ2 = 9.144, P = 0.015<0.05)、浸润深度(p16: χ2 = 6.229, P = 0.013<0.05; cyclin D1: χ2 = 8.023, P = 0.006<0.01)和淋巴结转移(p16: χ2 = 5.707, P = 0.016<0.05; cyclin D1: χ2 = 7.794, P = 0.005<0.01)上均有显著性差异(表3).
肿瘤细胞区别于正常细胞的基本特征是细胞生长失控和分化受阻, 细胞生长、分化的调节与细胞周期的许多因素密切相关, 细胞周期调控因子是一组调节细胞增殖、分化的正负信号, 他们通过相互促进或抑制作用维持细胞周期的正常进程. 在恶性肿瘤细胞中, 这些调控因子常发生过表达或失活, 从而引起细胞周期的正常调节紊乱, 促进肿瘤的形成与演进[15-18]. p16作为抑癌基因与细胞周期紧密相关, 通过细胞周期与其他癌基因、抑癌基因相互作用, 成为正常细胞增殖的负性调控因子[19]. 目前研究认为, 在与Rb, cyclin D1/CDK4共同组成的细胞增殖调控途径中, p16作为cyclin D1/CDK4复合物的特异抑制剂而发挥调节作用[20]. Rb作为CDK4的重要底物参与了细胞周期调控, 与多种细胞蛋白结合抑制其活性转录功能而有G1期阻滞作用, 作为抑癌基因阻止肿瘤的形成[21-22]. cyclin D1在细胞周期中与CDK结合, 协同CDK对Rb蛋白磷酸化, 磷酸化后的Rb蛋白失去其抑制活性[23-25], 从而促进细胞增殖成为细胞周期的正调控因子. 研究发现, 大肠癌中存在着多种细胞周期调控因子的异常表达, 但其中对于p16, Rb失活和cyclin D1 过表达的机制目前的报道有诸多差异[11,26-27]. 近来研究表明, 在肿瘤细胞中常存在DNA甲基化状态的改变, DNA甲基化异常分为甲基化增强和甲基化降低两种类型, 可引起与细胞增殖和分化有关基因的表达异常, 导致细胞恶变形成肿瘤[7-11]. DNA异常甲基化导致肿瘤发生的机制可能与基因突变、影响染色体凝聚及癌基因、抑癌基因的表达有关[28-29].
本研究结果显示, 在大肠癌发生过程中p16基因高甲基化和cyclin D1基因低甲基化可能是p16失活和cyclin D1高表达的主要机制, 其在大肠癌的发生、发展中发挥了重要作用, 对于大肠癌的早期诊断、恶性程度和预后判断有重要意义. 该结果与Norrie et al[26]的报道不同, 可能与实验中所用样品来源和检测方法不同等有关. 但与Dominguez et al[11]的报道一致. 值得注意的是, DNA甲基化改变可发生在大肠癌形成的早期甚至起始阶段, 因而建立检测DNA甲基化状态改变的准确而敏感的方法可早期诊断细胞恶变. 在细胞癌变过程中, 基因结构改变是不可逆的, DNA甲基化改变是可逆的, 尤其是癌变早期的异常改变可能使之向正常逆转[30], 这对肿瘤的防治尤为重要. Rb基因的甲基化率在大肠癌发生中虽然也呈增加趋势, 但阳性率很低, 并且甲基化程度与Rb蛋白表达也无相关性. 因此在大肠癌发生过程中Rb基因异常甲基化在Rb基因的失活上并不起主要作用, 而可能是由于突变、缺失等引起Rb基因的失活, 这有待于进一步研究.
DNA甲基化是哺乳动物细胞基因表达调控的重要方式之一, 对于肿瘤细胞, 基因组中出现高甲基化区域的同时, 还存在着低甲基化区域. 前者对抑癌基因的灭活起重要作用, 后者与癌基因的激活、过量表达有密切关系, 并由此引起细胞的异常增生, 最终导致肿瘤的发生. cyclinD1作为细胞周期的正调节因子可促进细胞增殖, 已被视为一种癌基因; p16和p27属于CKI家族, 通过抑制CDK的活性阻止细胞周期的进程, 是细胞周期的负性调节因子, 作为抑癌基因可阻止肿瘤的形成. 非磷酸化的Rb蛋白可结合E2F, 阻止G1/S期转换.
在多种人肿瘤中抑癌基因启动子区CpG岛高甲基化而灭活和癌基因低甲基化而过表达, 如肝细胞癌、非小细胞肺癌、胃癌、食管癌等, 在大肠癌中仅见p16基因高甲基化的检测及其机制的相关报道, 缺乏采用较大样本从正常肠黏膜→异常增生→腺瘤→大肠癌系统的多基因联合对比性研究. 为此, 本研究采用较大样本(包括大肠癌切缘正常黏膜上皮、癌旁组织、腺瘤和大肠癌标本)利用甲基化特异性PCR(methylationspecificPCR, MSP)技术和免疫组化染色检测了4种与细胞周期调控有关的基因(cyclinD1、p16、p27和Rb)启动子区CpG岛甲基化状态及其蛋白在大肠癌发生、发展过程中的表达情况.
在大肠癌发生过程中p16基因高甲基化和cyclinD1基因低甲基化可能是p16失活和cyclinD1高表达的主要机制, 其在大肠癌的发生、发展中发挥了重要作用, 对于大肠癌的早期诊断、恶性程度和预后判断有重要意义.
甲基化特异性PCR(methylationspecificPCR, MSP): 是用亚硫酸氢钠处理DNA, 则未甲基化胞嘧啶变为尿嘧啶, 而甲基化胞嘧啶则不变. 据此, 在基因启动子区富含CpG二核苷酸处设计甲基化和非甲基化特异性引物, 对所测基因的同一核苷酸序列进行PCR扩增, 扩增产物经凝胶电泳后进行结果分析. 此法克服了以往甲基化状态研究方法的局限性, 即使肿瘤组织中混有正常组织也不完全影响结果. 因此, MSP法是一种简单、敏感、高效的方法.
本文通过对56例大肠癌和42例腺瘤标本检测分析p16、cyclinD1和Rb基因的甲基化状态, 探讨基因甲基化与基因失活和蛋白表达异常的关系及其机制. 结果表明p16基因高甲基化和cyclinD1基因低甲基化可能是p16失活和cyclinD1高表达的主要机制, 不但对大肠癌早期诊断、恶性程度和预后判断有重要性, 可能还具有防治作用. Rb基因甲基化与蛋白表达无相关性, 因而Rb基因的失活机制尚有待进一步研究. 本文有临床参考价值.
电编: 张敏 编辑:潘伯荣
| 1. | Gatenby RA, Vincent TL. An evolutionary model of carcinogenesis. Cancer Res. 2003;63:6212-6220. [PubMed] |
| 2. | Leslie A, Pratt NR, Gillespie K, Sales M, Kernohan NM, Smith G, Wolf CR, Carey FA, Steele RJ. Mutations of APC, K-ras, and p53 are associated with specific chromosomal aberrations in colorectal adenocarcinomas. Cancer Res. 2003;63:4656-4661. [PubMed] |
| 3. | 郑 向奎, 李 春仲, 段 文都, 王 香琢, 王 毅, 郭 剑, 刘 会燕, 李 杰. Rb、p16、CyclinD1基因蛋白在大肠癌中的表达及临床意义. 中国肿瘤临床. 2005;32:1235-1238. |
| 4. | Cui X, Shirai Y, Wakai T, Yokoyama N, Hirano S, Hatakeyama K. Aberrant expression of pRb and p16(INK4), alone or in combination, indicates poor outcome after resection in patients with colorectal carcinoma. Hum Pathol. 2004;35:1189-1195. [PubMed] [DOI] |
| 5. | Carneiro FP, Ramalho LN, Britto-Garcia S, Ribeiro-Silva A, Zucoloto S. Immunohistochemical expre-ssion of p16, p53, and p63 in colorectal adenomas and adenocarcinomas. Dis Colon Rectum. 2006;49:588-594. [PubMed] [DOI] |
| 6. | Tada T, Watanabe T, Kazama S, Kanazawa T, Hata K, Komuro Y, Nagawa H. Reduced p16 expression correlates with lymphatic invasion in colorectal cancers. Hepatogastroenterology. 2003;50:1756-1760. [PubMed] |
| 7. | Kass SU, Landsberger N, Wolffe AP. DNA methyla-tion directs a time-dependent repression of transcri-ption initiation. Curr Biol. 1997;7:157-165. [PubMed] [DOI] |
| 8. | Bender CM, Pao MM, Jones PA. Inhibition of DNA methylation by 5-aza-2'-deoxycytidine suppresses the growth of human tumor cell lines. Cancer Res. 1998;58:95-101. [PubMed] |
| 9. | Gonzalez-Quevedo R, Garcia-Aranda C, Moran A, De Juan C, Sanchez-Pernaute A, Torres A, Diaz-Rubio E, Balibrea JL, Benito M, Iniesta P. Differential impact of p16 inactivation by promoter methylation in non-small cell lung and colorectal cancer: clinical implications. Int J Oncol. 2004;24:349-355. [PubMed] [DOI] |
| 10. | Fang XM, Sun LF, Peng JP, Dong Q, Zheng S. The study of 5-Aza-2'-deoxycytidine on transcription regulation of p16/CDKN2 gene demethylation in RKO human colorectal cell line. Zhonghua Yixue Zazhi. 2003;83:2077-2082. [PubMed] |
| 11. | Dominguez G, Silva J, Garcia JM, Silva JM, Rodrigu-ez R, Munoz C, Chacon I, Sanchez R, Carballido J, Colas A. Prevalence of aberrant methylation of p14ARF over p16INK4a in some human primary tumors. Mutat Res. 2003;530:9-17. [PubMed] [DOI] |
| 14. | Herman JG, Graff JR, Myohanen S, Nelkin BD, Baylin SB. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA. 1996;93:9821-9826. [PubMed] [DOI] |
| 15. | Murillo G, Salti GI, Kosmeder JW 2nd, Pezzuto JM, Mehta RG. Deguelin inhibits the growth of colon cancer cells through the induction of apoptosis and cell cycle arrest. Eur J Cancer. 2002;38:2446-2454. [PubMed] [DOI] |
| 16. | Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677-1684. [PubMed] [DOI] |
| 17. | Park WH, Kim ES, Jung CW, Kim BK, Lee YY. Monensin-mediated growth inhibition of SNU-C1 colon cancer cells via cell cycle arrest and apoptosis. Int J Oncol. 2003;22:377-382. [PubMed] [DOI] |
| 18. | Theocharis S, Kouraklis G, Margeli A, Agapitos E, Ninos S, Karatzas G, Koutselinis A. Glucocorticoid receptor (GR) immunohistochemical expression is correlated with cell cycle-related molecules in human colon cancer. Dig Dis Sci. 2003;48:1745-1750. [PubMed] [DOI] |
| 19. | Marx J. A challenge to p16 gene as a major tumor suppressor. Science. 1994;264:1846. [PubMed] [DOI] |
| 20. | Tam SW, Shay JW, Pagano M. Differential expres-sion and cell cycle regulation of the cyclin-depen-dent kinase 4 inhibitor p16Ink4. Cancer Res. 1994;54:5816-5820. [PubMed] |
| 21. | Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323-330. [PubMed] [DOI] |
| 22. | Hinds PW, Weinberg RA. Tumor suppressor genes. Curr Opin Genet Dev. 1994;4:135-141. [PubMed] [DOI] |
| 23. | Dowdy SF, Hinds PW, Louie K, Reed SI, Arnold A, Weinberg RA. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993;73:499-511. [PubMed] [DOI] |
| 24. | Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366-374. [PubMed] [DOI] |
| 25. | Borges HL, Bird J, Wasson K, Cardiff RD, Varki N, Eckmann L, Wang JY. Tumor promotion by caspase-resistant retinoblastoma protein. Proc Natl Acad Sci USA. 2005;102:15587-15592. [PubMed] [DOI] |
| 26. | Norrie MW, Hawkins NJ, Todd AV, Meagher AP, O'Connor TW, Ward RL. Inactivation of p16INK4a by CpG hypermethylation is not a frequent event in colorectal cancer. J Surg Oncol. 2003;84:143-150. [PubMed] [DOI] |
| 27. | Zhao P, Hu YC, Talbot IC. Expressing patterns of p16 and CDK4 correlated to prognosis in colorectal carcinoma. World J Gastroenterol. 2003;9:2202-2206. [PubMed] [DOI] |