修回日期: 2006-04-09
接受日期: 2006-04-13
在线出版日期: 2006-05-28
目的: 确认hIL-10基因修饰的L02肝细胞的克隆培养可实现hIL-10在L02肝细胞中的高效表达.
方法: 通过构建真核质粒表达载体pchIL-10, 并纯化后转染L02肝细胞. 通过G418的压力选择获得hIL-10高表达的克隆株, 并以ELISA测定其表达水平.
结果: 经过测序和酶切验证, 真核质粒表达载体pchIL-10构建成功. 电泳显示一长约540 bp条带. hIL-10基因转导可在L02肝细胞中实现hIL-10的高效表达. 最高表达株表达量为每小时69.875 ng/106细胞.
结论: hIL-10基因修饰的L02肝细胞的克隆培养可实现hIL-10的高效表达, 为抗肝纤维化、肝硬化提供有效途径.
引文著录: 代文杰, 胡震, 武林枫, 姜洪池, 吴耀华, 潘尚哈. hIL-10基因修饰的L02肝细胞的克隆培养和最高表达株的筛选. 世界华人消化杂志 2006; 14(15): 1458-1461
Revised: April 9, 2006
Accepted: April 13, 2006
Published online: May 28, 2006
AIM: To clone and culture human interleukin-10 (IL-10) gene modified L02 hepatocytes and select the cell strain highly expressing IL-10.
METHODS: With preparation of previously constructed and purified eukaryotic expression plasmid vector pchIL-10, L02 hepatocytes were transfected and then the positive clones were selected with the help of G418 pressure. Enzyme-linked immunosorbent assay (ELISA) was used to detect the expression of human IL-10 in the cells strain.
RESULTS: Sequencing and restriction endonuclease digestion confirmed that eukaryotic expression plasmid vector pchIL-10 was constructed successfully, and electrophoresis show a band of 540 bp. hIL-10 gene was highly expressed in L02 hepatocytes and the highest expression level was 69.875 ng/106 cell per hour.
CONCLUSION: Human interleukin-10 (IL-10) gene modified L02 hepatocytes can highly expresses hIl-10, which may be used in the antifibrotic or cirrhotic treatment.
- Citation: Dai WJ, Hu Z, Wu LF, Jiang HC, Wu YH, Pan SH. Clone culture of human interleukin-10 gene modified L02 hepatocytes and selection of cell strain with most interleukin-10 expression. Shijie Huaren Xiaohua Zazhi 2006; 14(15): 1458-1461
- URL: https://www.wjgnet.com/1009-3079/full/v14/i15/1458.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i15.1458
人白细胞介素-10(hIL-10)是肝纤维化、肝硬化进程中的重要调控因子, 具有潜在的抗纤维化的作用. 本研究通过构建真核质粒表达载体, 并纯化后感染L02肝细胞, 观察hIL-10的表达. 从而为肝纤维化和肝硬化的基因治疗提供有效途径.
真核表达载体pcDNA3.1(+)由黑龙江省肝脾外科中心实验部保存, 其全长5428 bp, 含CMV(巨细胞病毒)启动子(232-819). 其BglⅡ(A/GATCT)酶切位点为12, SmaⅠ(CCC/GGG)位点为2077, EcoRⅠ(G/AATTC)位点为952, BamHⅠ(G/GATCC)位点为929. L02肝细胞是永生化人肝细胞系, 由武汉大学中国典型培养物保藏中心提供.
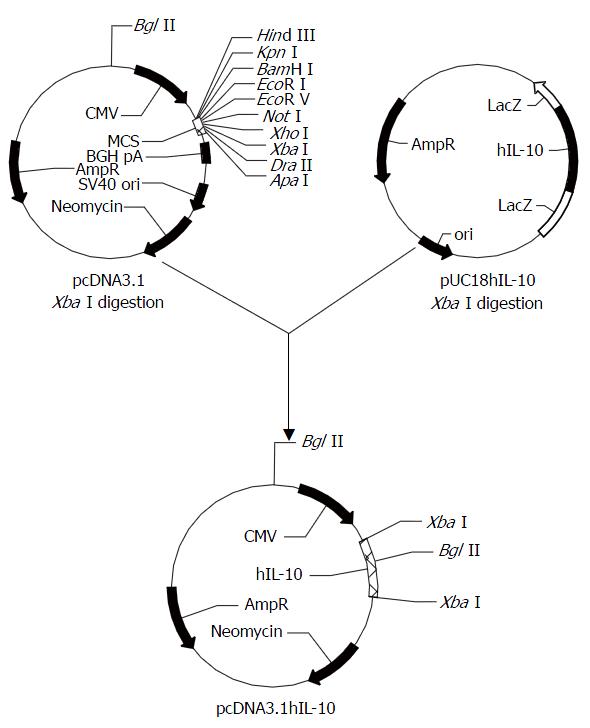
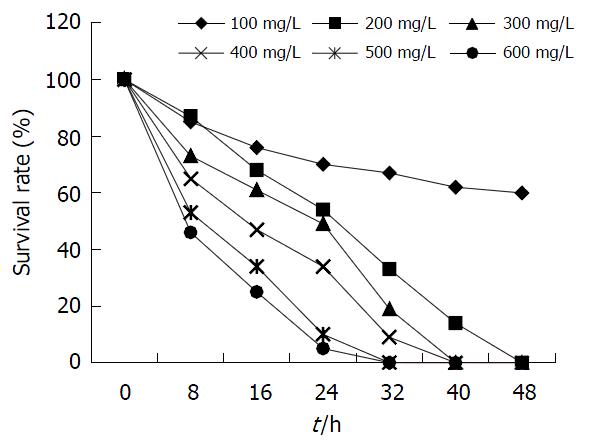
1.2.1 pcDNA3.1hIL-10真核表达载体的构建(图1): pUC18hIL-10和pcDNA3.1质粒的XbaⅠ酶切: 两种质粒载体, 分别取3 μg和1 μg, 总体系20 μL, 含5×酶切反应缓冲液4 μL, XbaⅠ 2 U, 加ddH2O至20 μL, 37 ℃反应2 h. pcDNA3.1/XbaⅠ继续以CIAP进行去磷酸化反应. 10 g/L琼脂糖凝胶电泳, 切胶后以Boehringer-Mannheim公司玻璃乳胶回收试剂盒回收目的片段. 14 ℃连接反应16 h后, 转化感受态大肠杆菌JM109. 挑取生长良好的菌落克隆行增菌培养, 对正向插入克隆进行测序分析[1]. 以Promega公司Wizard PureFection纯化转染质粒. 测序引物为T7启动子通用引物. 构建的pcDNA3.1 hIL-10以下简写为pchIL-10. 脂质体转染前24-36 h, 将L02肝细胞重新传代, 调整细胞数量, 使在转染时细胞贴壁生长状态良好, 达60%-70%汇合. 传代及转染时均使用无双抗的RPMI 1640培养液. 按LipofectAMINE Plus说明制备DNA-Plus-LipofectAMINE混合物并转染: 混合物500 μL加入培养瓶的2 μL RPMI 1640中, 轻轻混匀, 37 ℃, 50 mL/L CO2条件下培养3 h. 3 h后追加3-4 mL含100 mL/L胎牛血清的完全RPMI 1640培养基, 转染24 h后更换培养液为正常完全培养基继续培养. L02肝细胞正常传代后, 以含不同G418浓度的完全RPMI 1640培养, 绘制生长曲线, 以8 h内使L02肝细胞完全死亡的最低浓度作为筛选G418抗性阳性转染克隆的应用浓度. G418毒性试验的药物浓度依次为600, 500, 400, 300, 200, 100 mg/L. 转染后72 h将RPMI 1640培养液更换为含高浓度G418的完全RPMI 1640培养液.
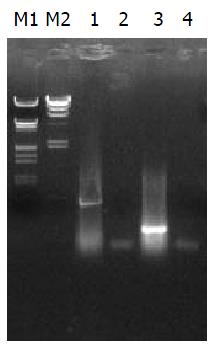
1.2.2 hIL-10的转录: 转染72 h与转染2 wk后, 收集细胞, 以GIBCO BRL TRIZOL法提取总RNA和DNA. PCR检测hIL-10在L02肝细胞中表达: hIL-10扩增引物为CMVP/hIL-10XP2, 循环条件: 94 ℃孵育5 min, 94 ℃变性40 s, 56 ℃退火50 s, 72 ℃延伸1 min, 共35个循环, 最后72 ℃延伸5 min. RT-PCR检测hIL-10在L02肝细胞中的转录: hIL-10扩增引物为hIL-10XP1/hIL-10XP2. 以hβ-actin为内对照, 扩增片段长度为232 bp(表1). λDNA/EcoRⅠ+Hind Ⅲ, λDNA/Hind Ⅲ及hALR为标记(380 bp).
| 引物 | 序列 |
| CMVP | 5'-GCTCGTTTAGTGAACCGT-3' |
| hIL-10×P1 | 5'-GCTCTAGAATGCACAGCTCAGCACTGCT-3' |
| hIL-10×P2 | 5'-GCTCTAGAGTCTCAGTTTCGTATCTTCAT-3' |
| hβ-actin1 | 5'-CATTGTGATGGACTCCGGAGACGG-3' |
| hβ-actin2 | 5'-CATCTCCTGCTCGAAGTCTAGAGC-3' |
1.2.3 ELISA法检测hIL-10的表达: 转染24 h后, 开始收集50 mL培养瓶中培养液行ELISA测定, 分别于1, 2, 3, 4, 5, 6, 7, 14, 21, 28 d取样测定. 最高表达株的筛选: 转染24 h后, 将细胞按有限稀释法单克隆至96孔培养板内, 待贴壁生长良好后, 以含400 mg/L G418的RPMI 1640作筛选. 待细胞生长为70%-90%汇合后, 收集新更换的24 h培养上清, 按hIL-10 ELISA检测说明书检测hIL-10含量, 同时计数相应孔别的细胞数.
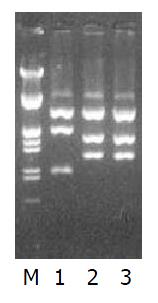
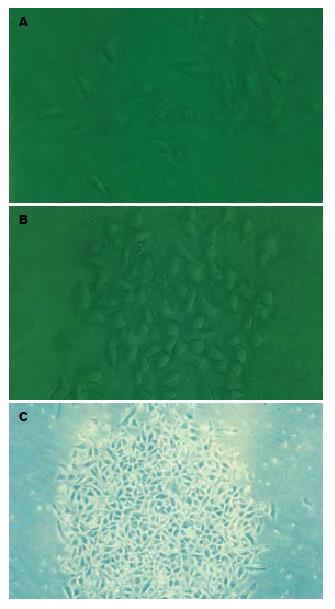
pchIL-10以XbaⅠ, BglⅡ酶切鉴定插入片段方向: BglⅡ酶切正向插入片段为1105 bp, 4860 bp, 反向插入片段为1390 bp和4575 bp(图2). 测序结果显示hIL-10 ORF完全与前述结果吻合[1]. 每隔8 h计数活细胞数, 绘制48 h存活率曲线(图3), 确定48 h内使培养L02肝细胞完全死亡的最低G418浓度为200 mg/L. L02肝细胞的克隆生长见图4.
电泳后出现约540 bp (hIL-10), 约380 bp (hALR)和232 bp (hβ-actin)电泳条带(图5).
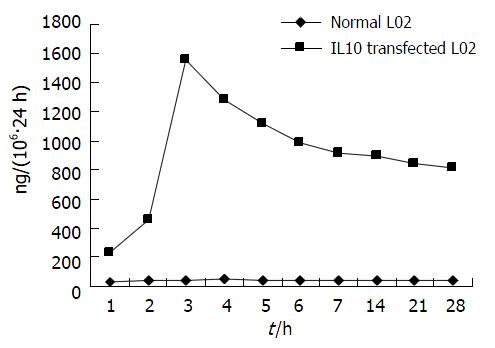
hIL-10浓度-时间曲线(图6). hIL-10最高表达株的筛选至96孔板内L02肝细胞克隆生长旺盛, 达80%-90%汇合时测定上清中hIL-10, 最高表达株表达量为69.875 ng/(106·h).
人白细胞介素-10基因定位于1号染色体, 为单拷贝基因, 全长8868 bp, 其mRNA为1.8 kb, 开放阅读框架为534 bp, 包括编码N-末端信号肽(18个)的序列. 研究证实hIL-10 ORF与EB病毒ORF有70%同源性[2], 并与马疱疹病毒亦有同源性, 推测EB病毒和马疱疹毒因具有哺乳动物IL-10同源序列, 在宿主细胞内可合成IL-10类似物, 抑制巨噬细胞和T细胞而逃避宿主抗病毒免疫. hIL-10蛋白质分子量约Mr 18 700, 活性形式常为二硫键形成的二聚体, 其中含有两个N-糖基化位点, 但无糖链. 另有3个半胱氨酸残基和9个甲硫氨酸残基. 本研究克隆的人IL-10开放阅读框架与GenBank登记序列完全吻合.
现已证实IL-10具有多种生物学功能, 参与了多种疾病的病理生理调节进程[3-18]. 作为炎症过程的负性调节因子, 其抑制功能主要表现在: 抑制Th1类细胞因子如IL-2、IL-1-β、IFN-γ、TNF-α等的合成及活性, 抑制单核细胞表面MHC Ⅱ类分子HLA-DR/DP及DQ的表达, 降低单核细胞的抗原提呈能力, 阻断抗原特异性的单核巨噬细胞因子如IL-2、IL-8和TNF-α的产生. 下调ICAM-1等黏附分子及核转录因子、核因子kB的表达, 正向调节IL-1R拮抗剂的表达. 抑制NK细胞的活性, 抑制单核细胞依赖性Th细胞的增生, 抑制反应性氮氧化物产生等.
大量证据表明IL-10参与了肝纤维化、肝硬化过程, 并具有重要的负性调控功能. 因IL-10具有强大的抑制炎症和巨噬细胞活性的功能, 具有潜在的抗肝纤维化、抗肝硬化能力而备受关注[3-5,9-10,16-18]. 以基因转导或基因修饰细胞移植方式将IL-10导入纤维化或硬化肝脏将成为治疗肝纤维化、肝硬化非常有前途的手段.
IL-10是Th2细胞因子的重要代表, 大量研究证实在肝硬化进程中发挥负性调节作用, 是肝硬化治疗中具有潜力的细胞因子.
采用IL-10基因转导方式是肝硬化基因治疗的重要方案之一. 本研究旨在克隆获取hIL-10并验证其在肝细胞中的表达.
Louis et al证实IL-10在肝纤维化肝硬化过程中发挥负性调节作用, 具有潜在抗肝硬化功能.
本文制备IL-10真核表达载体并将IL-10转导L02肝细胞后筛选高表达单克隆并计划扩增培养, 并应用于肝硬化的治疗, 以期为肝硬化基因治疗提供可选方案.
本研究有助于开发实用性IL-10抗肝硬化治疗产品.
本文通过构建表达载体、体外转染的方法研究hIL-10在L02肝细胞中的表达, 实验方法合理, 为肝硬化的治疗提供修饰化细胞, 具有一定临床实用意义.
电编: 张敏 编辑:潘伯荣
| 2. | Moore KW, Vieira P, Fiorentino DF, Trounstine ML, Khan TA, Mosmann TR. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science. 1990;248:1230-1234. [PubMed] [DOI] |
| 3. | Howard M, O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992;13:198-200. [PubMed] [DOI] |
| 4. | Thompson K, Maltby J, Fallowfield J, McAulay M, Millward-Sadler H, Sheron N. Interleukin-10 expression and function in experimental murine liver inflammation and fibrosis. Hepatology. 1998;28:1597-1606. [PubMed] [DOI] |
| 5. | Louis H, Van Laethem JL, Wu W, Quertinmont E, Degraef C, Van den Berg K, Demols A, Goldman M, Le Moine O, Geerts A. Interleukin-10 controls neutrophilic infiltration, hepatocyte proliferation, and liver fibrosis induced by carbon tetrachloride in mice. Hepatology. 1998;28:1607-1615. [PubMed] [DOI] |
| 6. | Wang SC, Ohata M, Schrum L, Rippe RA, Tsuka-moto H. Expression of interleukin-10 by in vitro and in vivo activated hepatic stellate cells. J Biol Chem. 1998;273:302-308. [PubMed] [DOI] |
| 7. | Thompson KC, Trowern A, Fowell A, Marathe M, Haycock C, Arthur MJ, Sheron N. Primary rat and mouse hepatic stellate cells express the macrophage inhibitor cytokine interleukin-10 during the course of activation In vitro. Hepatology. 1998;28:1518-1524. [PubMed] [DOI] |
| 8. | Rai RM, Loffreda S, Karp CL, Yang SQ, Lin HZ, Diehl AM. Kupffer cell depletion abolishes induction of interleukin-10 and permits sustained overexpression of tumor necrosis factor alpha messenger RNA in the regenerating rat liver. Hepatology. 1997;25:889-895. [PubMed] [DOI] |
| 9. | Meijer C, Wiezer MJ, Diehl AM, Schouten HJ, Schouten HJ, Meijer S, van Rooijen N, van Lambal-gen AA, Dijkstra CD, van Leeuwen PA. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20:66-77. [PubMed] [DOI] |
| 10. | Le Moine O, Marchant A, De Groote D, Azar C, Goldman M, Deviere J. Role of defective monocyte interleukin-10 release in tumor necrosis factor-alpha overproduction in alcoholics cirrhosis. Hepatology. 1995;22:1436-1439. [PubMed] [DOI] |
| 11. | Reitamo S, Remitz A, Tamai K, Uitto J. Interleu-kin-10 modulates type I collagen and matrix metall-oprotease gene expression in cultured human skin fibroblasts. J Clin Invest. 1994;94:2489-2492. [PubMed] [DOI] |
| 12. | Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM. IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest. 1995;96:2304-2310. [PubMed] [DOI] |
| 13. | Reitamo S, Remitz A, Tamai K, Ledo I, Uitto J. Interleukin 10 up-regulates elastin gene expression in vivo and in vitro at the transcriptional level. Biochem J. 1994;302:331-333. [PubMed] [DOI] |
| 14. | Van Vlasselaer P, Borremans B, van Gorp U, Dasch JR, De Waal-Malefyt R. Interleukin 10 inhibits transforming growth factor-beta (TGF-beta) synthe-sis required for osteogenic commitment of mouse bone marrow cells. J Cell Biol. 1994;124:569-577. [PubMed] [DOI] |
| 15. | Ertel W, Keel M, Steckholzer U, Ungethum U, Trentz O. Interleukin-10 attenuates the release of proinflammatory cytokines but depresses spleno-cyte functions in murine endotoxemia. Arch Surg. 1996;131:51-56. [PubMed] [DOI] |
| 16. | Zou XM, Yagihashi A, Hirata K, Tsuruma T, Matsuno T, Tarumi K, Asanuma K, Watanabe N. Downregu-lation of cytokine-induced neutrophil chemoattrac-tant and prolongation of rat liver allograft survival by interleukin-10. Surg Today. 1998;28:184-191. [PubMed] [DOI] |
| 17. | Louis H, Le Moine O, Peny MO, Quertinmont E, Fokan D, Goldman M, Deviere J. Production and role of interleukin-10 in concanavalin A-induced hepatitis in mice. Hepatology. 1997;25:1382-1389. [PubMed] [DOI] |
| 18. | Louis H, Le Moine A, Quertinmont E, Peny MO, Geerts A, Goldman M, Le Moine O, Deviere J. Repeated concanavalin A challenge in mice induces an interleukin 10-producing phenotype and liver fibrosis. Hepatology. 2000;31:381-390. [PubMed] [DOI] |