修回日期: 2006-01-11
接受日期: 2006-01-25
在线出版日期: 2006-04-18
目的: 构建TRF2小干扰RNA载体, 观察其在胃癌细胞中的表达.
方法: 根据pSilencer3.1-H1载体要求设计两对小干扰RNA, 退火后连接入载体相应位点. 经双酶切及测序鉴定后分别转染多药耐药衍生胃癌细胞SGC7901/ADR和SGC7901/VCR. G418选择培养液培养2 mo选取转染组及对照组细胞克隆. MTT法检测转染对细胞生长增殖速度的影响. 细胞用阿霉素和足叶乙甙处理6 h后Western blot法检测TRF2表达.
结果: 成功构建TRF2小干扰RNA载体, 建立稳定转染细胞株, 转染后TRF2表达明显降低, 对细胞的生长增殖速度无明显影响(P>0.05).
结论: 成功构建TRF2小干扰RNA载体及稳定转染细胞株.
引文著录: 宁寒冰, 李继昌, 刘志国, 樊代明. TRF2小干扰RNA载体的构建及表达. 世界华人消化杂志 2006; 14(11): 1044-1047
Revised: January 11, 2006
Accepted: January 25, 2006
Published online: April 18, 2006
AIM: To construct telomeric repeat binding factor 2 (TRF2) small interfering RNA (siRNA) eukaryotic expression vector and evaluate its expression in gastric cancer cells.
METHODS: Two pairs of hairpin siRNA template oligonucleotides for TRF2 based on pSilencer3.1-H1 vector were designed using the manufacturer's web-based tool. These oligonucleotides were annealed and ligated into vector respectively. After confirmation by double enzyme digestion analysis and DNA sequencing, positive recombinant plasmids were transfected into multidrug-resistant gastric cancer cells SGC7901/ADR and SGC7901/VCR respectively. Stable cell lines of G418-resistance were acquired after 2-month selection. The effect of transfection on cell growth was analyzed by MTT assay. The expression of TRF2 was detected by Western blot after the cells treated with adriamycin (ADR) or etoposide for 6 h.
RESULTS: We constructed two TRF2 siRNA eukaryotic expression vectors successfully and the transfected cells showed significantly suppressed TRF2 expression. The growth of cells were not significantly influenced by transfection (P > 0.05).
CONCLUSION: The TRF2 siRNA eukaryotic expression vectors are successfully constructed and stably expressed in gastric cancer cells.
- Citation: Ning HB, Li JC, Liu ZG, Fan DM. Construction and expression of telomeric repeat binding factor 2 small interfering RNA eukaryotic vector. Shijie Huaren Xiaohua Zazhi 2006; 14(11): 1044-1047
- URL: https://www.wjgnet.com/1009-3079/full/v14/i11/1044.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v14.i11.1044
端粒重复序列结合因子2(telomeric repeat binding factor 2, TRF2)能够通过维持端粒末端的T环结构, 防止端粒被DNA损伤修复机制检测为DSBs, 维持端粒的正常结构和功能[1]. 最新研究发现, 在非端粒部位TRF2也能够抑制ATM依赖的DNA损伤反应, 是通用的DNA损伤修复因子[2]. 我们在前期研究中发现阿霉素(ADR)和足叶乙甙(etoposide)引起的DNA损伤能使胃癌细胞TRF2的表达增高, 而且在多药耐药衍生胃癌细胞系SGC7901/ADR和SGC7901/VCR中的表达显著高于SGC7901. 为进一步探讨TRF2的功能, 我们构建了TRF2小干扰RNA载体, 并建立了稳定转染细胞株.
RPMI 1640培养基购自GIBCO公司; 抗TRF2抗体购自Upstate公司; etoposide和盐酸阿霉素为Sigma公司产品; pSilencer3.1-H1载体购自Ambion公司; T4 DNA连接酶、BamH Ⅰ、Hind Ⅲ等限制性内切酶购于TOYOBO公司; 脂质体LipofectamineTM2000为Invitrogen公司产品. 人胃癌细胞系SGC7901, 耐长春新碱(VCR)的胃癌细胞系SGC7901/VCR和耐阿霉素(ADR)的胃癌细胞系SGC7901/ADR均由第四军医大学全军消化病研究所诱导, 保存.
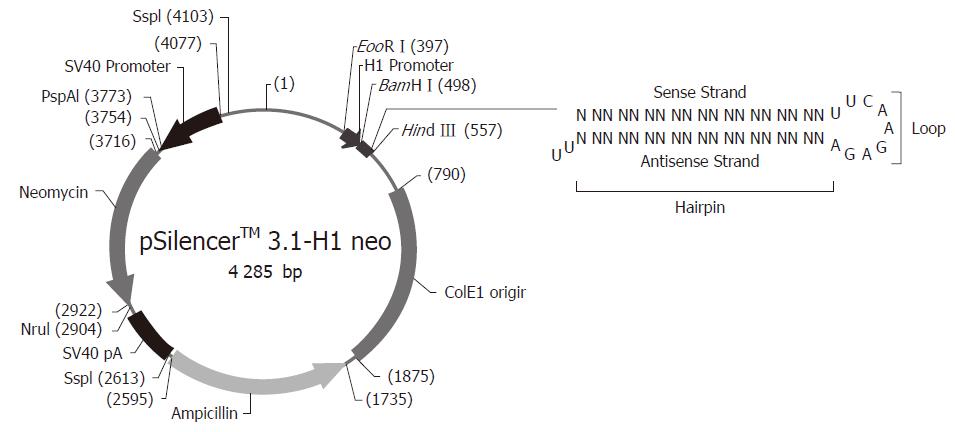
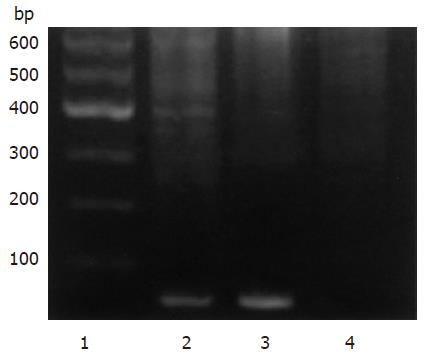
根据pSilencer3.1-H1载体要求和Ambion公司提供的网上设计软件设计两对小干扰RNA. 具体设计如下: Oligo-1: 5'-GATCCGTCCAACCACCATTGGATTCAAGAGATCCAATGGTGGTTGGAGGATTTTTTGGAAA-3'和5'-AGCTTTTCCAAAAAATCCTCCAACCACCATTGGATCTCTTGAATCCAATGGTGGTTGGAGGACG-3'; Oligo-2: 5'-GATCCGAACAAGCGCATGACAATATTCAAGAGATATTGTCATGCGCTTGTTCTTTTTTGGAAA-3'和5'-AGCTTTTCCAAAAAAGAACAAGCGCATGACAATATCTCTTGAATATTGTCATGCGCTTGTTCG-3'. 将Oligo1, Oligo2根据退火条件90 ℃退火3 min, 自然冷却得到退火产物. BamH Ⅰ, Hind Ⅲ双酶切pSilencer3.1-H1载体质粒, 胶回收大片段, 用T4 DNA连接酶连接胶回收片段和退火产物, 16 ℃连接12 h, 连接产物转化E. coli DH5α感受态细菌, 氨苄抗性平板过夜培养, 挑取单克隆. BamH Ⅰ, Hind Ⅲ双酶切后100 g/L SDS-PAGE凝胶电泳产生大小约为60 bp的片段, 表明连接正确. 进一步测序后证实序列完全正确, 质粒分别命名为TRF2-Si1和TRF2-Si2. 根据LipofectamineTM 2000说明书将TRF2-Si1, TRF2-Si2和空载体质粒分别转染SGC7901/VCR和SGC7901/ADR细胞, 转染48 h后改用G418选择培养液, 培养2 mo选取转染组及对照组细胞克隆, 并用Western blot检测转染细胞TRF2表达情况, 对转染细胞进行鉴定. 分别用20 mg/L etoposide或0.4 mg/L ADR处理各胃癌细胞, 在6 h收获细胞, 三去污裂解液提取细胞总蛋白, Bradford法进行蛋白定量. 120 g/L SDS-PAGE凝胶电泳分离蛋白, 将蛋白条带转移至硝酸纤维膜上, 50 g/L脱脂奶粉封闭后分别与TRF2、β-actin抗体及HRP标记的相应二抗孵育, ECL进行显色, Western blot检测蛋白表达. 细胞接种于96孔板, 分别在1, 2, 3, 4, 5, 6, 7, 8 d进行检测. 每孔加入新鲜配制的MTT溶液(5 g/L) 20 μL, 37 ℃培养4 h, 每孔加入DMSO 150 μL, 振荡10 min, 在490 nm波长处测吸光度值, 以时间为横坐标、平均吸光度值为纵坐标, 绘制细胞生长曲线.
统计学处理 采用SPSS13.0统计学软件, 进行ANOVA和GLM repeated measures分析.
根据pSilencer3.1-H1载体要求设计两对小干扰RNA, 与载体连接构建质粒TRF2-Si1和TRF2-Si2(图1). BamH Ⅰ, Hind Ⅲ双酶切鉴定, 产生大小约为60 bp的片段(图2泳道2和3), 表明为正确连接的TRF2-Si1和TRF2-Si2. 进一步测序后证实序列完全正确.
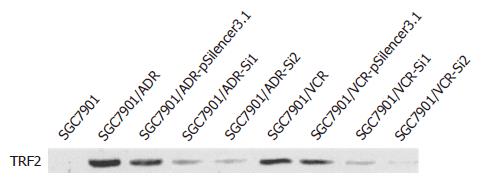
将TRF2-Si1, TRF2-Si2和空载体质粒分别转染SGC7901/VCR和SGC7901/ADR细胞, 经G418选择培养后建立稳定转染细胞系. 用Western blot检测etoposide或ADR处理后TRF2表达, 结果显示, 与空载体转染细胞及未转染对照细胞相比, 转染TRF2-Si1或TRF2-Si2后, 细胞TRF2表达明显降低(图3).
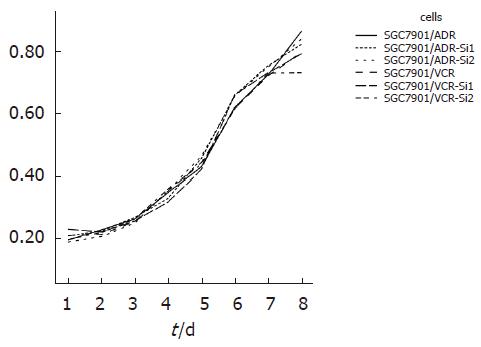
采用MTT法测定细胞生长曲线, 结果显示: 与空载体转染细胞及未转染对照细胞相比, 转染细胞的吸光值无明显改变(P>0.05), 对数生长期均出现在3-8 d(图4). 提示转染对胃癌细胞生长增生速度无明显影响.
胃癌是我国最常见的恶性肿瘤之一, 化疗仍为其主要的治疗手段, 但由于存在耐药性和毒副作用, 效果不尽人意. 因此迫切需要寻求新的治疗途径. 大部分的化疗药物通过损伤DNA发挥作用, 而有效的DNA修复是肿瘤细胞对治疗产生耐受的重要原因[3]. 因此, 抑制DNA修复是提高DNA损伤类治疗方式效果的有效方法, 最近已有一些抑制DNA修复蛋白的药物如MGMT抑制剂, PARP抑制剂, 甲氧胺等进入临床试验[4-6], 在与化疗或放疗的联合使用中显示了良好的前景. 不久的将来, DNA损伤修复抑制很可能成为肿瘤常规治疗的一部分[7].
端粒是位于染色体末端的DNA-蛋白结构, 由TTAGGG重复序列和大量的端粒结合蛋白组成[8]. 由六个端粒结合蛋白TRF1, TRF2, POT1, TIN2, TPP1和Rap1组成的复合体起着保护端粒的作用, 被称为是遮蔽蛋白[9]. 其中端粒重复序列结合因子TRF1和TRF2是两个主要的端粒结合蛋白, 他们通过相互作用来维持端粒的正常结构和功能[10-11]. TRF2在保护端粒中的作用有以下几个方面: 抑制非同源末端连接(nonhomologous end-joining, NHEJ), 防止不同染色体末端的融合[12]; 抑制端粒间的同源重组(homologous recombination, HR)[13]; 防止t-loop插入部位被认为是Holliday junction而被切除[14], 导致端粒的缩短. 关于端粒与肿瘤耐药的关系, 以往的研究集中于对端粒酶活性和端粒长度的检测, 但结论仍然存在争议[15-16]. 最新研究发现化疗药物或射线引起的DNA损伤能使TRF2表达增高, 或同时伴有端粒酶活性增高, 是DNA损伤反应的早期事件[17]. 而且TRF2在DNA损伤部位的聚集早于其他DNA损伤反应因子ATM、Nbs1等; TRF2的过表达能够抑制ATM依赖的DNA损伤反应[18-19]. 在端粒, TRF2与DSBs修复的蛋白如Ku, DNA PKcs, ERCC1/XPC, WRN等有相互作用[20-22]; TRF2还能与ATM直接作用[23]. 因此, TRF2可能是通用的DNA损伤修复因子, 而不仅仅是端粒结合因子[2]. 随着端粒酶和TRF2与DNA损伤修复关系的发现, 关于端粒功能的研究变得更加广泛, 也提示我们端粒可能通过干扰DNA损伤反应参与肿瘤耐药的发生.
ADR和etoposide是临床常用的肿瘤化疗药物, 主要作用机制为抑制DNA拓扑异构酶, 导致DNA双链断裂[24]. SGC7901/VCR和SGC7901/ADR是由SGC7901诱导的多药耐药衍生胃癌细胞系[25-26], 我们在前期研究中发现ADR和etoposide能使胃癌细胞TRF2的表达增高, 而且在SGC7901/ADR和SGC7901/VCR中的表达显著高于SGC7901. 此次实验, 我们设计了两个TRF2 siRNA真核表达载体, 分别转染SGC7901/ADR和SGC7901/VCR细胞建立稳定转染细胞株. 转染后均可明显抑制TRF2表达, 并对细胞的生长无明显影响. 本实验为进一步探索TRF2的功能及其与胃癌耐药的关系提供了材料和条件.
端粒及端粒相关因子与DNA损伤的关系是端粒功能研究的一个热点, 而DNA损伤修复抑制也正在成为肿瘤治疗的新的靶点. 我们在前期研究中发现, DNA损伤类化疗药可以诱导胃癌耐药细胞TRF2的过表达, 提示TRF2可能参与胃癌耐药的形成.
本研究中构建了TRF2siRNA载体, 并建立了稳定转染细胞株, 为进一步探索TRF2的功能及其与胃癌耐药的关系提供了材料和条件.
编辑: 潘伯荣 电编:张敏
| 1. | Houghtaling BR, Cuttonaro L, Chang W, Smith S. A dynamic molecular link between the telomere length regulator TRF1 and the chromosome end protector TRF2. Curr Biol. 2004;14:1621-1631. [PubMed] |
| 2. | Wright WE, Shay JW. Telomere-binding factors and general DNA repair. Nat Genet. 2005;37:116-118. [PubMed] |
| 3. | Gatti L, Zunino F. Overview of tumor cell chemore-sistance mechanisms. Methods Mol Med. 2005;111:127-148. [PubMed] |
| 4. | Tserng KY, Ingalls ST, Boczko EM, Spiro TP, Li X, Majka S, Gerson SL, Willson JK, Hoppel CL. Pharmacokinetics of O6-benzylguanine (NSC637037) and its metabolite, 8-oxo-O6-benzylguanine. J Clin Pharmacol. 2003;43:881-893. [PubMed] |
| 5. | Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265-7279. [PubMed] |
| 6. | Liu L, Gerson SL. Therapeutic impact of methoxya-mine: blocking repair of abasic sites in the base excision repair pathway. Curr Opin Investig Drugs. 2004;5:623-627. [PubMed] |
| 7. | Madhusudan S, Middleton MR. The emerging role of DNA repair proteins as predictive, prognostic and therapeutic targets in cancer. Cancer Treat Rev. 2005;31:603-617. [PubMed] |
| 8. | Liu D, O'Connor MS, Qin J, Songyang Z. Telosome, a mammalian telomere-associated complex formed by multiple telomeric proteins. J Biol Chem. 2004;279:51338-51342. [PubMed] |
| 9. | de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100-2110. [PubMed] |
| 10. | Ye JZ, Hockemeyer D, Krutchinsky AN, Loayza D, Hooper SM, Chait BT, de Lange T. POT1-interacting protein PIP1: a telomere length regulator that rec-ruits POT1 to the TIN2/TRF1 complex. Genes Dev. 2004;18:1649-1654. [PubMed] |
| 11. | Court R, Chapman L, Fairall L, Rhodes D. How the human telomeric proteins TRF1 and TRF2 recognize telomeric DNA: a view from high-resolution crystal structures. EMBO Rep. 2005;6:39-45. [PubMed] |
| 12. | van Steensel B, Smogorzewska A, de Lange T. TRF2 protects human telomeres from end-to-end fusions. Cell. 1998;92:401-413. [PubMed] |
| 13. | Wang RC, Smogorzewska A, de Lange T. Homolog-ous recombination generates T-loop-sized deletions at human telomeres. Cell. 2004;119:355-368. [PubMed] |
| 14. | Steinert S, Shay JW, Wright WE. Modification of subtelomeric DNA. Mol Cell Biol. 2004;24:4571-4580. [PubMed] |
| 15. | Kuranaga N, Shinomiya N, Mochizuki H. Long-term cultivation of colorectal carcinoma cells with anti-cancer drugs induces drug resistance and telomere elongation: an in vitro study. BMC Cancer. 2001;1:10. [PubMed] |
| 16. | Siva AC, Raval-Fernandes S, Stephen AG, LaFe-mina MJ, Scheper RJ, Kickhoefer VA, Rome LH. Up-regulation of vaults may be necessary but not sufficient for multidrug resistance. Int J Cancer. 2001;92:195-202. [PubMed] |
| 17. | Klapper W, Qian W, Schulte C, Parwaresch R. DNA damage transiently increases TRF2 mRNA expression and telomerase activity. Leukemia. 2003;17:2007-2015. [PubMed] |
| 18. | Bradshaw PS, Stavropoulos DJ, Meyn MS. Human telomeric protein TRF2 associates with genomic double-strand breaks as an early response to DNA damage. Nat Genet. 2005;37:193-197. [PubMed] |
| 19. | Karlseder J, Broccoli D, Dai Y, Hardy S, de Lange T. p53- and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science. 1999;283:1321-1325. [PubMed] |
| 20. | Zhu XD, Kuster B, Mann M, Petrini JH, de Lange T. Cell-cycle-regulated association of RAD50/MRE11/NBS1 with TRF2 and human telomeres. Nat Genet. 2000;25:347-352. [PubMed] |
| 21. | Song K, Jung D, Jung Y, Lee SG, Lee I. Interaction of human Ku70 with TRF2. FEBS Lett. 2000;481:81-85. [PubMed] |
| 22. | Opresko PL, von Kobbe C, Laine JP, Harrigan J, Hickson ID, Bohr VA. Telomere-binding protein TRF2 binds to and stimulates the Werner and Bloom syndrome helicases. J Biol Chem. 2002;277:41110-41119. [PubMed] |
| 23. | Karlseder J, Hoke K, Mirzoeva OK, Bakkenist C, Kastan MB, Petrini JH, de Lange T. The telomeric protein TRF2 binds the ATM kinase and can inhibit the ATM-dependent DNA damage response. PLoS Biol. 2004;2:E240. [PubMed] |
| 24. | Stavrovskaya AA. Cellular mechanisms of multi-drug resistance of tumor cells. Biochemistry. 2000;65:95-106. [PubMed] |
| 25. | Shen H, Pan Y, Han Z, Hong L, Liu N, Han S, Yao L, Xie H, Zhaxi C, Shi Y. Reversal of multidrug resistance of gastric cancer cells by downregulation of TSG101 with TSG101siRNA. Cancer Biol Ther. 2004;3:561-565. [PubMed] |
| 26. | Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, Liu N, Qiao T, Fan D. Overexpression and significance of prion protein in gastric cancer and multidrug-resistant gastric carcinoma cell line SGC7901/ADR. Int J Cancer. 2005;113:213-220. [PubMed] |