修回日期: 2004-01-28
接受日期: 2004-02-01
在线出版日期: 2005-05-01
目的: 研究原发性肝癌survivin基因表达及治疗靶点作用.
方法: 收集肝癌和癌旁组织40例. Western blot法检测survivin蛋白表达, RT-PCR法检测survivin mRNA. 原位末端标记法检测组织凋亡指数. 设计合成survivin反义寡核苷酸(antisense oligonucleotide, AS ODN)转染hepG2细胞, 设置空白对照组、脂质体组、正义链组和各浓度AS ODN组. 流式细胞术检测凋亡指数, MTT法检测化疗药物对细胞生长抑制率. 建立原位荷肝癌鼠模型并分为3组: 反义survivin链组、空白对照组及脂质体组. V = ab2/2计算肿瘤体积, 以V治疗后/V治疗前×100%计算生长指数.
结果: 肝癌细胞株和34/40例肝癌组织表达survivin蛋白及mRNA, 癌旁组织无survivin表达. 肝内转移组(93.5%)和门静脉癌栓组(92.8%)survivin蛋白表达阳性率显著高于阴性组(55.6%)和无癌栓组(66.7%)(P < 0.05). 肝内转移组(1.105% ± 0.396)和门静脉癌栓组(1.137% ± 0.404)survivin mRNA水平显著高于阴性组(0.572% ± 0.082)和无癌栓组(0.627% ± 0.122)(P < 0.05). Survivin阳性组织凋亡指数为1.15% ± 0.33%, 显著低于阴性组(4.50% ± 0.83%)(P = 0.00 002). AS ODN组survivin表达减弱, 凋亡指数增高, 200 nmol/L组为10.50% ± 0.76%, 400 nmol/L组为12.99% ± 0.42%, 600 nmol/L组为22.21% ± 1.26%, 明显高于对照组(P < 0.05). 化疗药物对AS ODN组生长抑制率增高, 200 nmol/L组为47.7% ± 4.6%(5-FU)、59.7% ± 3.5%(DDP), 400 nmol/L组为56.8% ± 4.0%(5-FU)、68.2% ± 2.1%(DDP), 600 nmol/L组为85.2% ± 2.3%(5-FU)、79.9% ± 3.7%(DDP), 明显高于对照组(P < 0.05).23/25裸鼠成功建立原位荷肝癌动物模型. AS ODN组肿瘤生长指数为51.0% ± 24.2%, 显著低于空白对照组(70.2% ± 23.4%)和脂质体组(168.0% ± 28.6%)(P < 0.05). AS ODN组凋亡指数为21.97% ± 2.11%, 显著高于空白对照组(3.21% ± 0.57%)和脂质体组(3.07% ± 0.81%)(P < 0.05).
结论: survivin在HCC发生中有重要作用. survivin AS ODN转染能下调survivin表达, 诱导细胞凋亡, 增加HCC细胞对化疗药物敏感性. survivin AS ODN对荷HCC鼠有一定治疗作用.
引文著录: 王颖, 王家马龙, 吴亮. 原发性肝癌survivin基因表达及靶向survivin治疗. 世界华人消化杂志 2005; 13(9): 1082-1088
Revised: January 28, 2004
Accepted: February 1, 2004
Published online: May 1, 2005
AIM: To investigate the role of survivin in the pathogenesis of hepatocelullar carcinoma (HCC), and to evaluate the therapeutic effect of antisense oligonucleotide (AS ODN) targeting surviving gene.
METHODS: Forty HCC specimens and their neighboring noncancerous tissues were obtained. Western blot were used to detect survivin protein. Semi-quantitative RT-PCR was used to detect survivin mRNA. TUNEL was employed to examine the apoptosis index (AI). Survivin AS ODN was designed and synthesized. HepG2 cells were divided into 6 groups: control group, lipofectin group, ODN group, and 200, 400 and 600 nmol/L AS ODN groups. AI was examined by flow cytometry. Rate of inhibition (IR) by chemotherapeutic drugs (5-Fu and DDP) was determined by the colorimetric MTT cell viability/proliferation assay. Orthotopic implant model was developed in BALB/nu/nu nude mouse. Tumor-take nude mice were divided into 3 groups: control group, lipofectin group, and AS ODN group. The size of tumor in situ was examined and the growth index (GI) was calculated.
RESULTS: Survivin protein and mRNA were detected in 2 HCC cell lines and 34 out of 40 patients, but not in nontumor tissues. The positive rate in patients with intrahepatic metastasis was 93.5%, higher than those without intrahepatic metastasis (55.6%, P < 0.05). The positive rate in the cases with portal vein invasion was 92.8%, higher than those without portal vein invasion (66.7%, P < 0.05). The levels of survivin mRNA in patients with intrahepatic metastasis (1.105% ± 0.396) or portal vein invasion (1.137% ± 0.404) were significantly higher than those without intrahepatic metastasis (0.572% ± 0.082) or portal vein invasion (0.627% ± 0.122)(P < 0.05). Survivin expression was significantly associated with reduced apoptosis index (1.15% ± 0.33% vs 4.50% ± 0.83%, P < 0.05). Expression of survivin in AS ODN groups was significantly decreased than that in the control group. The AI of AS ODN group was significantly higher than that of other groups. The AI were 10.500.76%, 12.99% ± 0.42%, and 22.21% ± 1.26% (P < 0.05)in the 200, 400, 600 nmol/L AS ODN group. The IR of chemotherapeutic drugs in AS ODN groups was higher than that of other groups. The IR of 200 nmol/L was 47.7% ± 4.6% (5-FU), 59.7% ± 3.5% (DDP), 400 nmol/L was 56.8% ± 4.0% (5-FU), 68.2% ± 2.1% (DDP) and 600 nmol/L was 85.2% ± 2.3% (5-FU), 79.9% ± 3.7% (DDP)(P < 0.05). Orthotopic implant model of hepatomacellular was developed in 23 of 25 nude mice. The GI of AS ODN group was 50.96% ± 24.20% , higher than that of other groups (P < 0.05). The AI of AS ODN group was 21.97% ± 2.11%, higher than those of control and lipofectin groups (P < 0.05).
CONCLUSION: Survivin may play an important role in HCC carcinogenesis. AS ODN targeting survivin induces apoptosis and sensitizes HCC cells to chemotherapy. In tumor-bearing nude mouse, survivin AS ODN may inhibit the growth of tumor and induce cell apoptosis.
- Citation: Wang Y, Wang JL, Wu L. Expression of survivin gene in hepatocellular carcinoma and survivin gene targeting therapy. Shijie Huaren Xiaohua Zazhi 2005; 13(9): 1082-1088
- URL: https://www.wjgnet.com/1009-3079/full/v13/i9/1082.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i9.1082
细胞凋亡是机体的重要生理过程[1-4], 细胞凋亡的异常抑制与恶性肿瘤的发生、进展以及耐药性的产生密切相关[5-10]. IAP(inhibitor of apoptosis proteins, 凋亡抑制蛋白)是一族结构、功能相似的凋亡抑制因子, 在凋亡的负性调控中有重要作用[11-15]. Survivin是近年来克隆出的IAP家族新成员, 其在正常情况下仅表达于胚胎组织, 在成熟分化的成人组织中表达消失, 但可再表达于多种恶性转化细胞株和恶性肿瘤组织[16-18], 目前认为其在恶性肿瘤的发生中有重要作用, 在肿瘤的诊断和治疗上具有一定价值. Survivin在原发性肝细胞癌中的表达及生物学意义尚不清楚, 我们检测了人HCC细胞株和组织标本中survivin的表达情况; 并以此为基础合成特异性靶向survivin的反义寡核苷酸(survivin AS ODN), 在细胞和动物水平检测其在体外、内的治疗作用.
收集HCC组织和相应癌旁组织标本40例. 所有标本均取自同济医院外科手术切除的肝脏, 均在术后经组织病理学检查证实. 人肝癌细胞株hepG2和SMMC-7721从同济医学院免疫教研室获得, 在含100 mL/L牛血清的DMEM中培养, 温度为37℃, CO2浓度为50 mL/L. 兔抗人survivin多克隆抗体为Santa Cruz公司产品; Gene Jammer脂质体转染试剂盒为stratagene公司产品; ECL显色试剂盒购自武汉亚法生物工程公司. 合成靶向survivin mRNA 232-251序列的反义寡核苷酸链(AS ODN链), 序列为: 5'CCAGCCTTCCAGCTCCTTG 3', 并同时合成正义链, 均采取全程硫代修饰, AS ODN链5'端以绿色荧光蛋白标记, 均由上海生工公司合成.
(1)检测survivin蛋白表达: 采用western blot法提取组织和细胞蛋白质样品, Bradford法测定浓度, 在120 g/LSDS-PAGE凝胶中进行电泳分离后, 电转移法将蛋白质转移至硝酸纤维素膜. 硝酸纤维素膜在含50 g/L脱脂奶粉的TTBS缓冲液中37℃封闭90 min, 依次加入一抗(兔抗人survivin抗体, 抗体的稀释度为1: 1 000)4℃孵育过夜, 二抗(辣根过氧化物酶标记的羊抗兔IgG, 抗体稀释度为1: 1 000)37℃作用40 min, TTBS充分漂洗(10 min×3次)后, 化学荧光法(ECL)显色, 观察结果. (2)RT-PCR法检测survivin mRNA表达: 采用RT-PCR法, 上游引物为5'-GGCATGGGTGCCCCGACGTT-3'; 下游引物为5'-AGAGGCCTCAATCCATGGCA-3', 以GAPDH作为内参照. PCR反应条件为94℃ 1 min, 55℃ 1 min, 72℃ 2 min, 共35个循环反应. 所得PCR产物在含EB的15 g/L的琼脂糖凝胶中电泳分离, 在紫外灯下分析结果; 应用分析软件作相对定量分析, 以survivin mRNA表达的吸光度值/GAPDH mRNA表达的吸光度值表示. (3)检测组织标本的凋亡指数: 采用原位末端转移酶标记(TUNEL)法操作步骤按试剂盒说明进行, DAB显色后, 光学显微镜下分析结果, 以阳性染色细胞数/总细胞数×100%计算凋亡指数(AI).(4)转染细胞凋亡指数: 取对数生长期的hepG2细胞, 接种于6孔板内, 使每孔细胞数为3×105, 培养24 h, 细胞融合约80%时开始实验. 设置空白对照组、脂质体转染对照组、正义链转染对照组和不同浓度的反义链转染组即200、400和600 nmol/L AS ODN转染组, 每组设3个复孔. 细胞转染过程按转染试剂盒说明书进行. 收获转染后各组细胞, 制成单细胞悬液, 冰乙醇-20℃固定24 h后使用; 取固定好的细胞, 碘化丙啶溶液避光染色30 min后待用; 采用Beckon/Dickinson Facssort型流式细胞仪, 在488 nm波长处进行检测. 应用相应程序软件进行资料的处理和分析, 细胞凋亡指数(AI) = 亚二倍峰细胞数/总细胞数×100%. (5)化疗药物对各组细胞的生长抑制率采用MTT法, 5-FU和顺铂(DDP)浓度根据韩锐(抗癌药物研究与实验技术. 北京: 人民卫生出版社1997)设定. 收获转染后各组细胞用含100 mL/L胎牛血清的DMEM重悬, 调整细胞浓度为1×107/L, 接种于96孔板内, 每组细胞均设化疗药物实验孔和空白对照孔. 常规条件下培养44 h后, 每孔加入5 g/L的MTT20 μL; 继续培养4 h, 倾尽上清. 每孔加入二甲基亚砜0.1 mL; 充分溶解后用酶标仪在波长570 nm处检测各孔吸光度A值, 化疗药物对细胞生长抑制率(IR) = (1-化疗药物实验孔A)/空白对照孔A×100%. (6)裸鼠移植瘤: 选用5周龄的BALB/nu/nu裸鼠25只, 均为雄性, 密闭无菌环境下饲养. 收集对数生长期hepG2细胞, 制备为浓度5×1010/L的细胞悬液, 以1只裸鼠建立皮下移植瘤, 取上述备好的细胞悬液0.2 mL; 用微量注射器注入到裸鼠颈背部的皮下, 作为供瘤鼠. 当皮下移植瘤长至1 cm左右时, 取出肿瘤, 将瘤组织剪切为约1 mm×1 mm×2 mm大小的小瘤块, 用套管针轻柔穿刺并注射入手术开腹的裸鼠肝脏包膜下. 建立模型2 wk后, 再次手术开腹, 观察肿瘤生长情况, 并测定肿瘤最大长径(a)和垂直短径(b). 将已证实成功建立模型的动物随机分为3组, 即(1)反义survivin寡核苷酸治疗组(8只): ip脂质体包裹的反义survivin寡核苷酸, 剂量8 ug/kg; (2)空白对照组(8只): ip等容积生理盐水; (3)脂质体对照组(7只): ip等容积空白脂质体; 均每日1次, 共连续7 d. 治疗结束后, 在第8 d处死动物, 以公式V = ab2/2计算肿瘤体积, 以V治疗后/V治疗前×100%计算肿瘤生长指数. 肿瘤组织的凋亡指数采用流式细胞术检测.
统计学处理 采用采用χ2检验、t检验和方差分析.
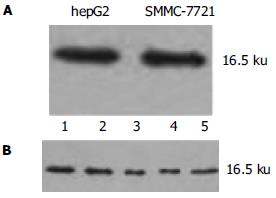
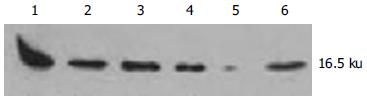
hepG2, SMMC-7721细胞和85.0%(34/40)肝癌组织中survivin表达均为阳性, 在16.5 KD可见到清晰条带(图1A-B). 相应40例癌旁组织中无1例有survivin阳性表达(图2). 肝内转移组survivin表达阳性为93.5%, 显著高于肝内转移阴性组(55.6%, P = 0.001<0.05); 门静脉癌栓组中survivin表达阳性为92.8%, 显著高于无门静脉癌栓组(66.7%, P = 0.002<0.05). survivin的阳性表达在男性(85.7%)与女性患者(80.0%)、年龄大于60岁(80.0%)与年龄小于60岁患者(86.7%)、肿瘤直径小于3 cm(87.5%)与肿瘤直径大于3 cm患者(83.3%)等组间比较均无显著差异(P = 0.02>0.05).40例HCC组织平均凋亡指数为1.65%±0.36%, 其中34例survivin表达阳性组织的平均凋亡指数为1.15%±0.33%, 6例survivin表达阴性组织的平均凋亡指数为4.50%±0.83%, 二者差异显著(P = 0.00 002<0.05).
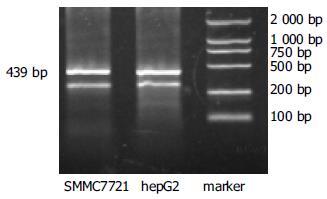
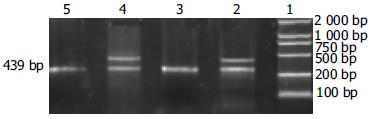
hepG2、SMMC-7721细胞(图3)和85.0%(34/40)HCC组织survivin mRNA表达为阳性, 相应的40例癌旁组织无1例有阳性表达(图4), 与Western blot所检测的survivin蛋白水平表达相一致. 并且肝内转移组和门静脉癌栓组中survivin mRNA表达显著高于肝内转移阴性组和无门静脉癌栓组(P<0.05, 表1).
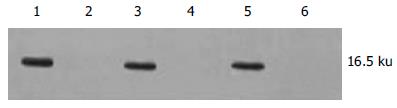
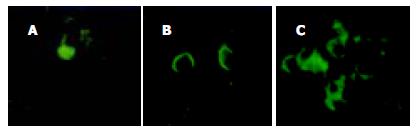
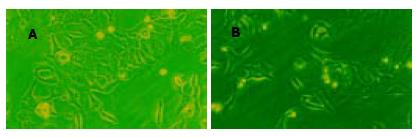
在各浓度AS ODN转染组均可见到转染后细胞内清晰的绿色荧光(图5A-C). 各浓度AS ODN转染组survivin蛋白表达有不同程度下调, 以600 nmol/L浓度组最明显, 在各对照组内survivin蛋白表达无明显改变(图6). 各浓度AS ODN转染组hepG2细胞形态有不同程度改变, 表现为细胞体积变小、形态不规则、细胞皱缩、核固缩、胞质粗糙、分裂相少见等改变, 而在各对照组中细胞形态基本正常, 贴壁生长良好(图7A-B). 各AS ODN转染组细胞凋亡指数明显高于各对照组(P<0.05), 以600 nmol/L浓度AS ODN转染时凋亡诱导最明显(P<0.05), 各对照组间比较无显著性差异(P>0.05, 表2). 等浓度化疗药物5-FU和DDP对各浓度AS ODN转染的hepG2细胞生长抑制率明显高于各对照组, 但在各对照组间比较无显著性差异(P>0.05, 表2).
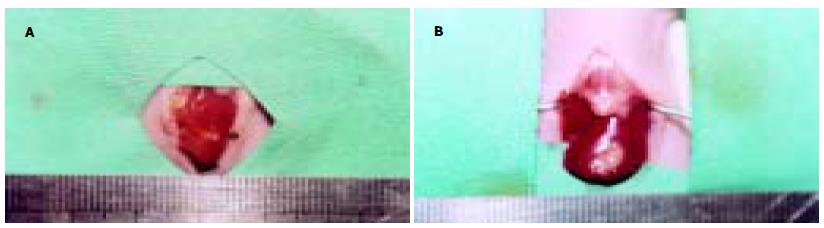
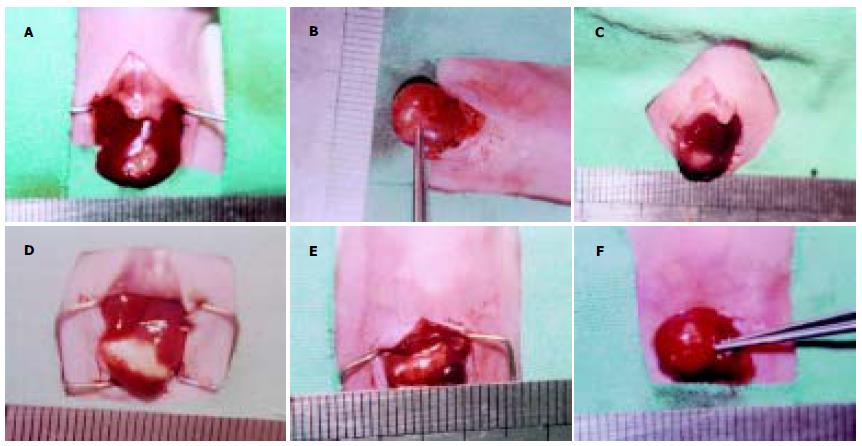
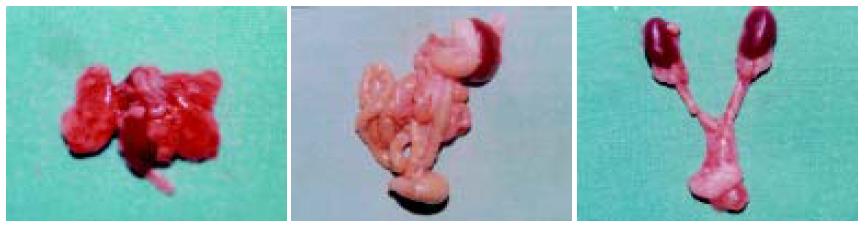
hepG2细胞接种于裸鼠皮下后, 可见皮下移植瘤逐渐增大, 约2 wk后其大小可达到0.8-1.0 cm左右. 移植术后2 wk上腹部可扪及明显的质地较硬结节. 开腹检查时可见23/25例裸鼠的肝脏有明显移植瘤形成, 模型建立的成功率为92.0%. 瘤块呈现出不规则结节状, 色灰白, 与周围肝组织分界较清晰, 质硬, 荷瘤鼠瘤结节平均体积为20.85±2.13 mm3(图8A-B). 反义survivin寡核苷酸治疗组与空白对照组和脂质体对照组相比, 其肿瘤生长指数显著降低, 生长明显受抑(P<0.05, 表3)(图9A-F). 而在心、肺、肾组织中未见明显的异常(图10A-C). Survivin AS ODN治疗组瘤组织凋亡指数显著高于空白对照组和脂质体对照组(P<0.05).
HCC临床进展快, 转移早, 预后差[19-20], 生存期短[21], 早期诊断尚存在着困难[22-25], 传统临床治疗的中远期疗效均不理想[26-28]. Survivin是近年来发现的IAP家族新成员, 其在包括胃肠道[29]、神经系统[30]、血液系统[31]在内的多种组织来源的恶性肿瘤中都具有选择性表达的特性. 我们的研究显示hepG2细胞和85%的HCC组织中survivin蛋白和mRNA表达为阳性, 并且其在蛋白和mRNA水平的表达是完全一致的, 而相应的癌旁组织无1例有survivin阳性表达, 提示survivin基因在HCC组织有选择性表达的特性, 其基因转录的异常启动可能是survivin再表达的关键机制. 进一步分析survivin表达与HCC各临床病理学参数的关系, 发现survivin表达与肿瘤患者的性别、年龄, 肿瘤大小等无显著关系, survivin的表达在HCC发生的早期即可出现, 可能是HCC发生中的早期事件, 这与在结肠癌、胰腺癌等中的研究报道是一致的[32-33]. 但survivin表达与肝内转移和门静脉癌栓形成明显相关, 对survivin mRNA表达做半定量分析显示肝内转移和门静脉癌栓组中survivin mRNA表达水平是显著高于无肝内转移和门静脉癌栓组, 这提示survivin表达与HCC的恶性生物学行为有关, survivin持续表达可能进一步参与恶性肿瘤的临床进展.
目前对于survivin参与肿瘤发生、发展的机制并不完全清楚, 可能涉及到细胞凋亡、增生等多个方面. 本文结果显示, survivin表达阳性HCC组织凋亡指数显著低于survivin表达阴性组织, 提示survivin表达可抑制HCC细胞凋亡, 这可能是其参与HCC发生的机制之一. 反义寡核苷酸是一类能够以Watson-Crick碱基配对原则与特定的靶mRNA互补结合的小分子量的, 可扩散的寡核苷酸序列, 多为人工合成, 他能够从翻译水平, 转录和核酸复制水平高度特异性地抑制靶基因的翻译和表达, 选择性地关闭特定的靶基因, 因其具有精确的选择性和高度的特异性, 而成为一项有价值的研究工具和治疗恶性肿瘤的重要的策略. 我们在证实了肝细胞癌中survivin具有选择性表达特性的基础上, 以survivin 作为靶点合成高特异性的反义寡核苷酸序列, 将其转染至survivin表达阳性的人HCC细胞株hepG2, 我们发现survivin AS ODN转染可抑制细胞survivin表达, 诱导凋亡, 以600 nmol/L AS ODN转染作用最为显著. 这与国外研究所报道的在皮肤癌、乳腺癌细胞株中转染survivin阴性突变质粒所诱导的细胞凋亡作用是相符的[34-35], 提示采用基因技术封闭survivin表达, 可以解除survivin的凋亡抑制作用, 诱导肿瘤细胞凋亡.
我们还发现AS ODN转染后的HCC细胞对化疗药物5-FU和DDP的敏感性明显增加, 提示survivin表达所引起的HCC细胞凋亡机制缺陷, 在一定程度上参与HCC细胞化疗耐药性的产生, 而AS ODN转染能封闭survivin表达, 在一定程度上逆转细胞耐药性. 我们还建立了原位荷HCC裸鼠模型, 以脂质体作为载体将survivin AS ODNip作为治疗手段, 结果显示AS ODN治疗组与空白对照组和脂质体对照组相比肿瘤生长明显缓慢, 生长指数显著降低. 进一步采用流式细胞术对肿瘤组织的凋亡指数进行检测, 我们发现AS ODN治疗组中肿瘤组织的凋亡指数显著高于空白对照组和脂质体对照组, 这表明采用survivin AS ODNip可有效诱导HCC细胞凋亡, 抑制肿瘤生长而达到治疗目的. 本实验中对心、肺以及肾脏等肝外器官组织的观察未见到明显异常改变, 表明survivin AS ODNip治疗较为安全.
总之, survivin基因在HCC中具有选择性表达的特性, 其表达与HCC的恶性生物学行为及凋亡抑制密切相关; survivin反义寡核苷酸可在体外诱导HCC细胞凋亡, 增加细胞对化疗药物的敏感性, 在体内可抑制荷HCC鼠肿瘤的生长, 诱导细胞凋亡, survivin可作为HCC诊断和治疗的有效靶点.
编辑: 张海宁 电编: 潘伯荣
| 1. | Guo F, Sigua C, Tao J, Bali P, George P, Li Y, Wittmann S, Moscinski L, Atadja P, Bhalla K. Cotreatment with histone deacetylase inhibitor LAQ824 enhances Apo-2L/tumor necrosis factor-related apoptosis inducing ligand-induced death inducing signaling complex activity and apoptosis of human acute leukemia cells. Cancer Res. 2004;64:2580-2589. [PubMed] |
| 8. | Zhou HB, Zhu JR. Paclitaxel induces apoptosis in human gastric carcinoma cells. World J Gastroenterol. 2003;9:442-445. |
| 9. | Chen XP, He SQ, Wang HP, Zhao YZ, Zhang WG. Expression of TNF-related apoptosis-inducing Ligand receptors and antitumor tumor effects of TNF-related apoptosis-inducing Ligand in human hepatocellular carcinoma. World J Gastroenterol. 2003;9:2433-2440. |
| 10. | Fu YG, Qu YJ, Wu KC, Zhai HH, Liu ZG, Fan DM. Apoptosis-inducing effect of recombinant Caspase-3 expressed by constructed eukaryotic vector on gastric cancer cell line SGC7901. World J Gastroenterol. 2003;9:1935-1939. |
| 11. | Gupta S. A role of inhibitor of apoptosis (IAP) proteins in increased lymphocyte apoptosis in aged humans. Mech Ageing Dev. 2004;125:99-101. [PubMed] |
| 12. | Lotocki G, Keane RW. Inhibitors of apoptosis proteins in injury and disease. IUBMB Life. 2002;54:231-240. [PubMed] |
| 13. | Martin SJ. Destabilizing influences in apoptosis: sowing the seeds of IAP destruction. Cell. 2002;109:793-796. [PubMed] |
| 14. | Salvesen GS, Duckett CS. IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol. 2002;3:401-410. [PubMed] |
| 15. | Verhagen AM, Vaux DL. Cell death regulation by the mammalian IAP antagonist Diablo/Smac. Apoptosis. 2002;7:163-166. [PubMed] |
| 17. | Chang Q, Liu ZR, Wang DY, Kumar M, Chen YB, Qin RY. Survivin expression induced by doxorubicin in cholangiocarcinoma. World J Gastroenterol. 2004;10:415-418. |
| 18. | Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-8589. [PubMed] |
| 28. | 凌 昌全, 陈 喆, 朱 德增, 俞 超芹, 黄 雪强, 翟 笑枫, 万 旭英, 李 瑾, 陈 坚, 沈 峰. 中西医结合治疗中晚期原发性肝癌313例. 世界华人消化杂志. 2001;9:114-115. [DOI] |
| 29. | Kawasaki H, Toyoda M, Shinohara H, Okuda J, Watanabe I, Yamamoto T, Tanaka K, Tenjo T, Tanigawa N. Expression of survivin correlates with apoptosis, proliferation, and angiogenesis during human colorectal tumorigenesis. Cancer. 2001;91:2026-2032. [PubMed] |
| 30. | Islam A, Kageyama H, Hashizume K, Kaneko Y, Nakagawara A. High expression of Survivin, mapped to 17q25, is significantly associated with poor prognostic factors and promotes cell survival in human neuroblastoma. Oncogene. 2000;19:617-623. [PubMed] |
| 31. | Shinozawa I, Inokuchi K, Wakabayashi I, Dan K. Disturbed expression of the anti-apoptosis gene, survivin, and EPR-1 in hematological malignancies. Leuk Res. 2000;24:965-970. [PubMed] |
| 32. | Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich SM, Fields JZ, Boman BM. Evidence that APC regulates survivin expression: a possible mechanism contributing to the stem cell origin of colon cancer. Cancer Res. 2001;62:8664-8667. [PubMed] |
| 33. | Satoh K, Kaneko K, Hirota M, Masamune A, Satoh A, Shimosegawa T. Expression of survivin is correlated with cancer cell apoptosis and is volved in the development of human pancreatic duct cell tumor. Cancer. 2001;92:271-278. [PubMed] |
| 34. | Mesri M, Wall NR, Li J, Kim RW, Altieri DC. Cancer gene therapy using a survivin mutant adenovirus. J Clin Invest. 2001;108:991-999. [PubMed] |
| 35. | Grossman D, Kim PJ, Blanc-Brude OP, Brash DE, Tognin S, Marchisio PC, Altieri DC. Transgenic expression of survivin in keratinocytes counteracts UVB-induced apoptosis and cooperates with loss of P53. J Clin Invest. 2001;108:981-990. [PubMed] |