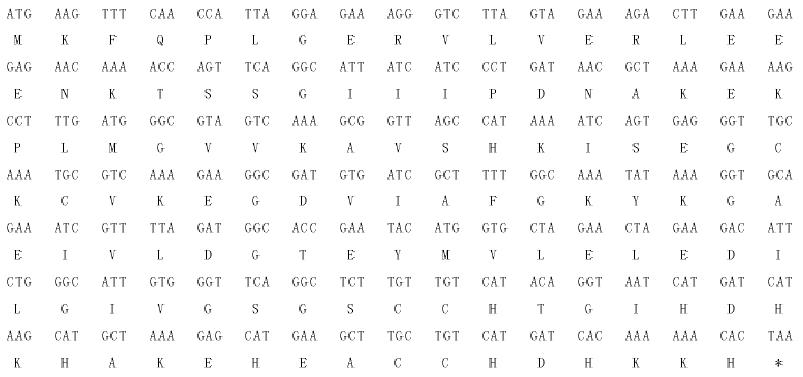
修回日期: 2005-01-01
接受日期: 2005-01-26
在线出版日期: 2005-03-01
目的: 利用原核表达载体高效表达幽门螺杆菌热休克蛋白A(HspA), 并进行纯化.
方法: PCR扩增hspA基因, 将其克隆至原核表达载体pET22b, pQE-60, pGEX-4T-2中, 转化大肠杆菌, 经IPTG诱导后, SDS-PAGE分析表达. 利用镍离子亲和层析对表达产物进行纯化. 免疫印迹检测蛋白的抗原性.
结果: PCR扩增得到hspA基因, 在载体pQE-60中以HspA和HspA-His6两种形式得到表达, 表达量高达菌体总蛋白的40%以上. 经镍离子亲和层析后, 纯化率分别为88.63%和 86.32%, 但HspA-His6在纯化过程中发生降解. 免疫印迹实验表明, HspA和HspA-His6都有很好的抗原性.
结论: 实现了HspA蛋白的高效表达和纯化, 为幽门螺杆菌HspA亚单位疫苗的免疫实验奠定基础.
引文著录: 刘秀丽, 张兆山, 陶好霞, 展德文, 刘纯杰. 幽门螺杆菌HspA亚基的表达与纯化. 世界华人消化杂志 2005; 13(5): 626-630
Revised: January 1, 2005
Accepted: January 26, 2005
Published online: March 1, 2005
AIM: To express and purify recombinant heat shock protein A subunit (HspA) of Helicobacter pylori (H. pyloi).
METHODS: Gene hspA was amplified by PCR, and inserted into the prokaryotic expression vector pET22b, pQE-60 and pGEX-4T-2 respectively. The plamids were transformed into the Escherichia coli JM109 and BL21 (DE3). Expression of hspA gene was induced by IPTG and was analyzed by SDS-PAGE. The recombinant proteins were purified through nickel-affinity chromatography. Antigenicity of the recombinant proteins was analyzed by Western blot.
RESULTS: The hspA gene was expressed in two forms (HspA and HspA-His6) in pQE-60. The expressed protein accounted for above 40% of the total bacterial protein. The purity of HspA and HspA-His6 were 88.63% and 86.32% respectively after purification. Western blot proved that the recombinant proteins could be recognized by the anti-H. pylori serum.
CONCLUSION: HspA of H. pylori has been expressed and purified with high efficiency, which can be used for vaccine development and immunological investigation.
- Citation: Liu XL, Zhang ZS, Tao HX, Zhan DW, Liu CJ. Expression and purification of recombinant heat shock protein A subunit of Helicobacter pylori. Shijie Huaren Xiaohua Zazhi 2005; 13(5): 626-630
- URL: https://www.wjgnet.com/1009-3079/full/v13/i5/626.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i5.626
幽门螺杆菌(Helicobacter pylori, H. pylori)是导致慢性胃炎、消化性溃疡及胃癌、胃黏膜相关性淋巴组织(MALT)淋巴瘤等胃部疾病的主要致病细菌, 1994年国际癌症研究所将H. pylori认定为第一类致癌因子[1-5]. 流行病学资料显示, H. pylori感染呈全球分布, 在发展中国家人群中, H. pylori感染率高达70%-80%, 即便在发达国家, H. pylori的感染率也达50%左右. 由于胃肠道黏膜组织很适合于H. pylori的定植, 一经感染H. pylori, 便很难治愈[6-10]. H. pylori复合抗生素疗法, 成本昂贵; 而且通用的抗生素一般都没有专性于胃肠环境, 因而对防治H. pylori感染的作用有限; 此外, H. pylori对抗生素的抗性日益增加, 将进一步限制抗生素疗法的应用. 因此, 疫苗是预防和控制H. pylori最有效的途径之一. H. pylori产生两种热休克蛋白, HspA和HspB, 其相对分子质量分别为Mr13 000和Mr58 200[11-15]. 由于HspA参与尿素酶的组装, 并使其发挥酶活性, 分解尿素产生氨, 从而中和胃酸有利于H. pylori的定值[16-20]. 由此可见HspA是H. pylori的重要致病因子, 同时研究已证明HspA是一种理想的保护性抗原成分, 并为有效的亚单位疫苗候选抗原. 我们将HspA基因克隆至原核表达载体, 实现HspA蛋白的初步纯化, 为研究其生物学特性和制备H. pylori亚单位疫苗奠定基础.
H. pylori NCTC11637为国际标准株, 大肠杆菌JM109, BL21(λDE3), TOPO10; 质粒pET22b, pQE-60, pGEX-4T-2. 由本室保存. 限制性内切酶Nco I, BamH I和Xho I, Ex Taq DNA聚合酶和T4 DNA连接酶, 购自宝生物工程大连有限公司. 辣根过氧化物酶标记的第二抗体, 购自Sigma公司. 镍离子亲和层析柱(5 mL), 购自安玛西亚公司.
1.2.1 HspA表达载体的构建: 收集用平板厌氧培养的H. pylori菌体, 用TE缓冲液(pH8.0)洗涤和重悬后, 沸水浴10 min, 离心取上清; 用等体积抽提液(酚: 氯仿: 异物醇的比例为25: 24: 1)抽提两次, 并用2倍乙醇沉淀和预冷700 mL/L乙醇洗涤; 沉淀抽干后溶于TE中, 制备H. pylori NCTC11637基因组模板. PCR扩增引物为: P1: 5'-CTG CCA TGG AGT TTC AAC CAT TAG-3', P2: -5'-CGG GAT CCT TAG TGT TTT TTG TG-3', P3-5'-CGG GAT CCG TGT TTT TTG TGA TC-3', P4: -5'-CGG GAT CCA TGA AGT TTC AAC C-3', P5: -5'-CCG CTC GAG TTA GTG TTT TTT GTG-3'. P1和P2扩增的基因用于克隆至载体pET22b, pQE-60上, 此引物为匹配载体上的起始密码字, 将HspA基因上的第4个碱基由A变为G, 从而使HspA蛋白的第二个氨基酸由Lys变为Glu. P3和P2扩增的基因用于克隆至载体pQE-60上, 由此HspA蛋白不仅第二个氨基酸由Lys变为Glu, 而且C末端加入两个酶切位点的4个氨基酸和(His)6, 为融合表达基因. P3和P2扩增的基因用于克隆至载体pGEX-4T-2上, 与GST蛋白形成融和表达. PCR反应体系: 取10×PCR buffer 5 μL, 模板DNA 1 μL, 50 ng/μL 5'和3'端引物1 μL, Ex Taq 0.5 μL, dNTP 4 μL, 加无菌水至50 μL.94℃热变性5 min后, 进行30个"变性-复性-延伸"循环, 94℃变性30 s, 56℃复性30 s, 72℃延伸30 s. 然后72℃延伸10 min. 利用凝胶回收试剂盒割胶回收PCR产物. 用Xho I和Eco RI双酶切HspA基因和质粒pET22b, pQE-60, pGEX-4T-2, 酶切产物用凝胶回收试剂盒割胶回收, 并在T4 DNA连接酶作用下于16℃过夜连接, 连接产物转化大肠杆菌TOPO10后挑选阳性克隆, 经过抽提质粒、酶切分析鉴定. 将得到的重组质粒分别命名为pETH、pQEH、pQEHS和pGEXH. 将重组质粒进行测序鉴定.
1.2.2 HspA基因的表达及鉴定: 将重组质粒pQEH, pQEHS转化E. coli JM109感受态, 将pETH、pGEXH转化E. coli BL21感受态, 筛选转化重组子. 将转化菌的单克隆菌落接种至LB培养基37℃培养3 h, A600为0.4-0.6时, 加入终浓度为1 mmol/L的IPTG, 诱导表达4 h后, 离心收集1 mL菌体, 然后加入水70 μL, 5上样缓冲液20 μL, 1 mol/LDTT 10 μL. 煮沸10 min, 离心10 min, 取上清上样, 进行SDS-PAGE电泳, 鉴定转化菌的表达. 在LB液体培养基中大量培养400 mL转化菌, 诱导表达离心收集菌体, 加入0.02 mol/L的PB缓冲液(0.2 mol/L Na2HPO4, 80 mL; 0.2 mol/L NaH2PO4, 20 mL; H2O, 900 mL; )12 mL, 超声破碎10 min, 15 000 g离心10 min, 收取上清和沉淀, SDS-PAGE电泳检测表达蛋白的分布情况.
1.2.3 HspA基因的纯化: 用10 mL一次性注射器吸满10 mL水与镍柱进口进行无气泡连接, 拧去柱子末尾的堵头, 冲洗柱中的200 mL/L乙醇, 冲洗20 mL, 再使柱中充满0.1 mol/L NiSO4 约10 mL, 然后再用去离子水冲洗15 mL. 用Binging Buffer(0.016 mol/L Na2HPO4, 0.004 mol/L NaH2PO4, 0.05 mol/L NaCl, pH7.4)冲洗10个柱体积, 约50 mL. 样品先用0.45 μm的滤膜过滤, 上样. 用Eluting Buffer1(10 mmol/L咪唑, 0.016 mol/L Na2HPO4, 0.004 mol/L NaH2PO4, 0.05 mol/L NaCl, pH7.4)平衡25 mL, 用Eluting Buffer2(0.25 mol/L咪唑, 0.016 mol/L Na2HPO4, 0.004 mol/L NaH2PO4, 0.05 mol/L NaCl, pH7.4)洗脱, 收集1 mL/管, SDS-PAGE电泳检测.
1.2.4 Western印迹: SDS-PAGE结束后, 将胶用转移缓冲液(39 mmol/L甘氨酸, 48 mmol/L Tris, 37 mg/LSDS, 200 mL/L甲醇, 加水至1 000 mL)漂洗. 同时剪一与胶大小相同的硝酸纤维素膜及滤纸. 以半干核酸蛋白转移仪0.8 mA/cm2转移2 h. 取出硝酸纤维素膜, 用封闭液(0.05 mol/L PBS, 0.5 mol/L NaCl, 50 g/LBSA, 5 g/LTween20, )4℃封闭过夜. 然后抗H. pylori血清稀释液, 室温下作用2-3 h. 漂洗液(0.05 mol/L PBS, 0.5 mol/L NaCl, 0.5% Tween-20, )漂洗4次, 每次10 min. 加入稀释液稀释的辣根过氧化物酶标记的第二抗体(1: 5 000稀释, 稀释液成分: 0.01 mol/L PBS, 0.25 mol/L NaCl, 0.5% Tween-20, 0.5% BSA), 室温作用2 h. 漂洗后, 加入底物溶液(Na2HPO4 0.05 mol/L柠檬酸 0.24 mol/L, 10 mL; 二胺基联苯氨4 mg, 300 mL/L30% H2O2 15 μL)室温下显色. 待显色适度时, 用2 mol/L H2SO4终止反应, 水漂洗后阴干.
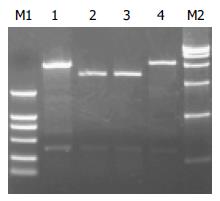
重组质粒pETH, pQEH, pQEHS和pGEXH经酶切分析鉴定, 表明已成功将hsp A抗原基因克隆至原核表达载体(图1, 2). 用引物从两侧进行序列测定, 即可通读. 序列分析表明, 该序列含hsp A基因357 bp, 克隆至pETH、pQEH上的HspA基因的第四个碱基由A变为G, 从而使HspA蛋白的第二个氨基酸由Lys变为Glu. 克隆至pQEHS末尾增加GGA TCC AGA TCT CAT CAT CAT CAT CAT CAT 30个核甘酸. 克隆至pGEXH上的HspA基因GST基因融合, 序列没有改变.
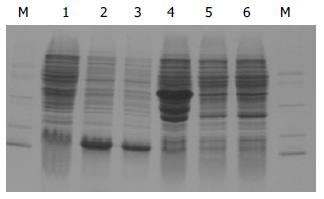
重组的基因工程菌经IPTG诱导后, 经SDS-PAGE检测发现: 与对照JM109菌株相比pQEH和pQEHS均在14 000处表达处一条蛋白带. 初步判断应为重组HspA蛋白. HspA蛋白占总蛋白的42.51%, HspA-His6蛋白占总蛋白的43.07%. 与对照BL21相比pGEXH在43 ku左右表达处3条带, 而pETH没有表达带(图3).
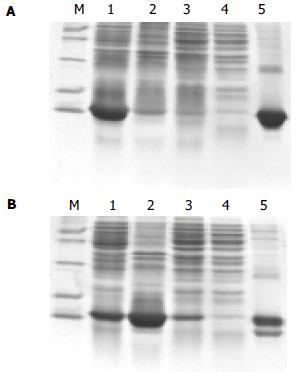
质粒pETH未表达目的蛋白, 而重组pGEXH未明原因的表达三条带. 本实验以pQEH和pQEHS为纯化的研究对象. 取大量培养的超声离心上清和沉淀样品、纯化时的上样穿流峰样品、平衡峰样品和洗脱峰样品进行SDS-PAGE电泳分析, 结果发现pQEH表达的HspA蛋白均以可溶形式表达, 而pQEHS表达的HspA-His6蛋白部分以可溶形式表达, 部分以包涵体形式表达. HspA蛋白与镍柱结合效果好, 在穿流峰中未检测到HspA蛋白. 而HspA-His6蛋白与镍柱结合效果差, 在穿流峰中检测到HspA-His6蛋白, 且在洗脱峰中HspA-His6蛋白出现降解带. 纯化HspA蛋白的纯度为88.63%, 而HspA-His6蛋白的纯度为86.32%(包括降解带). 从400 mL的培养菌中可得到HspA约12 mg, 相同的菌量HspA-His6只能得到3 mg左右(图4).
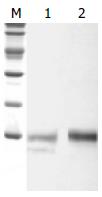
以150 g/L的SDS-PAGE将HspA和HspA-His6 蛋白进行分离, 然后用抗H. pylori血清进行免疫印迹, 结果发现HspA纯化产物在14 KD左右有一阳性印迹, 而HspA-His6纯化产物在14 ku左右有2条阳性印迹带, 由此可见HspA-His6在纯化过程中有降解现象(图5).
H. pylori中含有一个编码13 ku的热休克蛋白A(HspA), 他与大肠杆菌GroES高度同源动物实验研究表明用HspA(佐剂为霍乱毒素CT)免疫小鼠, 可使小鼠对猫胃螺杆菌攻击的保护率为80%, 说明HspA是有效的保护性抗原[21]. 另外由于HspA蛋白序列在不同H. pylori菌株间高度保守, 因此将其单独使用或与其他抗原联用, 均可激发机体产生保护免疫, 是H. pylori疫苗的理想组分[22]. 我们将hspA基因克隆至不同的表达载体中, 目的是实现该基因的高度表达并利于纯化, 在pET22b中, 克隆的基因经测序后序列正确, 但未能实现表达. 将该基因克隆至pGEX-4T-2中, 使其与GST融合, 然后利用GST亲和层析对其纯化. 虽然克隆的基因序列完整无误, 但融合蛋白在表达或在检测处理过程中出现降解, 使得纯化出现困难. 可喜的是 HspA在pQE-60中以HspA和 HspA-His6的形式得到高效表达, 因此在纯化过程中我们以纯化HspA和HspA-His6为主.
Hsp A 位于H. pylori的表面, 其C端含有4个组氨酸和4个半胱氨酸, 能够特异性结合镍离子, 起到镍离子库的作用[23-29]. 尿素酶是H. pylori中重要的定植因子, 他通过分解尿素形成氨以中和胃酸, 为H. pylori的定值和生存创造条件. 尿素酶的活性与镍离子的存在有密切关系. 因此Ure和Hsp A之间通过镍离子建立起一定的联系, 在决定H. pylori定植效率方面起协同作用[30]. 我们将hspA基因克隆至pQE-60中, 因pQE-60中含有6个组氨酸, 以利于用镍亲和柱纯化, 因此我们将hspA基因以带有和不带有his6尾巴两种形式表达. 通过纯化实验发现, HspA无论在表达形式(可溶表达或包涵体)还是在与亲和柱结合上比 HspA-His6的效果都要好. 这可能是his6尾巴的加入不但没有增强与镍亲和柱的结合, 反而干扰了HspA可溶性表达和HspA原有的结合镍离子的作用区域, 实属画蛇添足. 实验研究表明HspA对镍离子有很强的网络能力, 这为H. pylori含有HspA的融合疫苗通过镍离子亲和层析纯化打下基础. 实验中所获得的高纯度的HspA蛋白为进一步进行动物免疫实验提供了大量抗原.
电编: 潘伯荣
| 1. | Nishibayashi H, Kanayama S, Kiyohara T, Yamamoto K, Miyazaki Y, Yasunaga Y, Shinomura Y, Takeshita T, Takeuchi T, Morimoto K. Helicobacter pylori-induced enlarged-fold gastritis is associated with increased mutagenicity of gastric juice, increased oxidative DNA damage, and an increased risk of gastric carcinoma. J Gastroenterol Hepatol. 2003;18:1384-1391. [PubMed] |
| 2. | Suleymanov Z. Expression of class I and II MHC receptors in Helicobacter pylori-positive patients with active gastritis and duodenal ulcer. Turk J Gastroenterol. 2003;14:168-172. [PubMed] |
| 3. | Loughlin MF. Novel therapeutic targets in Helicobacter pylori. Expert Opin Ther Targets. 2003;7:725-735. [PubMed] |
| 4. | Perri F, Qasim A, Marras L, O'Morain C. Treatment of Helicobacter pylori infection. Helicobacter. 2003;8:53-60. [PubMed] |
| 5. | Lamarque D, M Peek R Jr. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2003;8:21-30. [PubMed] |
| 6. | Nardone G, Morgner A. Helicobacter pylori and gastric malignancies. Helicobacter. 2003;8:44-52. [PubMed] |
| 8. | Jimenez FP, Estevez MP. Role of cytokines in chronic gastritis by Helicobacter pylori Acta. Gastroenterol Latinoam. 2001;31:137-141. [PubMed] |
| 9. | Tytgat G. Helicobacter pylori: past, present and future. J Gastroenterol Hepatol. 2000;15:G30-33. [PubMed] |
| 10. | Kagawa J, Honda S, Kodama M, Sato R, Murakami K, Fujioka T. Enterocromaffin-like cell tumor induced by Helicobacter pylori infection in Mongolian gerbils. Helicobacter. 2002;7:390-397. [PubMed] |
| 11. | Eamranond PP, Torres J, Munoz O, Perez-Perez GI. Age-specific immune response to HspA in Helicobacter pylori-positive persons in Mexico. Clin Diagn Lab Immunol. 2004;11:983-985. [PubMed] |
| 12. | Jiang Z, Huang AL, Tao XH, Wang PL. Construction and characterization of bivalent vaccine candidate expressing HspA and Mr18 000 OMP from Helicobacter pylori. World J Gastroenterol. 2003;9:1756-1761. [PubMed] |
| 13. | Jiang Z, Pu D, Huang AL, Tao XH, Wang PL. Construction, expression and antigenic study of bivalent vaccine candidate with 26 000 OMP and heat short protein A of human Helicobacter pylori. Zhonghua Yixue Zazhi. 2003;83:862-867. [PubMed] |
| 14. | Yi P, Li G, Liu S, Luo S, Tao X. Effect of xiaokuiling prescription on the expression of HSP72, HSP B in gastric mucosa of patients with Helicobacter pylori-associated duodenal ulcer. J Tongji Med Univ. 2001;21:310-313. [PubMed] |
| 15. | Lock RA, Coombs GW, McWilliams TM, Pearman JW, Grubb WB, Melrose GJ, Forbes GM. Proteome analysis of highly immunoreactive proteins of Helicobacter pylori. Helicobacter. 2002;7:175-182. [PubMed] |
| 16. | Yan J, Wang Y, Shao SH, Mao YF, Li HW, Luo YH. Construction of prokaryotic expression system of ltB-ureB fusion gene and identification of the recombinant protein immunity and adjuvanticity. World J Gastroenterol. 2004;10:2675-2679. [PubMed] |
| 17. | Fujii R, Morihara F, Fukushima K, Oku T, Hifumi E, Uda T. Recombinant antigen from Helicobacter pylori urease as vaccine against H. pylori-associated disease. Biotechnol Bioeng. 2004;86:737-746. [PubMed] |
| 18. | Mao YF, Yan J. Construction of prokaryotic expression system of ureB gene from a clinical Helicobacter pylori strain and identification of the recombinant protein immunity. World J Gastroenterol. 2004;10:977-984. [PubMed] |
| 19. | Mehta N, Benoit S, Maier RJ. Roles of conserved nucleotide-binding domains in accessory proteins, HypB and UreG, in the maturation of nickel-enzymes required for efficient Helicobacter pylori colonization. Microb Pathog. 2003;35:229-234. [PubMed] |
| 20. | Lu DS, Mao XH, Zou QM, Wu C, Yang J, Zhang WJ, Wang FK, Xie QH, Luo P. Recombinant Helicobacter pylori urease B subunit and its biological properties. Diyi Junyi Daxue Xuebao. 2003;23:549-552. [PubMed] |
| 21. | Ferrero RL, Thiberge JM, Kansau I, Wuscher N, Huerre M, Labigne A. The GroES homolog of Helicobacter pylori confers protective immunity against mucosal infection in mice. Proc Natl Acad Sci USA. 1995;92:6499-6503. [PubMed] |
| 22. | Suerbaum S, Thiberge JM, Kansau I, Ferrero RL, Labigne A. Helicobacter pylori hspA-hspB heat-shock gene cluster: nucleotide sequence, expression, putative function and immunogenicity. Mol Microbiol. 1994;14:959-974. [PubMed] |
| 23. | Ren H, Yi C. Role of Helicobacter pylori infection in pathogenesis of gastric adenocarcinoma. J Tongji Med Univ. 1999;19:127-130. [PubMed] |
| 24. | Presecki V, Katicic M, Kalenic S, Strnad M, Plecko V, Babus V, Dominis M. A vaccine against Helicobacter pylori infection. Lijec Vjesn. 2002;124:79-82. [PubMed] |
| 25. | Li MF, Ling Z, Zhang YC, Ma AY, Sun JX, Shi HJ, Wu XF. Helicobacter pylori HspA Heat-shock protein gene cloning, Expression and immunogenicity. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 1999;31:264-268. [PubMed] |
| 26. | Liu CJ, Zhang ZS, Li SQ, Huang CF. Cloning and secretion expression of heat-shock protein 70 gene of Helicobacter pylori. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2000;32:524-528. [PubMed] |
| 27. | Li MF, He ZY, Ling Z, Wang JY, Sheng XD, Yang GZ, Wu XF. A Candidate oral vaccine to Helicobacter pylori fusion protein of HspA and CtxB. Shengwu Huaxue Yu Shengwu Wuli Xuebao (Shanghai). 2001;33:360-364. [PubMed] |
| 28. | Rokutan K. Role of heat shock proteins in gastric mucosal protection. J Gastroenterol Hepatol. 2000;15:D12-19. [PubMed] |
| 29. | Asante MA, Mendall MA, Ballam L, Morris J, Northfield TC. Relationship between Helicobacter pylori, gastric parietal cell antibodies and heat shock proteins. Eur J Gastroenterol Hepatol. 1999;11:1365-1370. [PubMed] |
| 30. | Lee CK, Weltzin R, Thomas WD Jr, Kleanthous H, Ermak TH, Soman G, Hill JE, Ackerman SK, Monath TP. Oral immunization with recombinant Helicobacter pylori urease induces secretory IgA antibodies and protects mice from challenge with Helicobacter felis. J Infect Dis. 1995;172:161-172. [PubMed] |