修回日期: 2005-11-25
接受日期: 2005-12-08
在线出版日期: 2005-12-28
目的: 检测γ-氨基丁酸 (gamma-aminobutyric acid, GABA) 和谷氨酸脱羧酶 (glutamic acid decarboxylase, GAD) 在大鼠降结肠上皮的表达及分布特征, 并探讨GABA与上皮细胞分化增殖的关系.
方法: 用免疫荧光及激光共聚焦显微扫描技术, 检测GABA、GAD65及GAD67在大鼠降结肠上皮中的表达, 并以麦芽凝聚素组织化学染色与免疫荧光结合的双重染色显示GABA和GAD65表达细胞的分布特征. 同时, 用RT-PCR和原位分子杂交方法检测GAD mRNA的表达. 此外, 用3H-胸腺嘧啶放射自显影法显示降结肠上皮的增殖带.
结果: RT-PCR显示降结肠黏膜中GAD65及GAD67mRNA均阳性, 原位杂交显示阳性杂交信号主要分布在上皮细胞的隐窝和腔面, 且GAD65信号较GAD67强. GABA及GAD65 免疫反应阳性细胞主要分布在降结肠的腔面和隐窝的上1/3上皮细胞的胞质, 而GAD67阳性细胞仅分布腔面, 此外, GABA及GAD65阳性染色也见于黏膜固有层. 双重染色显示杯状细胞中GABA及GAD65均阴性. 3H-胸腺嘧啶标记阳性细胞主要在隐窝的中下段.
结论: GABA及GAD65分布在大鼠降结肠上皮的成熟带及功能带, GABA系统可能参与上皮细胞的分化与增殖的调节.
引文著录: 汪芳裕, MaemuraKentaro, 朱人敏, HirataIchiro, Katsu Ken-ichi, WatanabeMasahito. 大鼠降结肠上皮γ-氨基丁酸及谷氨酸脱羧酶的表达特征. 世界华人消化杂志 2005; 13(24): 2833-2837
Revised: November 25, 2005
Accepted: December 8, 2005
Published online: December 28, 2005
AIM: To detect the expression of γ-aminobutyric acid (GABA) and glutamate decarboxylases (GADs) in the epithelial growth zones of rat descending colon, and to investigate their relations with cell differentiation and proliferation.
METHODS: Immunocytochemical expression of GABA and GADs in the epithelial cells of rat descending colon was investigated by immunofluorescent staining and confocal laser scanning techniques, and the goblet cells were further revealed by wheat-germ agglutinin (WGA) histochemistry. GAD65 and GAD67 mRNAS were also detected by reverse transcription-polymerase chain reaction (RT-PCR) and in situ hybridization. Furthermore, evaluation of cell kinetics in colonic epithelia was conducted by [3H]-thymidine autoradiography.
RESULTS: Immunoreactive GABA and GAD65 were distributed in the upper third of the crypts and at the luminal surface in the rat descending colon. Strong staining for GABA and GAD65 was localized mainly in the cytoplasm of epithelial cells near the neck of the crypts and along the luminal surface. Immunoreactivity of GAD67, however, was localized only on the luminal surface. In addition, GABA and GAD65 were detected at lamina propria in clonic mucosa. No staining for GABA or GADs was found in goblet cells. GAD65 and GAD67 mRNAs were both identified in the homogenates of rat descending colon, and the epithelium showed stronger hybridization signal for GAD65 mRNA than that for GAD67. Meanwhile, [3H]-thymidine labeled cells were found in the lower two-thirds of the crypts.
CONCLUSION: The expression of GABA and GADs in the maturation and function zones suggests that GABA might be involved in the differentiation and proliferation of the colonic epithelial cells.
- Citation: Wang FY, Maemura K, Zhu RM, Hirata I, Katsu KI, Watanabe M. Characteristic expression of γ-aminobutyric acid and glutamic acid decarboxylase in epithelial cells of rat descending colon. Shijie Huaren Xiaohua Zazhi 2005; 13(24): 2833-2837
- URL: https://www.wjgnet.com/1009-3079/full/v13/i24/2833.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i24.2833
γ-氨基丁酸 (gamma-aminobutyric acid, GABA) 是哺乳动物大脑中主要的抑制性神经介质, 但也存在于全身许多组织器官中并发挥着生物活性作用[1-3]. 在胚胎发育中, GABA已被证明对中枢神经以外多种组织的形态发生及成熟起重要作用[4,5], GABA及谷胺酸脱羧酶 (glutamic acid decarboxylase, GAD, 包括GAD65和GAD67) 在大鼠骨骺生长板中亦有表达, 而且主要分布于成熟带而非储备带或增殖带, 提示GABA在骨骼的生长及分化过程中发挥着一定的功能[6]. 生物化学及免疫组化研究证明, GABA及GAD在人类大肠癌组织中的表达明显高于癌周组织[7,8]. 然而, GABA及GAD在大肠上皮的表达和分布方式尚未明了, 我们检测大鼠降结肠上皮中GABA及GAD的表达, 探讨GABA系统与大肠上皮增殖和分化之间的关系.
兔抗大鼠GAD65多克隆抗体购自Sigma公司, 兔抗大鼠GABA多克隆抗体及兔抗大鼠GAD67多克隆抗体购自Chemicon International Co.. 荧光标记羊抗兔IgG (二抗) Alexa Fluo TM 488 (H+L)及Alexa Fluo R594麦芽凝聚素 (wheat germ agglutinin, WGA) 购自Molecular Probes. 雄性Wistar大鼠 (4-6 wk), 体质量80-100g饲养于光照 (6: 00/18: 00) 及温度控制条件 (23℃), 给予动物正常进食及自由饮水. 将大鼠 (n = 5) 以苯巴比妥 (50 mg/kg) 深度麻醉, 然后以40 g/L中性缓冲甲醇任氏液经心内灌流固定, 待大鼠整体固定后, 切取降结肠节段 (距肛门口5 cm处)浸于4 g/L中性缓冲甲醇PBS 溶液中, 然后转至300 g/L蔗糖溶液中过夜, 常规冰冻切片 (厚5 µm).
1.2.1 3H-胸腺嘧啶放射自显影: 于10: 00给大鼠 (n = 2) 3.7MBq 3H-胸腺嘧啶, ip, 90 min后, 麻醉动物并以25 g/L戊二醛心内灌流固定, 降结肠标本取材同上. 常规石蜡切片 (厚5 µm). 显影方法[9]: 组织切片常规脱蜡, 用等体积双蒸水稀释的NM-2乳胶液涂片, 置于暗冰箱暴光10 d后将切片依次置于Kodak D-19中显影 (20℃, 8 min)、10 ml/L醋酸中1 min终止反应及300 g/L硫代硫酸钠中固定 (20℃, 8 min). 最后, 以苏木素淡染细胞核.
1.2.2 RNA提取和逆转录-多聚酶链式反应(RT-PCR): 将大鼠麻醉后行Ringer液行心内灌注. 然后, 切取降结肠组织, 并用Rneasy mini kit制剂盒提取总RNA. GAD65扩增片段长度209 bp, 基因组编号M27422, 引物系列为上游5′-GTCATCTCAAACCCTGCA-3′, 下游5′-ACTGGACTCTACTGTGAC-3'; GAD67扩增片段为303 bp, 编号M76177, 引物系列为上游5′-GCTGGAAGGATGGAAGGTTTTAA-3′, 下游 5′-AATATCCCATCACCATCTTTATTTGACC-3′. PCR 反应在GeneAmy 9700 PCR仪进行. 反应条件为: 先94℃变性 2 min; 然后, 再94℃ 变性30 s, 60℃ 退火30 s, 72℃延伸60 s, 共38个循环; 最后, 72℃延伸7 min. 取PCR反应物10 µL加样于15 g/L琼脂糖凝胶电泳, 显色紫外灯观察.
1.2.3 原位分子杂交: GAD65 的互补寡聚核苷酸探针系列为5'-GGCTTCTCAGAGTCTCCGTAGAGCAGAGCGCATAGCTTGTTTCCGATGCC-3′, GAD67为: 5′-TAGTATTAGGATCCGCTCCCGCGTTCGAGGAGGTTGCAGGCGAAGGCG-3′, 均由UltraFast Parallel Synthesizer合成 (Genset), 并且于3′-末端以地高辛标记(DIG-ddUTP, Roche Diagnostics GmbH), 有意义探针作为阴性对照. 杂交方法[10]: 将石蜡切片常规脱蜡、脱水后, 置于0.2 mol/L HCl/PBS中在室温下作用15 min, 然后以蛋白酶K消化 (10 g/L) 10 min. 再用PBS 洗涤后以40 g/L中心缓冲福尔马林重新固定. 杂交过程: 依次用2 mL/L甘油/PBS 洗涤2次、2.5 g/L乙酸酐/0.1 mmol/L三乙醇胺 (pH 8.0) 乙酰化10 min、乙醇脱水后加入杂交缓冲液 (450 g/L去离子甲酰胺, 10 mol/L Tris-HCl pH 7.6, 200 µg/mL t RNA 1倍数Denhard 液, 100 g/L糖酐硫酸酯, 600 mmol /L NaCl, 2.5 g/L SDS, 1 mmol/L EDTA, pH 8.0), 分别加入杂交探针至其终浓度为5-20 mg/L. 杂交条件为37℃、16 h. 杂交后将切片分别以500 mL/L甲酰胺、5 倍 SSC 溶液、1 倍 SCC 溶液 和 0.1 mmol/L Tris-HCl (pH 7.5) 缓冲液中于50℃洗涤1次, 再与阻断剂 (100 mmol/L Tris-HCl, 1 mmol/L EDTA, pH 8.0)作用 60 min. 最后将切片与碱性磷酸酶-抗地高辛抗体孵育后显色.
1.2.4 免疫荧光与WGA组织化学双重染色: 抗GABA、GAD65及GAD67多克隆抗体分别稀释至1︰800、1︰1 000及1︰1 000. 首先将冰冻切片置于预冷的丙酮中固定10 min, 再用100 g/L绵羊血清室温下封闭60 min, 然后, 加入一抗于4℃冰箱中过夜; 再将二抗加入切片中室温下孵育60 min. 最后, 以0.01 mol/L PBS 洗涤2次, 再加入Alexa Fluo R594 WGA在暗室中孵育60 min, 然后以0.01 mol/L PBS冲洗3次, 封片固定保存于4℃冰箱中待观察. 以PBS代替一抗的切片作阴性对照. 荧光染色等切片均用氩激光共聚焦显微镜观察 (Radiance 2 000), 采用相差扫描模式 (DIC) 及共聚焦扫描模式[6], 分别使用氩激光波长为488 nm及590 nm. 为叙述方便, 将结肠上皮分为腔面以及隐窝上、中、下三个部分[11], 3H-胸腺嘧啶标记指数为10个隐窝中阳性细胞数占总数的百分比之平均值[12].
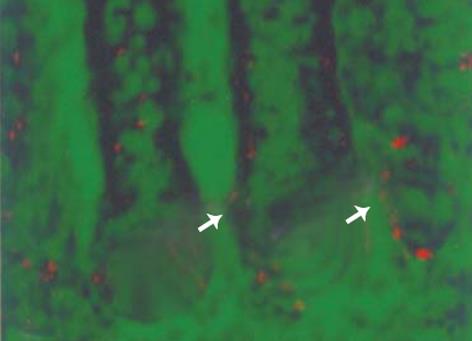
阳性标记细胞核主要位于降结肠隐窝的中部和下部, 而上部及腔面均为阴性 (图1). 降结肠隐窝上皮细胞3H-胸腺嘧啶阳性标记指数为 23.3%±6.5%.
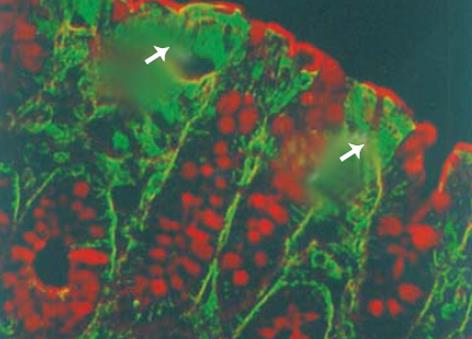
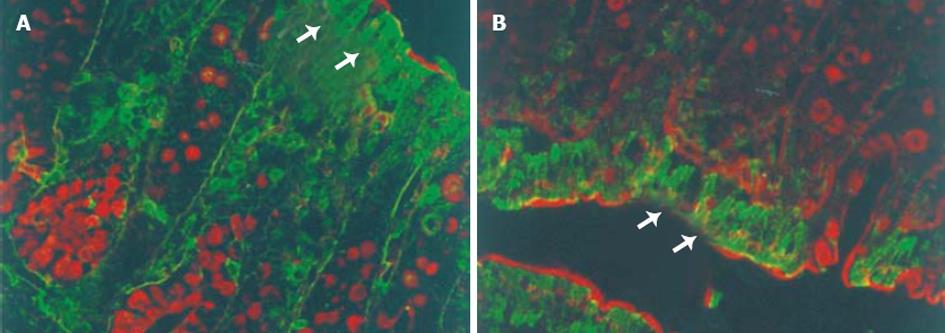
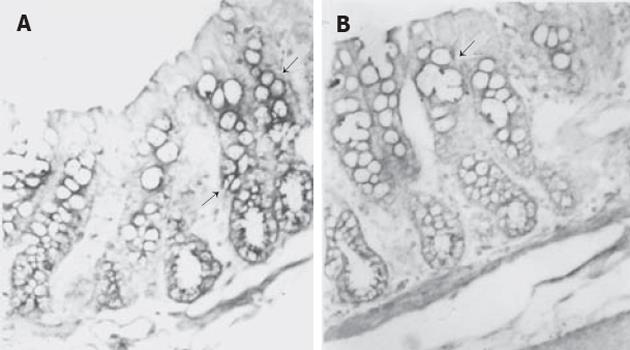
大鼠降结肠上皮GABA和GAD65免疫反应阳性细胞分布于腔面和隐窝的上1/3的上皮细胞 (图2, 3A), 强阳性染色主要位于腔面及隐窝颈部上皮细胞的细胞质中. 此外, 黏膜固有层还散布着少许GABA和GAD65强阳性着色. WGA荧光染色将杯状细胞清晰地显示出来, 黏膜上皮表面的黏液层也为WGA阳性, 双重染色显示GABA和GAD65强阳性细胞WGA阴性, 即不是杯状细胞 (图2,3A). 然而, GAD67免疫反应阳性细胞仅分布于腔面上皮细胞, 且着色程度较GAD65弱 (图3B). 免疫反应阳性GABA和 GAD的分布 (表1) 与3H-胸腺嘧啶标记细胞的分布正好相反, 即GABA和 GAD65阳性表达见于降结肠的成熟带和功能带, 而不是增殖带.
| 隐窝 | 腔面 | |||
| 下 | 中 | 上 | ||
| 3H-Thymidine | +++ | ++ | ± | - |
| GABA | ± | + | ++ | +++ |
| GAD65 | - | + | ++ | +++ |
| GAD67 | - | - | - | ++ |
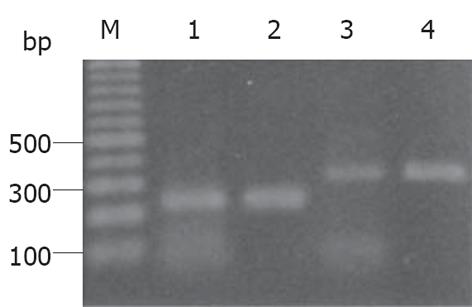
mRNA在大鼠降结肠组织提取物中均有表达(图4), 大鼠大脑RNA提取物作为阳性对照.
GAD65及 GAD67 mRNA在大鼠降结肠黏膜上皮细胞中均有表达, 而各增殖带之间的区别不明显, 并且GAD67 mRNA信号较之GAD65弱 (图5). 有意义探针对照切片无特异信号表达.
哺乳动物结肠上皮细胞的分化增殖模式与小肠类似, 大鼠及小鼠大肠的增殖带干细胞位于隐窝的下部, 向腔面生长和移动的同时逐步增殖和分化, 更新时间为3-5 d[13], 传统的细胞动力学检测方法为3H-胸腺嘧啶放射自显影法, 其原理为DNA合成期细胞摄取胸腺嘧啶, 故3H-胸腺嘧啶可作为S期细胞的标志, 新近有作者用5-溴脱氧尿苷、增殖细胞核抗原 (PCNA) 和其他细胞周期蛋白作为增殖细胞的标记物, 研究结果与传统理论一致[14-16]. 基于这些研究, Bartkowa et al[17]将肠上皮细胞分为4个部分: 隐窝基底部的干细胞, 隐窝下部与中部的增殖带, 隐窝上部的成熟带, 以及整个腔面的功能区. 我们用3H-胸腺嘧啶放射自显影法得到了与经典模式一致的结果, 即结肠上皮细胞的增殖带位于隐窝的下部及中部. 免疫荧光染色的结果显示, GABA及GAD65阳性分布主要在结肠腔面和隐窝的上1/3, GAD67分布于腔面, 表明GABA及GAD阳性细胞主要见于分化良好的上皮而非增殖的上皮, 即位于成熟带及功能带而非增殖带. 杯状细胞用植物血凝素组织化学技术清晰地显示出来, 因为WGA可特异性地与杯状细胞黏液中的糖基结合[18]. 双重染色显示, GABA和GAD强阳性不是杯状细胞. 已知肠上皮细胞经由干细胞分化成吸收上皮细胞、杯状细胞、内分泌细胞和潘氏细胞[19], 我们推测这些表达GABA和GAD的细胞为结肠吸收上皮或内分泌细胞. 我们首次报道大鼠降结肠上皮细胞有GABA和GAD表达, 而这种特征性分布的意义尚待进一步探索.
Kaita et al[20]报道GABA-A 受体拮抗剂环丙沙星在乙醇性肝硬化动物模型中能显著增强肝脏再生活性, PCNA染色结果显示, 生理盐水组和环丙沙星组PCNA 阳性肝细胞分别为13.4±3.7和47.4±7.3. 化学诱癌动物模型研究则表明, GABA-A和GABA-B受体激动剂可抑制大肠肿瘤的发生与生长[21]. Gilon et al[4]研究发现, 在发育胚胎的胰腺及十二指肠快速生长及成熟结束时可检测到一个GABA高峰, 这一结果与早先关于大脑发育的研究一致[22], 大鼠大脑发育过程中, GABA可诱导细胞膜去极化而抑制皮层细胞的增殖, 并且可能通过GABA-A受体介导的细胞内钙离子浓度升高而起作用, 然而, GABA引起细胞膜去极化及抑制DNA合成的机制尚未明了[3,23]. 已有实验证实, GABA和GABA-A受体在大鼠和人类的结肠上皮均有表达[24,25];我们的前期研究表明, GABA和GAD在大鼠空肠上皮增殖带分布也有类似的特征, 即主要表达在成熟带或功能带而非增殖带[26]. 这些结果提示, GABA和GAD的特异性分布GABA可能通过GABA-GABA受体途径参与上皮细胞增殖和分化的调节.
已知GABA主要由GAD催化谷氨酸脱羧生成, GAD有GAD65及GAD67两种异构体, 因其分子量分别为65 300及66 600得名[27], 二者由不同基因编码, 且在进化上高度保守, 基因发生学研究表明, 脊椎动物中枢神经系统GAD基因的多样性源于500万年前[28], 虽然GAD的两种异构体催化同一生化反应, 其分布特征却不尽相同, 并可能发挥着不同的功能[29,30]. 我们的结果表明, 大鼠降结肠黏膜上皮主要表达GAD65而非GAD67, 进一步支持GAD在哺乳动物神经组织以外分布的多样性.
电编: 张勇 编辑: 潘伯荣 审读: 张海宁
| 1. | Watanabe M, Maemura K, Kanbara K, Tamayama T, Hayasaki H. GABA and GABA receptors in the central nervous system and other organs. Int Rev Cytol. 2002;213:1-47. [PubMed] [DOI] |
| 2. | Fujimura S, Shimakage H, Tanioka H, Yoshida M, Suzuki-Kusaba M, Hisa H, Satoh S. Effects of GABA on noradrenaline release and vasoconstriction induced by renal nerve stimulation in isolated perfused rat kidney. Br J Pharmacol. 1999;127:109-114. [PubMed] [DOI] |
| 3. | Azuma H, Inamoto T, Sakamoto T, Kiyama S, Ubai T, Shinohara Y, Maemura K, Tsuji M, Segawa N, Masuda H. Gamma-aminobutyric acid as a promoting factor of cancer metastasis; induction of matrix metalloproteinase production is potentially its underlying mechanism. Cancer Res. 2003;63:8090-8096. [PubMed] |
| 4. | Gilon P, Mallefet J, De Vriendt C, Pauwels S, Geffard M, Campistron G, Remacle C. Immunocytochemical and autoradiographic studies of the endocrine cells interacting with GABA in the rat stomach. Histochemistry. 1990;93:645-654. [PubMed] [DOI] |
| 5. | Tozuka Y, Fukuda S, Namba T, Seki T, Hisatsune T. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803-815. [PubMed] [DOI] |
| 6. | Tamayama T, Kanbara K, Maemura K, Kuno M, Watanabe M. Localization of GABA, GAD65 and GAD67 in rat epiphyseal growth plate chondrocytes. Acta Histochem Cytochem. 2001;34:201-206. [DOI] |
| 7. | Kleinrok Z, Matuszek M, Jesipowicz J, Matuszek B, Opolski A, Radzikowski C. GABA content and GAD activity in colon tumors taken from patients with colon cancer or from xenografted human colon cancer cells growing as s.c. tumors in athymic nu/nu mice. J Physiol Pharmacol. 1998;49:303-310. [PubMed] |
| 8. | Maemura K, Yamauchi H, Hayasaki H, Kanbara K, Tamayama T, Hirata I, Watanabe M. Gamma-amino-butyric acid immunoreactivity in intramucosal colonic tumors. J Gastroenterol Hepatol. 2003;18:1089-1094. [PubMed] [DOI] |
| 9. | Kuroda E, Watanabe M, Tamayama T, Shimada M. Autoradiographic distribution of radioactivity from 14C-GABA in the mouse. Micros Res Tech. 2000;48:116-126. [PubMed] [DOI] |
| 10. | Sun Z, Wang HB, Laverghetta A, Yamamoto K, Reiner A The distribution and cellular localization of glutamic acid decarboxylase-65 (GAD65) mRNA in the forebrain and midbrain of domestic chick. J Chem Neuroanat. 2005;29:265-281. [PubMed] [DOI] |
| 11. | Bunpo P, Kataoka K, Arimochi H, Nakayama H, Kuwahara T, Bando Y, Izumi K, Vinitketkumnuen U, Ohnishi Y. Inhibitory effects of Centella asiatica on azoxymethane-induced aberrant crypt focus formation and carcinogenesis in the intestines of F344 rats. Food Chem Toxicol. 2004;42:1987-1997. [PubMed] [DOI] |
| 13. | Thompson JS, Saxena SK, Sharp JG. Regulation of intestinal regeneration: new insights. Microsc Res Tech. 2000;51:129-137. [PubMed] [DOI] |
| 14. | Shpitz B, Bomstein Y, Mekori Y, Cohen R, Kaufman Z, Grankin M, Bernheim J. Proliferating cell nuclear antigen as a marker of cell kinetics in aberrant crypt foci, hyperplastic polyps, adenomas, and adenocarcinomas of the human colon. Am J Surg. 1997;174:425-430. [PubMed] [DOI] |
| 15. | Kim SJ, Turner S, Killion S, Hellerstein MK. In vivo measurement of DNA synthesis rates of colon epithelial cells in carcinogenesis. Biochem Biophys Res Commun. 2005;331:203-309. [PubMed] [DOI] |
| 16. | Sun BC, Zhao XL, Zhang SW, Liu YX, Wang L, Wang X. Sulindac induces apoptosis and protects against colon carcinoma in mice. World J Gastroenterol. 2005;11:2822-2826. [PubMed] [DOI] |
| 17. | Bartkova J, Thullberg M, Slezak P, Jaramillo E, Rubio C, Thomassen LH, Bartek J. Aberrant expression of G1-phase cell cycle regulators in flat and exophytic adenomas of the human colon. Gastroenterology. 2001;120:1680-1688. [PubMed] [DOI] |
| 18. | Bryk SG, Sgambati E, Gheri-Bryk G. Lectin histochemistry of goblet cell sugar residues in the gut of the chick embryo and of the newborn. Tissue-Cell. 1999;31:170-175. [PubMed] [DOI] |
| 19. | Kong SE, Heel K, McCauley R, Hall J. The role of enterocytes in gut dysfunction. Pathol Res Pract. 1998;194:741-751. [PubMed] [DOI] |
| 20. | Kaita KD, Assy N, Gauthier T, Zhang M, Meyers AF, Minuk GY. The beneficial effects of ciprofloxacin on survival and hepatic regenerative activity in a rat model of fulminant hepatic failure. Hepatology. 1998;27:533-536. [PubMed] [DOI] |
| 21. | Tatsuta M, Iishi H, Baba M, Taniguchi H. Attenuation by the GABA receptor agonist baclofen of experimental carcinogenesis in rat colon by azoxymethane. Oncology. 1992;49:241-245. [PubMed] [DOI] |
| 22. | LoTurco JJ, Owens DF, Heath MJ, Davis MB, Kriegstein AR. GABA and glutamate depolarize cortical progenitor cells and inhibit DNA synthesis. Neuron. 1995;15:1287-1298. [PubMed] [DOI] |
| 23. | Deisseroth K, Malenka RC. GABA excitation in the adult brain: a mechanism for excitation- neurogenesis coupling. Neuron. 2005;47:775-777. [PubMed] [DOI] |
| 24. | Bayer S, Crenner F, Aunis D, Angel F. Effects of GABA on circular smooth muscle spontaneous activities of rat distal colon. Life Sci. 2002;71:911-925. [PubMed] [DOI] |
| 25. | Tedeschi L, Carai MA, Frison G, Favretto D, Colombo G, Ferrara SD, Gessa GL. Endogenous gamma-hydroxybutyric acid is in the rat, mouse and human gastrointestinal tract. Life Sci. 2003;72:2481-2488. [PubMed] [DOI] |
| 26. | Wang FY, Watanabe M, Zhu RM, Maemura K. Characteristic expression of gamma-aminobutyric acid and glutamate decarboxylase in rat jejunum and its relation to differentiation of epithelial cells. World J Gastroenterol. 2004;10:3608-3611. [PubMed] [DOI] |
| 27. | Ahman AK, Wagberg F, Mattsson MO. Two glutamate decarboxylase forms corresponding to the mammalian GAD65 and GAD67 are expressed during development of the chick telencephalon. Eur J Neurosci. 1996;8:2111-2117. [PubMed] [DOI] |
| 28. | Bosma PT, Blazquez M, Collins MA, Bishop JD, Drouin G, Priede IG, Docherty K, Trudeau VL. Multiplicity of glutamic acid decarboxylases (GAD) in vertebrates: molecular phylogeny and evidence for a new GAD paralog. Mol Biol Evol. 1999;16:397-404. [PubMed] [DOI] |
| 29. | Cram DS, Faulkner-Jones B, Kun J, Harrison LC. Glutamic acid decarboxylase-67 (GAD67): expression relative to GAD65 in human islets and mapping of autoantibody epitopes. Endocrinology. 1995;136:1111-1119. [PubMed] [DOI] |
| 30. | Katarova Z, Sekerkova G, Prodan S, Mugnaini E, Szabo G. Domain-restricted expression of two glutamic acid decarboxylase genes in midgestation mouse embryos. J Comp Neurol. 2000;424:607-627. [PubMed] [DOI] |