修回日期: 2005-08-20
接受日期: 2005-08-26
在线出版日期: 2005-11-28
目的: 研究胃癌高、低发区差异基因型幽门螺杆菌(Helicobacter pylori, H. pylori)菌株对永生化的非肿瘤胃上皮细胞GES-1的损伤作用; 并阐明H. pylori致细胞损伤的分子机制.
方法: 通过常规HE染色、Gimsa染色、W-S银染色鉴定H. pylori菌株, 采用蛋白酶K及酚-氯仿抽提法提取DNA, PCR法扩增cagA、vacAs1、s2、m1a、m1b、m2基因, 对H. pylori基因亚型进行检测. 利用细胞和幽门螺杆菌共培养技术, 观察胃癌高低发区差异基因亚型H. pylori以及其他两种非差异基因型H. pylori对胃上皮细胞系GES-1的损伤作用, 并通过免疫细胞化学方法检测GES-1细胞8-OHdG的表达.
结果: 幽门螺杆菌对GES-1细胞具有损伤作用, 随着作用时间的延长, GES-1细胞形态由贴壁的梭形变为圆形, 细胞核染色质浓缩, 呈新月形聚集于核缘, 细胞质浓缩, 可见空泡变性, 死亡细胞逐渐增多. 差异基因型H. pylori对GES-1细胞的损伤程度比非差异基因型的损伤作用重. 正常对照组8-OHdG表达基本阴性(0.5%), 而H. pylori处理组GES-1细胞8-OHdG表达阳性达98.5%, 二者差异显著(P<0.01), 有统计学意义.
结论: 胃癌高发区差异基因型H. pylori即cagA+, vacAs1+/m1b+对胃上皮细胞GES-1具有更强损伤作用, 其损伤具有时间依赖关系; H. pylori可能通过增加胃上皮细胞8-OhdG表达而导致正常胃黏膜上皮细胞恶性转化..
引文著录: 何红梅, 宫月华, 袁媛. 胃癌高低发区差异基因型幽门螺杆菌菌株对人胃上皮细胞系GES-1的损伤作用. 世界华人消化杂志 2005; 13(22): 2681-2684
Revised: August 20, 2005
Accepted: August 26, 2005
Published online: November 28, 2005
AIM: To investigate the damage effect of the different genotypes of Helicobacter pylori (H. pylori) on human gastric epit-helial cell line GES-1 in high- and low-risk areas of ga-stric cancer, and to explore its related mechanism.
METHODS: H. pylori were identified by hematoxylin-eosin (HE) staining, Gimsa staining, and Warthin-Starry silver staining. The DNA was obtained by proteinase K and phenol-chloroform extraction method. The cagA, vacAs1/s2, m1a, m1b, and m2 gene were amplified by polymerase chain reaction (PCR). The damage eff-ects of H. pylori with differential sub-genotypes and 2 other non-differential genotypes on GES-1 cells were observed by cell and H. pylori co-culture. The expression of 8-OHdG in GES-1 cells was detected by S-P immun-ohistochemistry.
RESULTS: GES-1 cells were seriously damaged by H. pylori. With the prolongation of the co-culture time, the morphology of GES-1 cells were changed from spindle to round, and the nuclei showed chromatin pyknosis and clustered on the inner border of karyon. The cytoplasm condensed and blebbing appeared. The numbers of the dead and damaged cells were increas-ing. The damage effect of H. pylori with differential ge-notypes on GES-1 cells was more serious than that of the non-differential genotypes. The expression of 8-OHdG were almost all negative (0.5% positive) in GES-1 cells of the normal controls, while the positive rate was 98.5% in the H. pylori treatment cells (P < 0.01).
CONCLUSION: H. pylori with cagA+, vacAs1+/m1b+ ge-notypes in the high-risk area of gastric cancer have more serious damage effects on gastric cancer cell line GES-1, and they can promote the transformation of normal gastric epithelial cells to malignant cells by up-regulation of 8-OHdG expression.
- Citation: He HM, Gong YH, Yuan Y. Damage effect of different genotype of Helicobacter pylori on human gastric epithelial cell line GES-1 in high- and low-risk areas of gastric cancer. Shijie Huaren Xiaohua Zazhi 2005; 13(22): 2681-2684
- URL: https://www.wjgnet.com/1009-3079/full/v13/i22/2681.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i22.2681
幽门螺杆菌(Helicobacter pylori, H. pylori)是慢性胃炎、消化性溃疡的病因, 并与胃腺癌和黏膜相关性淋巴瘤等疾病密切相关[1-5]. 但H. pylori致癌的机制目前还不是很清楚, 目前研究也不足以解释H. pylori感染后发生胃癌的差异性, 有人认为这可能与研究中所应用的菌株或细胞系不同有关.
有证据表明, H. pylori基因亚型的遗传学差别对产生不同的临床结局具有重要作用, 特别是致病相关基因如: cagA、vacA和iceA等的差异, 与感染的临床结局密切相关[6]. 辽宁庄河是我国北方胃癌高发区, 胃癌死亡率为50/10万, 本室前期工作发现当地H. pylori检出率高达79.3%[7], 明显高于胃癌低发区沈阳, 胃癌高低发区H. pylori菌株基因型的分布存在明显差异, 其差异基因型组合为cagA+, vacAs1+/m1b+[8].
研究提示, 庄河胃癌高发区的胃癌高发与H. pylori感染有密切关系, 是否是由于差异基因型H. pylori是庄河地区胃癌高发的重要危险因素, 还不清楚. 我们通过比较胃癌高低发区差异基因型H. pylori菌株对永生化的非肿瘤胃上皮细胞GES-1的损伤作用, 以明确差异基因型H. pylori的致病能力; 检测共培养后GES-1细胞8-OHdG的表达, 以阐明其损伤的分子机制.
胃癌高发区高危人群胃黏膜培养出的不同基因亚型H. pylori(中国医科大学肿瘤研究所第三研究室提供), 胃黏膜上皮细胞系GES-1(北京市肿瘤防治研究所细胞研究室提供).
1.2.1 细菌培养: 我们在微需氧的条件下, 把本室菌库中的菌株接种于含70 mL/L羊血的脑心浸液琼脂培养基上, 置于37℃, 50 mL/L O2, 100 mL/L CO2, 相对湿度为97%的三气细菌培养箱内培养, 3-4 d后挑取阳性菌落传代, 涂于新板上, 48 h后收集.
1.2.2 幽门螺杆菌菌株鉴定: 形态学观察: 用常规HE染色、Gimsa染色、W-S银染色鉴定菌株. 用接种环挑取一个菌落, 涂片, 火焰固定细菌. 光学显微镜下观察, HE染色呈蓝色, Gimsa染色呈蓝紫色, W-S银染色见棕黑色或黑色的"S"型、弯曲状、海鸥状、杆状细菌者为H. pylori阳性. 聚合酶链反应: 采用蛋白酶K及酚-氯仿抽提法提取DNA, PCR法扩增cagA、vacAs1、s2、m1a、m1b、m2基因, 对H. pylori基因亚型进行检测. PCR反应条件如下: cagA: 94℃ 1 min, 50℃ 1 min, 72℃ 1 min, 72℃ 5 min; vacA: 94℃ 1 min, 52℃ 1 min, 72℃ 1 min, 72℃ 5 min; PCR扩增产物经20 g/L琼脂糖凝胶电泳分析, 紫外凝胶成像系统观察, 照相.
1.2.3 胃黏膜上皮细胞GES-1培养: 采用胃上皮永生化非肿瘤细胞系GES-1置于细胞培养箱, 于37℃, 50 mL/L CO2, 饱和湿度的环境培养, 用含10%胎牛血清的RPMI 1640培养基, 每周更换3次营养液, 并于细胞生长约80%融合时, 用2.5 g/L胰蛋白酶消化并以1∶3传代.
1.2.4 H. pylori与GES-1细胞共培养: 细胞处于对数生长期时, 用PBS调节H. pylori浓度6×108 CFU/mL, 按细胞/细菌(1∶200)比例加入不同亚型H. pylori, 共同孵育. 实验分组根据PCR结果进行: A组细胞加cagA+, vacAs1+/m1b+型H. pylori, B组细胞加cagA+, vacAs1-/m1b-型H. pylori, C组细胞加cagA-, vacAs1+/m1b+型H. pylori, D组细胞只加PBS空白对照.
1.2.5 S-P免疫组化法检测GES-1细胞8-OHdG表达: 细胞爬片后用冰丙酮固定15 min, 4℃ PBS冲洗3遍后, 利用S-P免疫组化法检测各组细胞8-OHdG表达. 经DAB显色, 细胞质内出现棕黄色为阳性.
统计学处理 用SPSS软件进行统计学处理: χ2检验, P<0.05, 认为8-OHdG在H. pylori阳性和阴性组细胞间表达差别有统计学意义.
细菌培养和形态学染色结果证实, 培养细菌为H. pylori, 基因亚型A组菌: cagA+, vacAs1+/m1b+, B组菌, cagA+, vacAs1-/m1b-, C组菌H. pylori即cagA-, vacAs1+/m1b+.
2.2.1 幽门螺杆菌对GES-1细胞的损伤作用: 正常情况下, GES-1细胞在倒置显微镜下观察呈菱形或多边形等不规则形状, 贴壁, 生长旺盛, 偶见漂浮细胞. 随着作用时间的延长, GES-1细胞形态由正常贴壁的多角形、梭形变为圆形, 贴壁细胞减少, 悬浮细胞增多, 并且细胞周围出现碎片; 细胞核染色质浓缩, 呈新月形聚集于核缘, 细胞质浓缩, 可见空泡变性, 死亡细胞逐渐增多; 死亡和受损伤的细胞逐渐增多; 随着时间的延长, 细胞培养液颜色变黄, 变混.
2.2.2 高低发区差异基因型H. pylori与非差异基因型H. pylori致损伤作用的比较: 倒置显微镜下, 观察到A组细胞加入高低发区差异基因型H. pylori后, 随着时间的变化, 细胞数明显减少, 细胞折光性减弱, 变圆, 与培养瓶附着没有以前紧密, 部分细胞脱离瓶壁, 悬浮细胞逐渐增多, 最后大多数细胞悬浮、死亡, 生长液中有大量的死细胞及细胞碎片悬浮; 存活细胞贴壁状态也不佳, 细胞轮廓变粗, 细胞中出现黑色颗粒, 空泡变性, 老化细胞增多, 细胞培养液变黄, 变浑. A组、B组、C组、D组细胞损伤程度比较, 以A组(差异基因型H. pylori)对GES-1细胞的损伤程度最重, 而非差异基因型的损伤作用较轻.
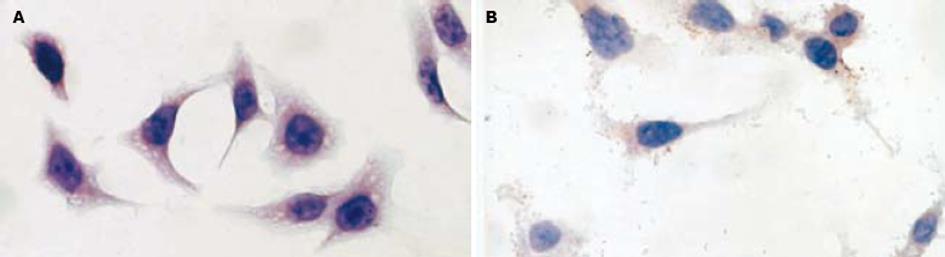
2.2.3 免疫组化8-OHdG表达的改变: 阴性对照组8-OHdG表达基本阴性, 阳性率0.5%(图1A), 而H. pylori处理组8-OHdG表达阳性率达98.5%, 二者差别有统计学意义 (P<0.05, 图1B).
GES-1是胎儿胃黏膜上皮细胞经SV40转化而建立的人胃黏膜上皮细胞永生化细胞株, 现已证明GES-1为一基本正常的胃黏膜细胞株[9-11]. GES-1是了解正常胃黏膜细胞离体特征以及胃癌致病机制的良好细胞株. 以往对H. pylori致病性研究多使用胃癌细胞系, 本研究使用接近正常胃上皮细胞的 GES-1, 更能了解H. pylori的体内致病性.
H. pylori的DNA指纹图和限制性片段长度多肽等方法研究表明在H. pylori不同分离株间存在巨大的遗传变异, 其遗传异质性大于多种细菌[7,8]. 这种遗传学的差别产生不同的临床结局, 特别是H. pylori致病相关基因如: cagA、vacA和iceA等的差异, 与感染的临床结局密切相关[9]. 来自中国胃癌高发区研究发现cagA+菌株感染与各型胃疾病、胃癌前病变等均呈明显正相关[10]. 流行病学结果分析, vacA等位基因各种组合的产毒能力不同: s1m1型vacA基因的菌株产生高水平毒素; s1m2型菌株产生低至中等水平的毒素; 而s2m2型菌株不产生有活性的毒素 [11].
中国医科大学肿瘤研究所第三研究室在十五期间的分析发现庄河地区H. pylori感染率高达79.3%, 差异基因型为cagA+, vacAs1+/m1b+, 与沈阳低发区的H. pylori菌株类型差异显著; vacA基因亚型两地具有显著性差异. 本研究通过H. pylori对体外人胃黏膜细胞作用发现, 胃癌高低发区差异基因型H. pylori cagA+, vacAs1+/m1b+, 对非肿瘤细胞GES-1具有明显的DNA损伤作用, 而且比非差异基因型H. pylori损伤强度更大, 结果提示, 在胃癌高发区, cagA+, vacAs1+/m1b+型H. pylori可能具有更大的细胞毒性, 从而导致胃癌的发生, 庄河地区的cagA+, vacAs1+/m1b+型H. pylori可能为辽宁庄河地区胃癌高发的环境致癌因素.
研究认为, H. pylori代谢产物对胃上皮的基因毒性作用在H. pylori致癌作用中占重要地位, 特别是在由多因素多步骤参与的致癌始动阶段[12,13]. 机体内细胞的氧化损伤广泛存在, 8-羟基脱氧鸟苷(8-hydroxy-2'-deoxyguanine, 8-OHdG)是活性氧代谢产物之一, 目前已成为DNA氧化损伤中最常采用的生物标志物. 8-OHdG通过改变碱基配对的性质, 导致抑癌基因p53基因和ras原癌基因等基因发生难以修复的DNA G→T或A→C碱基颠换, 导致DNA误读, 突变, 从而启动细胞突变, 是癌变过程中的重要事件. H. pylori对胃上皮的基因毒性作用在致癌过程中占重要地位, 特别是在由多因素多步骤参与的致癌始动阶段[14,15]. 我们的研究结果表明, 胃上皮8-OHdG在H. pylori处理组明显高于无H. pylori组, 提示H. pylori使胃上皮细胞8-OHdG表达增加, 引起细胞DNA损伤, 这可能是H. pylori导致胃黏膜上皮细胞恶性转化的机制之一.
本研究GES-1细胞系由北京市肿瘤所遗传研究室柯杨教授提供.
电编: 张敏 编辑: 菅鑫妍 审读: 张海宁
| 1. | Graham DY. Helicobacter pylori infection in the pathogenesis of duodenal ulcer and gastric cancer: a model. Gastroenterology. 1997;113:1983-1991. [PubMed] [DOI] |
| 2. | Graham DY, Yamaoka Y. H pylori and cagA: relationships with gastric cancer, duodenal ulcer, and reflux esophagitis and its complications. Helicobacter. 1998;3:145-151. [PubMed] [DOI] |
| 3. | Isaacson PG, Spencer J. Is gastric lymphoma an infectious disease? Hum Pathol. 1993;24:569-570. [PubMed] [DOI] |
| 4. | Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991;325:1132-1136. [PubMed] [DOI] |
| 5. | Parsonnet J, Friedman GD, Vandersteen DP, Chang Y, Vogel-man JH, Orentreich N, Sibley RK. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127-1131. [PubMed] [DOI] |
| 6. | Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Gra-ham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999;37:2274-2279. [PubMed] |
| 8. | Gong YH, Wang Y, Yuan Y. Distribution of Helicobacter pylori in north China. World J Gastroenterol. 2005;11:3523-3527. [PubMed] [DOI] |
| 9. | Ke Y, Ning T, Wang B. Establishment and characterization of a SV40 transformed human fetal gastric epithelial cell line-GES-1. Zhonghua Zhongliu Zazhi. 1994;16:7-10. |
| 10. | Zhang J, Ke Y, Ning T. Glucocorticoid-induced apoptosis of human gastric epithelial cells transfected with p53 genes. Zhonghua Zhongliu Zazhi. 1996;18:328-330. |
| 11. | Zheng S, Ke Y. Study of APC, Rb, c-met gene copy numbers of human gastric mucosa epithelial cell line GES-1. Zhonghua Zhongliu Zazhi. 1999;21:409-411. |
| 12. | Baik SC, Youn HS, Chung MH, Lee WK, Cho MJ, Ko GH, Park CK, Kasai H, Rhee KH. Increased oxidative DNA damage in Helicobacter pylori-infected human gastric mucosa. Cancer Res. 1996;56:1279-1282. [PubMed] |
| 13. | Farinati F, Cardin R, Degan P, Rugge M, Mario FD, Bonvicini P, Naccarato R. Oxidative DNA damage accumulation in gastric carcinogenesis. Gut. 1998;42:351-356. [PubMed] [DOI] |
| 14. | Akopyanz N, Bukanov NO, Westblom TU, Kresovich S, Berg DE. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137-5142. [PubMed] [DOI] |
| 15. | Marshall DG, Coleman DC, Sullivan DJ, Xia H, O'Morain CA, Smyth CJ. Genomic DNA fingerprinting of clinical isolates of Helicobacter pylori using short oligonucleotide probes containing repetitive sequences. J Appl Bacteriol. 1996;81:509-517. [PubMed] [DOI] |