修回日期: 2005-09-10
接受日期: 2005-10-11
在线出版日期: 2005-11-28
目的: 设计人端粒酶逆转录酶(hTERT)的新型病毒样颗粒疫苗, 并鉴定其表达蛋白的免疫原性和疫苗转染效率 .
方法: 合成阳离子抗原肽K18P9, 同时将人粒细胞、巨噬细胞集落刺激因子(hGM-CSF)和hTERT的克隆入真核双表达载体pTCAE中, 再将多肽和核酸疫苗结合于同一疫苗颗粒, 并将其转染入真核细胞内, ELISA法和免疫印迹法检测hGM-CSF和hTERT的免疫原性, 同时评价疫苗的转染效率 .
结果: 酶切及测序结果证实已成功构建含hGM-CSF和hTERT的载体pTGH, 电镜等结果证实核酸被包装肽包裹形成病毒样颗粒, ELISA法和免疫印迹法证实疫苗在体外对真核细胞的转染效应以及hGM-CSF和hTERT的免疫原性, 与阳性对照DOTAP组比较, 疫苗转染效率约可达78.5% .
结论: 成功构建了具有免疫原性的hTERT肽-核酸病毒样颗粒疫苗 .
引文著录: 郭红, 郝嘉, 吴超, 房殿春. 端粒酶逆转录酶肽-核酸病毒样颗粒疫苗的制备及免疫原性鉴定. 世界华人消化杂志 2005; 13(22): 2645-2649
Revised: September 10, 2005
Accepted: October 11, 2005
Published online: November 28, 2005
AIM: To construct a novel virus-like particle peptide-nucleic acid vaccine (VPNV) of human telomerase reverse transcriptase (hTERT), and to identify its imm-unogenicity and transfection activity.
METHODS: Cationic antigenic peptide K18P9 was syn-thesized and purified, then human GM-CSF and TERT gene were cloned into eukaryotic expression vector pTCAE. The peptide was combined with the nucleic acid vaccine to make VPNV, which were transfected into eukaryotic cells COS-7. The immunogenicity of hGM-CSF and hTERT were detected by enzyme linked imm-unosorbent assay (ELISA) and Western blotting.
RESULTS: Restriction enzyme digestion and sequen-ce analysis confirmed that hGM-CSF and hTERT were cloned into pTCAE and the nucleic acid vaccine of hTERT gene was constructed successfully. Under ele-ctronic microscopy, nucleic acid was packaged by pep-tide, forming into virus-like particle. Furthermore, the transfection activity of VPNV and the immunogenic-ity of hGM-CSF and hTERT could reach 78.5% as co-mpared with the positive controls.
CONCLUSION: The VPNV is successfully constructed, and its immunogenicity is also identified.
- Citation: Guo H, Hao J, Wu C, Fang DC. Construction of virus-like particle peptide-nucleic acid vaccine of human telomerase reverse transcriptase and identification of its immunogenicity. Shijie Huaren Xiaohua Zazhi 2005; 13(22): 2645-2649
- URL: https://www.wjgnet.com/1009-3079/full/v13/i22/2645.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i22.2645
人端粒酶逆转录酶(human telomerase reverse transcriptase, hTERT)为人端粒酶催化亚单位, 是癌细胞永生化的必要途径, 在消化道恶性肿瘤中大多过度表达[1-5], 且具有高度保守性[6-11], 因此如以hTERT为靶标制备消化道肿瘤疫苗, 将有望激发机体特异性抗肿瘤的免疫应答. 利用肿瘤疫苗有效激活特异性细胞毒T淋巴细胞(cytotoxic T lymphocyte, CTL), 是实现肿瘤主动免疫治疗的关键所在[12-17]. 目前高效的新型CTL肿瘤疫苗仍处于积极的研究和探索中. 我们结合基因治疗领域富正电荷肽类DNA转运载体的进展, 将正电荷包装肽与带负电荷质粒DNA结合, 并同时采用hTERT全长抗原蛋白及其低亲和力表位异形肽p572Y[18-20], 以人粒细胞、巨噬细胞集落刺激因子(human granulocyte macrophage colony stimulating factor, hGM-CSF)为免疫佐剂[21,22], 制备并鉴定了新型的病毒样颗粒肽-核酸疫苗(virus-like particulate peptide-nucleic acid vaccine, VPNV), 为进一步探索其抗肿瘤免疫作用奠定了基础 .
以AB431A型多肽合成仪(Pelin Elmer公司)按照标准Fomc方案合成阳离子抗原肽(K)18 YLFFYRKSV(其N端含18个赖氨酸, C端为P572Y, K18P9), 用ÄKTA explorer 100型中压液相色谱仪纯化, Delta 600型反向高压液相色谱仪鉴定纯度, 纯度>95%; API 2000LC/MS/MS型质谱仪测定相对分子质量为3528.8. 纯化产物冻干, -70℃冻存备用. 以pcDNA3.1-GM-CSF为模板扩增人GM-CSF cDNA, 并将其克隆入双表达真核载体pTCAE[23]的BglⅡ-EcoRⅠ表达盒中, 构建重组质粒pTG. 再将克隆有人端粒酶催化亚单位hTERT cDNA的质粒pGRN145(美国Geron公司惠赠)用EcoRⅠ酶切后克隆入载体pET28a中, 再行SalⅠ-BglⅡ消化, 并将含有hTERT的片段连接入pTG的SalⅠ-BamHⅠ(BglⅡ同尾酶)表达盒中, 形成hGM-CSF和hTERT双表达的重组质粒, 并命名为pTGH. 酶切鉴定pTGH并送公司测序. Kl8P9多肽分子中每个赖氨酸(K)的氨基(NH4+)携带一个正电荷, 故每个多肽Kl8P9分子携带18个正电荷; 而DNA分子中每个脱氧核糖核酸的平均分子量为330 Da, 含1个磷酸根(PO4-), 携带一个负电荷. 因此, 制备本疫苗时二者正负电荷的比值r反映了质粒与肽的用量关系为: M肽: MDNA = r×3 528.8/18∶330/1, 即M肽 = 0.594×r×MDNA, 同时可通过DNA阻滞试验和DNaseⅠ保护试验获得最佳r值, 以确定制备疫苗时肽与质粒的最佳相对用量.
参照文献[24]方案制备疫苗, 将不同量的阳离子抗原肽和2 μg质粒DNA分别溶于50 μL的150 mmol/L的NaC1水溶液中, 将肽溶液逐滴滴人混旋的DNA溶液中, 5 μL/min, 滴定完毕后产物继续混旋30 min, 室温静置60 min, 形成不同电荷比的多肽包裹的DNA聚合物. 制备r值分别为0, 0.5, 1.0, 2.0, 4.0, 8.0的不同阳离子肽-DNA聚合物, 各取3 μL样品于8 g/L琼脂糖凝胶电泳加样后电泳, 观察不同疫苗产物的泳动情况. 取制备产物50 μL, 与DNase Ⅰ(Sigma)在1×工作液(10 mmol/L CaC12, 5 mmol/L MgC12), 37℃孵15 min. 加入2 μL 0.5 mol/L的EDTA灭活, 再加入等体积25 g/L胰酶溶液消化40 min, 释放未降解的质粒DNA, 经2次1∶1酚氯仿抽提、乙醇沉淀后溶于水中. 8 g/L琼脂糖凝胶电泳后, 观察质粒留存情况. 6孔培养板内将COS-7细胞单层培养至覆盖面积30%. 更换培养液为2 mL无血清培养基, 加入100 μL制备好的疫苗, 同时用DOTAP法将5 μg质粒pTGH转入另组细胞, 孵育4 h, 更换完全培养基培养48 h, 收获细胞. 用ELISA检测hGM-CSF的表达水平, 由此分析疫苗体外转染效应的高低. 用常规Western方案, 以兔抗人hTERT抗体, 以辣根过氧化物酶标记的羊抗兔IgG为二抗, 经二氨基联苯胺(3, 3-diaminobenzidine, DAB)化学显色, 检测hTERT的表达. 将新鲜制备的疫苗15 μL滴于200目Í网的碳膜上, 室温5 min. 吸水纸吸干, 晾干30 s. 以20 g/L磷酸钨盐溶液负染30 s, 吸水纸吸干, 晾干30 s. 80 KV透射电镜观察.
1.2.1 真核表达载体的细胞转染: 用FuGENE6转染试剂将2 μg pcDNA3.1(-)-DNAPTP1及pcDNA3.1(-)空载体分别转染35 mm平皿HepG2细胞, 48 h后收获细胞.
1.2.2 细胞mRNA提取: 用mRNA纯化试剂盒, 直接提取转染了重组表达质粒及空载体的HepG2细胞mRNA, 经琼脂糖凝胶电泳及分光光度计进行定性、定量分析.
1.2.3 消减杂交文库的建立: 采用PCR-Select cDNA Subtraction Kit, 常规SSH方法按说明书进行: 转染了重组表达质粒及空载体的HepG2细胞cDNA分别标记为Tester和Driver, 经Rsa Ⅰ消化, 产生相对较短的平端片段, 纯化酶切产物. 将Tester的cDNA分为两份, 分别连接试剂盒提供的特殊设计的寡核苷酸接头Adapter 1和Adapter 2, 然后与过量的Driver cDNA进行杂交; 合并两种杂交产物后再与Driver cDNA做第2次杂交; 最后将杂交产物做选择性PCR扩增, 使Tester cDNA中特异性表达或高表达的片段得到特异性扩增.
1.2.4 消减文库扩增及克隆鉴定分析: 扩增产物与pGEM-Teasy载体连接, 转化DH5α感受态细菌, 在含氨苄青霉素的LB/X-gal/IPTG培养板上, 37℃培养16-18 h. 挑取白色菌落, 增菌, 以pGEM-Teasy载体多克隆位点两端T7/SP6引物进行菌落PCR扩增, 产物经20 g/L琼脂糖凝胶电泳鉴定, 证明含有插入片段(200-1 000 bp)后, 测序(上海联合基因公司). 应用生物信息学将测得序列与GenBank数据库进行同源性分析.
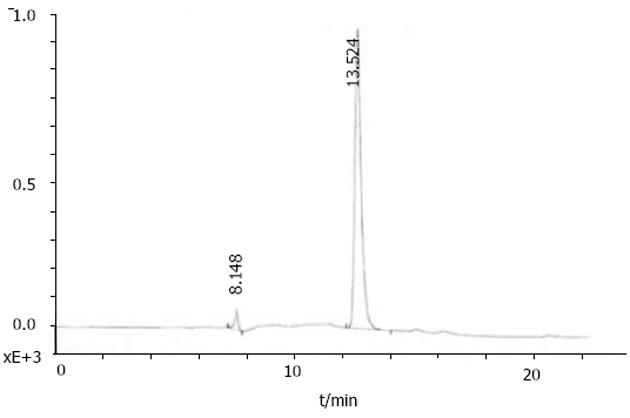
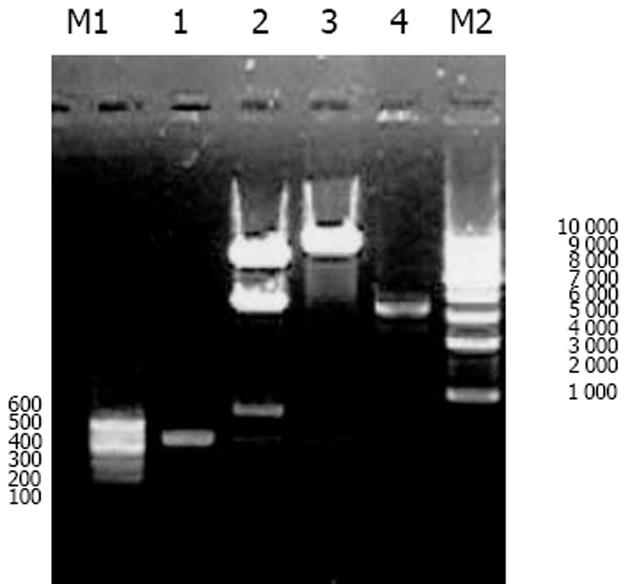
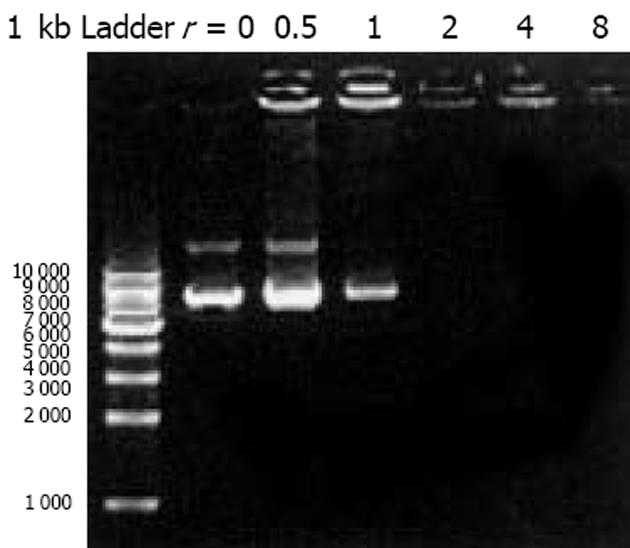
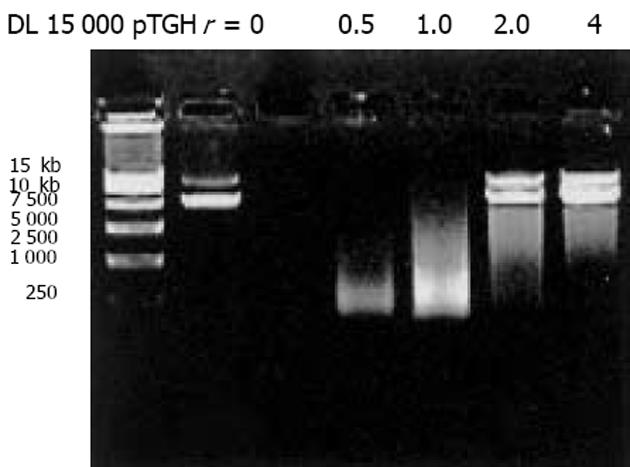
我们按照标准Fmoc方案合成阳离子双功能线性肽K18P9, 其中由18个多聚赖氨酸构成的K18可形成一个正电荷富集的DNA结合区, 而P9则为人端粒酶逆转录酶(hTERT)的隐性表位异形肽P572Y(YLFFYRKSV). 多肽经中压液相色谱纯化, 高压液相色谱鉴定, 其纯度达到国际多肽试验标准; 相对分子质量以质谱鉴定, 经质谱算法测定M为3 528, 与理论计算值基本相符, 证实合成纯化产物为目标多肽K18P9(图1). PCR扩增人GM-CSF, 产物大小与预期453 bp相符(泳道1), 并经BglⅡ和EcoRⅠ消化后克隆于双表达载体pTCAE相应位点, 产生重组质粒pTG. hTERT SalⅠ经和BglⅡ酶切, 回收片段为3 453 bp, 与预期相符(泳道4), 将其克隆入pTG的SalⅠ和BamHⅠ(BglⅡ的同尾酶)之间, 产生重组质粒pTGH. pTG和pTGH经BglⅡ和EcoRⅠ酶切鉴定(泳道3、2), pTG所得片段大小与hGM-CSF一致, pTGH所得片段分别为6 398 bp, 3 453 bp(泳道4)、726 bp和453 bp(泳道1), 与预期一致. pTGH中hGM-CSF和hTERT测序分别与Genebank中人GM-CSF和TERT序列一致(数据未列出), 证实pTGH构建成功. 采用DNA阻滞试验分析不同量多肽与DNA发生完全阻滞质粒泳动所需的最小r值(图3), 当r<2时, 阳离子肽-核酸疫苗的泳动被部分阻滞, 随着r值的逐渐增大, 泳动的聚合物数量迅速减少, 滞留在加样孔中的疫苗的DNA量相应增多. r>2时, 聚合物被完全阻滞, 提示质粒DNA被充分包装所需的最小r值为2. 以DNaseⅠ保护试验分析聚合物中多肽有效保护质粒DNA, 避免受DNase酶降解所需的最小r值(图4), 当r = 0时, 游离的DNA被DNA酶完全降解; 当r = 0.5和1.0时, 质粒DNA不能被阳离子肽有效保护; 但随着r值的增大被DNaseⅠ酶解的片段有所增大, 保护效应逐渐增加, 在r≥2.0时出现了完全保护的DNA条带, 因此确定DNA有效保护所需最小r值为2.
为鉴定肽-DNA聚合物在体外对哺乳细胞的转染效应以及双表达质粒编码hGM-CSF和hTERT表达后的免疫原性, 分别以r值为0, 1, 2, 4, 8的疫苗转染COS-7, 以DOTAP法转染裸质粒pTGH作为阳性对照, 通过ELISA法检测转染细胞上清中的GM-CSF的表达量, 从而评价其转染效应; 同时采用Western杂交分析hTERT表达的有效性. 结果发现当r = 4时, 可见有较高水平的GM-CSF表达, 即此时转染效率最高, 与阳性对照DOTAP组比较, 疫苗转染效率约为其78.5%, 当r为2或8时, GM-CSF表达水平明显下降. 本实验证实了双表达质粒编码的GM-CSF基因和hTERT基因均能在真核细胞内有效表达, 有望诱导较理想的免疫应答.
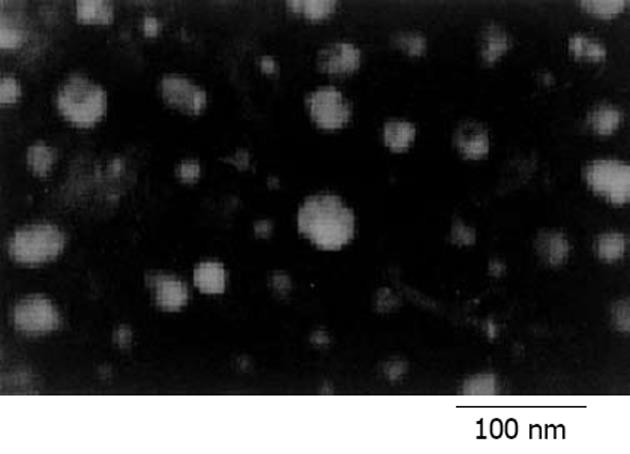
VPNV能否通过细胞核膜是基因表达的关键环节. 以r = 2.0时制备疫苗, 质粒DNA被阳离子肽充分包裹, 能有效抵抗DNA酶的降解, 这时才具备基因转染的能力, 但电镜下直径较大, 致使其转染效率较低, 因此相应细胞上清中的GM-CSF水平也较低; 当r = 4.0时, 形成的病毒样颗粒呈大小均一的近圆形颗粒, 且直径较小, 约10-40 nm, 绝大多数颗粒直径<25 nm, 如图5显示. 当r = 8时, 因过多的多肽包裹DNA, 使颗粒的直径更小而致密.
阳离子肽类DNA转运载体(cationic peptides DNA delivery systems)是基因治疗领域中一类倍受关注的技术[23-28], 他由一些富含正电荷氨基酸的多肽组成, 可通过电性中和作用与富含负电荷的质粒DNA发生聚合并压缩, 可将直径约数百纳米且松散的质粒DNA缩聚成几十纳米的致密颗粒, 从而易于被真核细胞摄入, 并可保证DNA不被核酸酶降解, 以达到基因转染的目的. 我们以端粒酶逆转录酶hTERT为靶标, 合成其隐性表位异形肽P572Y加多聚赖氨酸的多肽, 并制备含有编码hTERT和hGM-CSF基因的双表达真核质粒, 使二者结合形成一种新型的病毒样颗粒肽-核酸疫苗VPNV. 当二者正负电荷的比值r≥2后, 在DNA阻滞试验中阳离子抗原肽一方面中和了DNA的电荷; 另一方面, 使DNA分子在电场中泳动的阻力增加, 从而有效包装质粒DNA; 同时也保护质粒DNA免受DNaseⅠ的破坏. 有关阳离子肽类DNA转运载体的研究表明, 阳离子肽与质粒DNA的结合, 取决于多肽内含赖氨酸等碱性氨基酸的数目、多肽长度及其在溶液中的构象等因素, 还与所在溶液的离子强度有关[29,30]. VPNV经负染后裸质粒可见结构松散的闭环质粒分子, 缠绕折叠呈不规则形, 骨架疏松呈灰黑色高密度影, 此因质粒分子内部附着较多染料钨盐所致; 随着多肽/核酸比例增加, 二者形成的聚合物就越致密, 最终形成直径约几十纳米的致密圆形颗粒, 结构致密呈低密度影(灰白色), 其周围则为高密度影(灰黑色), 此因多肽和质粒聚缩成内部空隙少的致密颗粒, 无法附着钨盐, 而周围的碳膜上却吸附了相对较多的钨盐故呈高密度影. 这种因电性中和作用而形成的致密颗粒, 将有利于被抗原递呈细胞摄入加工[31]. 结合DNA阻滞实验、电镜以及体外转染效应试验的结果分析: 当r = 2时, 多肽可完全中和质粒的负电荷, 并形成聚合物, 此时才具备基因转染的能力, 但此时聚合物较松散, 直径较大(电镜); 当r = 4.0时, VPNV较易于被细胞摄入, 进入胞内的VPNV在溶酶体中被蛋白酶降解, 可释放出质粒DNA, 部分DNA可转移入胞质和胞核内, 进而被转录和·译, 由此出现较高的转染效率, 相应细胞上清中也可检测到较高水平的GM-CSF; 当r = 8.0时, 颗粒更致密, 但这样的颗粒进入胞内后, 其中的质粒可能反而不易释放处理, 进而阻碍了GM-CSF的表达, 使得细胞上清中的GM-CSF水平降低. 基于上述结果, 决定了在下一步的疫苗制备中采用r = 4.0, 以保证该疫苗在体内具备一定的转染活性. 本研究为下一步研究体内诱发特异性CTL应答奠定了基础, 该疫苗将有望激发机体特异性抗肿瘤的免疫应答.
电编: 张敏 编辑: 潘伯荣 审读: 张海宁
| 1. | Yang SM, Fang DC, Luo YH, Lu R, Battle PD, Liu WW. Alter-ations of telomerase activity and terminal restriction fragment in gastric cancer and its premalignant lesions. J Gastroenterol Hepatol. 2001;16:876-882. [PubMed] [DOI] |
| 6. | Vonderheide RH. Telomerase as a universal tumor-associated antigen for cancer immunotherapy. Oncogene. 2002;21:674-679. [PubMed] [DOI] |
| 7. | Vonderheide RH, Domchek SM, Schultze JL, George DJ, Hoar KM, Chen DY, Stephans KF, Masutomi K, Loda M, Xia Z. Vaccination of cancer patients against telomerase induces functional antitumor CD8+ T lymphocytes. Clin Cancer Res. 2004;10:828-839. [PubMed] [DOI] |
| 8. | Frolkis M, Fischer MB, Wang Z, Lebkowski JS, Chiu CP, Majumdar AS. Dendritic cells reconstituted with human telo-merase gene induce potent cytotoxic T-cell response against different types of tumors. Cancer Gene Ther. 2003;10:239-249. [PubMed] [DOI] |
| 9. | Amarnath SM, Dyer CE, Ramesh A, Iwuagwu O, Drew PJ, Greenman J. In vitro quantification of the cytotoxic T lymph-ocyte response against human telomerase reverse transcriptase in breast cancer. Int J Oncol. 2004;25:211-217. [PubMed] |
| 10. | Verra NC, Jorritsma A, Weijer K, Ruizendaal JJ, Voordouw A, Weder P, Hooijberg E, Schumacher TN, Haanen JB, Spits H. Human telomerase reverse transcriptase-trans-duced human cytotoxic T cells suppress the growth of human melanoma in immunodeficient mice. Cancer Res. 2004;64:2153-2161. [PubMed] [DOI] |
| 11. | Gordan JD, Vonderheide RH. Universal tumor antigens as targets for immunotherapy. Cytotherapy. 2002;4:317-327. [PubMed] [DOI] |
| 12. | Berzofsky JA, Terabe M, Oh S, Belyakov IM, Ahlers JD, Janik JE, Morris JC. Progress on new vaccine strategies for the imm-unotherapy and prevention of cancer. J Clin Invest. 2004;113:1515-1525. [PubMed] [DOI] |
| 13. | Finn OJ. Cancer vaccines: between the idea and the reality. Nat Rev Immunol. 2003;3:630-641. [PubMed] [DOI] |
| 15. | Odunsi K, Lele S, Savalgi R. Vaccine therapy for cancer: fact or fiction? Surg Technol Int. 2004;13:39-47. [PubMed] |
| 16. | Radvanyi L. Discovery and immunologic validation of new antigens for therapeutic cancer vaccines. Int Arch Allergy Immunol. 2004;133:179-197. [PubMed] [DOI] |
| 17. | Ribas A, Butterfield LH, Glaspy JA, Economou JS. Current developments in cancer vaccines and cellular immunotherapy. J Clin Oncol. 2003;21:2415-2432. [PubMed] [DOI] |
| 18. | Gross DA, Graff-Dubois S, Opolon P, Cornet S, Alves P, Benna-ceur-Griscelli A, Faure O, Guillaume P, Firat H, Chouaib S. High vaccination efficiency of low-affinity epitopes in antitumor immunotherapy. J Clin Invest. 2004;113:425-433. [PubMed] [DOI] |
| 19. | Scardino A, Gross DA, Alves P, Schultze JL, Graff-Dubois S, Faure O, Tourdot S, Chouaib S, Nadler LM, Lemonnier FA. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168:5900-5906. [PubMed] [DOI] |
| 20. | Hernandez J, Schoeder K, Blondelle SE, Pons FG, Lone YC, Simora A, Langlade-Demoyen P, Wilson DB, Zanetti M. Anti-genicity and immunogenicity of peptide analogues of a low affinity peptide of the human telomerase reverse transcriptase tumor antigen. Eur J Immunol. 2004;34:2331-2341. [PubMed] [DOI] |
| 21. | Chang DZ, Lomazow W, Joy Somberg C, Stan R, Perales MA. Granulocyte-macrophage colony stimulating factor: an adju-vant for cancer vaccines. Hematology. 2004;9:207-215. [PubMed] [DOI] |
| 22. | Ullenhag GJ, Frodin JE, Mosolits S, Kiaii S, Hassan M, Bonnet MC, Moingeon P, Mellstedt H, Rabbani H. Immunization of colorectal carcinoma patients with a recombinant canarypox virus expressing the tumor antigen Ep-CAM/KSA (ALVAC-KSA) and granulocyte macrophage colony- stimulating factor induced a tumor-specific cellular immune response. Clin Cancer Res. 2003;9:2447-2456. [PubMed] |
| 23. | Chow YH, Huang WL, Chi WK, Chu YD, Tao MH. Impro-vement of hepatitis B virus DNA vaccines by plasmids coex-pressing hepatitis B surface antigen and interleukin-2. J Virol. 1997;71:169-178. [PubMed] |
| 24. | Liu G, Molas M, Grossmann GA, Pasumarthy M, Perales JC, Cooper MJ, Hanson RW. Biological properties of poly-L-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276:34379-34387. [PubMed] [DOI] |
| 25. | Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33-37. [PubMed] [DOI] |
| 26. | Cho YW, Kim JD, Park K. Polycation gene delivery systems: escape from endosomes to cytosol. J Pharm Pharmacol. 2003;55:721-734. [PubMed] [DOI] |
| 27. | Thomas M, Klibanov AM. Non-viral gene therapy: polycation-mediated DNA delivery. Appl Microbiol Biotechnol. 2003;62:27-34. [PubMed] [DOI] |
| 28. | Brown MD, Schatzlein AG, Uchegbu IF. Gene delivery with synthetic (non viral) carriers. Int J Pharm. 2001;229:1-21. [PubMed] [DOI] |
| 29. | Vaysse L, Arveiler B. Transfection using synthetic peptides: comparison of three DNA-compacting peptides and effect of centrifugation. Biochim Biophys Acta. 2000;1474:244-250. [PubMed] [DOI] |
| 30. | Ewert K, Slack NL, Ahmad A, Evans HM, Lin AJ, Samuel CE, Safinya CR. Cationic lipid-DNA complexes for gene therapy: understanding the relationship between complex structure and gene delivery pathways at the molecular level. Curr Med Chem. 2004;11:133-149. [PubMed] [DOI] |
| 31. | Sheng KC, Pietersz GA, Wright MD, Apostolopoulos V. Den-dritic cells: activation and maturation-applications for cancer immunotherapy. Curr Med Chem. 2005;12:1783-1800. [PubMed] [DOI] |