修回日期: 2005-08-03
接受日期: 2005-08-06
在线出版日期: 2005-10-15
目的: 应用siRNA表达载体介导的RNAi技术, 观察RNA干扰沉默生存素基因对胃癌MGC-803细胞增殖的影响.
方法: 用脂质体介导将生存素siRNA表达质粒转染MGC-803细胞, 通过倒置显微镜观察转染后细胞形态学的变化, 四甲基偶氮唑蓝(MTT)法检测细胞增殖活性, 流式细胞术分析细胞周期的变化, 逆转录聚合酶链式反应(RT-PCR)方法检测生存素 mRNA的表达水平.
结果: 生存素siRNA表达质粒转染组MGC-803细胞变圆、浮起, 生存素siRNA表达质粒明显下调MGC-803细胞内生存素mRNA的表达, 与空白对照组相比降低了48.2%, 阻断细胞周期在G1期(77.4%), 显著抑制细胞增殖, siRNA转染组细胞吸光度比空白组显著降低(24 h: 0.272±0.048 vs 0.576±0.018; 48 h: 0.270±0.060 vs 0.809±0.027; 72 h: 0.143±0.046 vs1.015±0.075; 均P<0.01). 转染24、48和72 h后的抑制率分别为53.4%、66.7%和86.3%.
结论: 应用siRNA表达载体介导的RNAi技术, 可有效下调生存素在MGC-803细胞中的表达, 并在体外抑制细胞的增殖.
引文著录: 赵伟红, 郭俊明, 肖丙秀, 管忠, 肖东升. 生存素 siRNA 表达质粒对MGC-803 细胞增殖的影响. 世界华人消化杂志 2005; 13(19): 2302-2305
Revised: August 3, 2005
Accepted: August 6, 2005
Published online: October 15, 2005
AIM: To silence the expression of survivin gene in MGC-803 cells by the siRNA expression vector-based RNA interference (RNAi) technique, and to investigate its effects on the proliferation of MGC-803 cells.
METHODS: The survivin siRNA expression plasmid was transfected into MGC-803 cells by lipofectamine. Morphological changes of the cells were observed under invert microscope. The expression of survivin mRNA was determined by reverse transcription-polymerase chain reaction (RT-PCR). The changes of cell cycle and the cell proliferation were analyzed by flow cytometry and MTT assay, respectively.
RESULTS: Abnormal morphological changes of MGC-803 cells were observed in the group transfected with the survivin siRNA expression plasmid. The survivin siRNA expression plasmid significantly down-regulated the expression of survivin mRNA in MGC-803 cells with a percentage of 48.2% ( vs empty controls), and it arrested the cell cycle in G1 phase (77.4%). The cell proliferation was significantly inhibited, and the optical density in siRNA-transfected cells was markedly lower than that in the empty controls (24 h: 0.272 ± 0.048 vs 0.576 ± 0.018; 48 h: 0.270 ± 0.060 vs 0.809 ± 0.027; 72 h: 0.143 ± 0.046 vs 1.015 ± 0.075; all P < 0.01). The growth inhibitory rates of MGC-803 cells were 53.4%, 66.7%, and 86.3% after 24, 48, and 72 h of the transfection, respectively.
CONCLUSION: The expression of survivin in MGC-803 cells can be down-regulated by the plasmid-based RNAi technique, and the down-regulation can inhibit the cell proliferation in vitro.
- Citation: Zhao WH, Guo JM, Xiao BX, Guan Z, Xiao DS. Effects of survivin siRNA expression plasmid on proliferation of MGC-803 cells. Shijie Huaren Xiaohua Zazhi 2005; 13(19): 2302-2305
- URL: https://www.wjgnet.com/1009-3079/full/v13/i19/2302.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i19.2302
细胞凋亡和细胞周期的调控异常是恶性肿瘤无限增殖的主要原因, 近年来研究发现, 凋亡蛋白抑制因子(IAPs)家族中的最小成员生存素(survivin)在细胞凋亡和周期调控中发挥重要作用[1-3]. 胃癌是我国最常见的恶性肿瘤之一, 研究显示生存素广泛表达于胃癌组织[4-7], 生存素的表达水平与胃癌患者的预后呈负相关[8,9]. RNA干扰(RNA interference, RNAi)是一项新兴的基因阻断技术, 具有特异性和高效性[10]. 为了探讨靶向生存素在胃癌治疗中的意义和价值, 本文运用RNAi技术, 将生存素基因的小分子干扰(small interfering RNA, siRNA)表达质粒转染至生存素表达阳性的胃癌细胞株MGC-803中, 观察其对胃癌细胞增殖的影响, 为胃癌的基因治疗提供基础.
1.1.1 细胞株: 人胃癌细胞株MGC-803, 购自中科院上海细胞所细胞库.
1.1.2 生存素siRNA表达质粒: 由广东医学院生物化学与分子生物学研究所构建惠赠, 序列[11]为: 正义链5'-TTT GTT CTT GAA TGT AGA GAT GCT GTG GTC CTT CAA GAG AGG ACC ACC GCA TCT CTA CAT TCA AGA ACT TTT T-3', 反义链5'-CTA GAA AAA GTT CTT GAA TGT AGA GAT GCG GTG GTC CTC TCT TGA AGG ACC ACC GCA TCT CTA CAT TCA AGA A-3'.
1.1.3 主要试剂: 脂质体(Lipofectamine 2 000)为Invitrogen公司产品, DMEM细胞培养基为Gibco BRL公司产品, 标准分子量DNA、Trizol、RT-PCR试剂盒为Takara公司产品, survivin和β-actin两对引物由上海生工公司合成.
1.2.1 细胞培养及转染: 人胃癌细胞MGC-803在含有100 mL/L小牛血清的DMEM中常规培养(37 ℃, 50 mL/L CO2), 取对数生长期细胞用于实验. 转染前1 d, 将MGC-803细胞接种到96、24或6孔板, 转染前将板内培养基更换为无血清DMEM培养基, 具体转染步骤按Lipofectamine 2 000转染说明书进行, 转染的质粒量为1.3 mg/L. 转染后孵育4 h. 4 h后换用完全培养液. 在37 ℃、50 mL/L CO2培养箱中分别培养24、48或72 h后, 收集所有贴壁和不贴壁细胞, 作进一步检测.
1.2.2 MTT法检测细胞增殖: 转染前1 d, 将细胞以1× 104/孔浓度接种于96孔板. 转染后分别培养24、48和72 h, 每孔加入20 mL MTT(5 g/L), 继续培养4 h, 倾去培养液, 每孔加入150 mL DMSO, 室温下振荡10 min, 使结晶充分溶解, 于492 nm波长在酶标仪上测定各孔吸光度, 检测细胞增殖情况.
1.2.3 流式细胞术检测细胞周期: 转染48 h后收集细胞, 用PBS洗涤, 加入100 mL RNase(1 g/L), 37 ℃孵育30 min, 加100mL PI(400 mg/L)染色, 流式细胞仪检测, 分析细胞周期, 结果用Multicycle软件处理.
1.2.4 RT-PCR检测生存素mRNA表达: h后收集细胞, 应用Trizol试剂抽提MGC-803细胞的总RNA, 经紫外分光光度计检测其纯度和含量, 标本总RNA 转染48 A260nm/280nmkit试剂盒和0.1 均在1.8-2.0. 用One Step RT-PCR mg总RNA合成RT-PCR产物. Survivin上游引物相对应核苷酸号为47-66(5'-GGCATGGGTGCC CCGACGTT-3'), 下游引物相对应核苷酸号为466-485(5'-AGAGGCCTCAATCCATGGCA-3'). 扩增β-actin作为内参照, β-actin上游引物相对应核苷酸号为578-609(5'-ATCTGGCACCACACCTTCTACAATGAGCTGCG-3'), 下游引物相对应核苷酸号为1 415-1 384(5'-CGTCATACTCCTGCTTCCTGATCCACATCTGC-3'). 扩增片段分别为439 bp和838 bp. 逆转录50 ℃ 30 min, 预变性94 ℃ 2 min, 之后变性94 ℃ 1 min, 退火62 ℃ 1 min, 延伸72 ℃ 1 min, 反应30个循环, 最后延伸72 ℃ 8 min. RT-PCR产物以15 g/L琼脂糖凝胶电泳, 溴化乙锭(EB)显色, 置于Gel Doc2 000凝胶图像分析仪, 用Quantity One 4.1软件分析. RT-PCR的最终结果以survivin产物量与β-actin产物量的比值表示.
统计学处理 实验数据以mean±SD表示, 应用SPSS 10.0统计软件进行t检验和单因素方差分析.
在倒置显微镜下观察, 转染了生存素siRNA表达质粒的MGC-803细胞经培养后, 细胞形态由原来的梭状或多角状变为缩水的圆形, 并部分从培养瓶脱落浮起, 而脂质体对照组和空载体质粒对照组的细胞形态正常, 贴壁生长良好(图1).
MGC-803细胞转染生存素siRNA表达质粒后, 细胞的生长受到明显的抑制(P<0.01, 表1), 转染后24、48和72 h的抑制率分别为53.4%、66.7%和86.3%. 脂质体对照组和空载体质粒对照组则无明显的抑制作用.
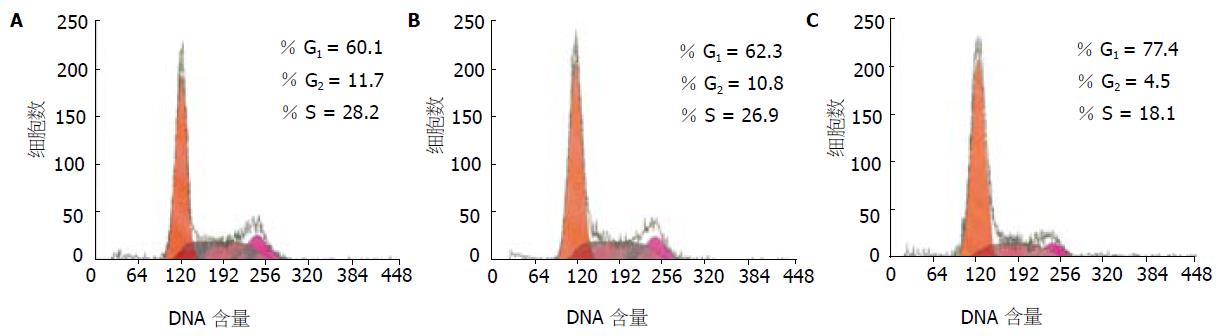
经流式细胞仪检测分析, 转染了生存素siRNA表达质粒的MGC-803细胞周期分布发生了显著变化, G1期细胞数比例增加, 由空白对照组的60.1%上升至77.4%, S期和G2期细胞数比例减少, S期由空白对照组的28.2%下降至18.1%, G2期由空白对照组的11.7%下降至4.5%,凋亡峰不明显(图2).
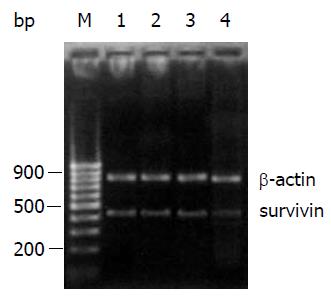
RT-PCR产物电泳结果显示, 生存素siRNA表达质粒能有效下调MGC-803细胞内生存素mRNA的表达水平(图3), 与空白对照组比较下调了48.2%.
目前RNAi作为基因功能研究的新手段, 已被广泛运用于生命科学研究的多个领域[12-14]. 它是一种由双链RNA(double-stranded RNA, dsRNA)介导同源序列mRNA降解的现象, 可以使基因转录后沉默. 细胞内dsRNA的来源可以是体外人工合成的dsRNA, 或经表达载体转染后在细胞内转录生成dsRNA. 由于体外合成的长链dsRNA在大多数哺乳动物细胞中会产生强烈的毒性反应[15], 而人工合成的短链RNA虽可避免这种非特异性作用, 但其维持时效短, 表达载体可以帮助克服RNAi上述缺点[16,17], 所以利用载体在细胞内转录生成dsRNA成为RNAi技术运用的主要手段. 生存素是一个在调控细胞内平衡中发挥多效应能力的重要分子. 有很多文献报道[18-21], 多种生存素反义寡核苷酸可以在体外下调生存素基因的表达, 并进一步抑制肿瘤细胞增殖, 促进细胞凋亡的发生. 研究表明[22,23], RNAi比传统的反义核酸技术能更有效地抑制基因表达. 本文运用RNAi技术, 将生存素siRNA表达质粒在脂质体介导下转染胃癌细胞MGC-803, 对生存素表达进行特异性抑制. 实验结果显示, 生存素siRNA表达质粒转染细胞后使细胞内生存素mRNA表达下降, 而且细胞周期分布发生变化, G1期细胞数比例增加, 细胞的增殖活性明显减弱. 其机制可能为生存素siRNA表达质粒转染后在细胞内转录生成dsRNA, dsRNA与RNAi核酸酶结合, 并被分解成为21-23 nt的siRNA, 解旋酶区域催化mRNA与siRNA的正义链交换, 最终mRNA被降解. 生存素广泛存在于肿瘤细胞中[24-28], 具有抑制细胞凋亡和调节细胞增殖的双重作用. 它选择性表达于细胞周期的G2/M期[29], 在G2/M期, 生存素表达可上调40多倍. Giodini et al[30]认为生存素控制微管系统的稳定性和有丝分裂纺锤体的形成, 因而影响细胞分裂. 生存素的高表达可能使得细胞更容易逃避G2/M期的分子检查机制而不发生细胞凋亡. 因此, 将生存素基因作为RNA干涉的靶点对诱导细胞凋亡和阻断细胞周期非常有意义.
本文结果在体外实验中证实了运用RNAi技术抑制细胞内生存素可以抑制胃癌细胞增殖, 这为将来以生存素为靶基因进一步研究胃癌治疗的方法提供了一种新的选择, 同时也为质粒介导的RNAi技术运用于肿瘤的基因治疗提供了一定的理论依据.
电编: 张敏 编辑:张海宁
| 1. | Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003;22:8581-8589. [PubMed] [DOI] |
| 2. | Johnson ME, Howerth EW. Survivin: a bifunctional inhibitor of apoptosis protein. Vet Pathol. 2004;41:599-607. [PubMed] [DOI] |
| 3. | Morinaga S, Nakamura Y, Ishiwa N, Yoshikawa T, Noguchi Y, Yamamoto Y, Rino Y, Imada T, Takanashi Y, Akaike M. Expression of survivin mRNA associates with apoptosis, proliferation and histologically aggressive features in hepatocellular carcinoma. Oncol Rep. 2004;12:1189-1194. [PubMed] [DOI] |
| 4. | Wang ZN, Xu HM, Jiang L, Zhou X, Lu C, Zhang X. Expression of survivin in primary and metastatic gastric cancer cells obtained by laser capture microdissection. World J Gastroenterol. 2004;10:3094-3098. [PubMed] [DOI] |
| 5. | Zhu XD, Lin GJ, Qian LP, Chen ZQ. Expression of survivin in human gastric carcinoma and gastric carcinoma model of rats. World J Gastroenterol. 2003;9:1435-1438. [PubMed] [DOI] |
| 6. | Yu J, Leung WK, Ebert MP, Ng EK, Go MY, Wang HB, Chung SC, Malfertheiner P, Sung JJ. Increased expression of survivin in gastric cancer patients and in first degree relatives. Br J Cancer. 2002;87:91-97. [PubMed] [DOI] |
| 7. | Krieg A, Mahotka C, Krieg T, Grabsch H, Müller W, Takeno S, Suschek CV, Heydthausen M, Gabbert HE, Gerharz CD. Expression of different survivin variants in gastric carcinomas: first clues to a role of survivin-2B in tumour progression. Br J Cancer. 2002;86:737-743. [PubMed] [DOI] |
| 8. | Li YH, Wang C, Meng K, Chen LB, Zhou XJ. Influence of survivin and caspase-3 on cell apoptosis and prognosis in gastric carcinoma. World J Gastroenterol. 2004;10:1984-1988. [PubMed] [DOI] |
| 9. | Meng H, Lu CD, Sun YL, Dai DJ, Lee SW, Tanigawa N. Expression level of wild-type survivin in gastric cancer is an independent predictor of survival. World J Gastroenterol. 2004;10:3245-3250. [PubMed] [DOI] |
| 10. | Agrawal N, Dasaradhi PV, Mohmmed A, Malhotra P, Bhatnagar RK, Mukherjee SK. RNA interference: biology, mechanism, and applications. Microbiol Mol Biol Rev. 2003;67:657-685. [PubMed] [DOI] |
| 11. | Li LP, Liang NC, Luo CQ. Construction of survivin siRNA expression vector and its regulation on cell cycle and proliferation in MCF-7 cells. Ai Zheng. 2004;23:742-748. [PubMed] |
| 12. | Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63:1515-1519. [PubMed] |
| 13. | Ning S, Fuessel S, Kotzsch M, Kraemer K, Kappler M, Schmidt U, Taubert H, Wirth MP, Meye A. siRNA-mediated down-regulation of survivin inhibits bladder cancer cell growth. Int J Oncol. 2004;25:1065-1071. [PubMed] [DOI] |
| 14. | Wilda M, Fuchs U, Wössmann W, Borkhardt A. Killing of leukemic cells with a BCR/ABL fusion gene by RNA interference (RNAi). Oncogene. 2002;21:5716-5724. [PubMed] [DOI] |
| 15. | Scherr M, Morgan MA, Eder M. Gene silencing mediated by small interfering RNAs in mammalian cells. Curr Med Chem. 2003;10:245-256. [PubMed] [DOI] |
| 16. | Hannon GJ, Conklin DS. RNA interference by short hairpin RNAs expressed in vertebrate cells. Methods Mol Biol. 2004;257:255-266. [PubMed] [DOI] |
| 17. | Yu JY, DeRuiter SL, Turner DL. RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA. 2002;99:6047-6052. [PubMed] [DOI] |
| 18. | Tong QS, Zheng LD, Chen FM, Zeng FQ, Wang L, Dong JH, Lu GC. Selection of optimal antisense accessible sites of survivin and its application in treatment of gastric cancer. World J Gastroenterol. 2005;11:634-640. [PubMed] [DOI] |
| 19. | Dai DJ, Lu CD, Lai RY, Guo JM, Meng H, Chen WS, Gu J. Survivin antisense compound inhibits proliferation and promotes apoptosis in liver cancer cells. World J Gastroenterol. 2005;11:193-199. [PubMed] [DOI] |
| 20. | Yang JH, Zhang YC, Qian HQ. Survivin antisense oligodeoxynucleotide inhibits growth of gastric cancer cells. World J Gastroenterol. 2004;10:1121-1124. [PubMed] |
| 21. | Ma X, Wang S, Zhou J, Xing H, Xu G, Wang B, Chen G, Lu YP, Ma D. Induction of apoptosis in human ovarian epithelial cancer cells by antisurvivin oligonucleotides. Oncol Rep. 2005;14:275-279. [PubMed] |
| 22. | Aoki Y, Cioca DP, Oidaira H, Kamiya J, Kiyosawa K. RNA interference may be more potent than antisense RNA in human cancer cell lines. Clin Exp Pharmacol Physiol. 2003;30:96-102. [PubMed] [DOI] |
| 23. | Cioca DP, Aoki Y, Kiyosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10:125-133. [PubMed] [DOI] |
| 24. | Falleni M, Pellegrini C, Marchetti A, Oprandi B, Buttitta F, Barassi F, Santambrogio L, Coggi G, Bosari S. Survivin gene expression in early-stage non-small cell lung cancer. J Pathol. 2003;200:620-626. [PubMed] [DOI] |
| 25. | Chen WC, Liu Q, Fu JX, Kang SY. Expression of survivin and its significance in colorectal cancer. World J Gastroenterol. 2004;10:2886-2889. [PubMed] [DOI] |
| 26. | Fields AC, Cotsonis G, Sexton D, Santoianni R, Cohen C. Survivin expression in hepatocellular carcinoma: correlation with proliferation, prognostic parameters, and outcome. Mod Pathol. 2004;17:1378-1385. [PubMed] [DOI] |
| 27. | Nasu S, Yagihashi A, Izawa A, Saito K, Asanuma K, Nakamura M, Kobayashi D, Okazaki M, Watanabe N. Survivin mRNA expression in patients with breast cancer. Anticancer Res. 2002;22:1839-1843. [PubMed] |
| 28. | Sarela AI, Verbeke CS, Ramsdale J, Davies CL, Markham AF, Guillou PJ. Expression of survivin, a novel inhibitor of apoptosis and cell cycle regulatory protein, in pancreatic adenocarcinoma. Br J Cancer. 2002;86:886-892. [PubMed] [DOI] |
| 29. | Suzuki A, Hayashida M, Ito T, Kawano H, Nakano T, Miura M, Akahane K, Shiraki K. Survivin initiates cell cycle entry by the competitive interaction with Cdk4/p16(INK4a) and Cdk2/cyclin E complex activation. Oncogene. 2000;19:3225-3234. [PubMed] [DOI] |
| 30. | Giodini A, Kallio MJ, Wall NR, Gorbsky GJ, Tognin S, Marchisio PC, Symons M, Altieri DC. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462-2467. [PubMed] |