修回日期: 2005-07-24
接受日期: 2005-07-28
在线出版日期: 2005-09-28
目的: 观察选择性环氧合酶-2抑制剂塞来昔布对胃癌细胞株SGC7901增殖的影响, 及细胞增殖与细胞外信号调节激酶2(ERK2) 表达的关系.
方法: MTT法检测塞来昔布对胃癌细胞生长的影响; TUNEL法及流式细胞仪检测细胞凋亡率; 免疫组化(SP法)检测细胞磷酸化ERK2表达, 图像分析系统计量.
结果: 塞来昔布呈剂量依赖方式抑制胃癌细胞的生长, 诱导细胞凋亡, 抑制胃癌细胞磷酸化ERK2表达. 塞来昔布10, 20, 40, 80, 160 μmol/L作用48 h后其胃癌细胞生长抑制率分别为9.8%, 30%, 58.9%, 76.3%, 88.3%; TUNEL染色显示胃癌细胞具有典型的凋亡细胞形态学特征; 流式细胞仪显示胃癌细胞凋亡率(4.23±0.81%, 15.5±2.1%, 24.35±2.32%, 31.52±3.64%, 45.82±5.92%)显著高于对照组(1.85±0.31%, P<0.01); 其磷酸化ERK2平均吸光度(2.96±0.24, P>0.05; 2.56±0.24, P<0.05; 2.04±0.20, P<0.01; 1.68±0.16, P<0.01; 1.52±0.09, P<0.01)显著低于对照组(3.32 ±0.28).
结论: 塞来昔布抑制胃癌细胞增殖并诱导细胞凋亡, 这可能与其抑制ERK2信号传导通路有关.
引文著录: 张勇, 蒋明德, 曾维政, 徐辉, 熊碧君, 翁敏. 塞来昔布对胃癌细胞生长及 ERK2 表达的影响. 世界华人消化杂志 2005; 13(18): 2213-2216
Revised: July 24, 2005
Accepted: July 28, 2005
Published online: September 28, 2005
AIM: To explore the role of celecoxib, a selective COX-2 inhibitor, in the proliferation of human gastric carcinoma cells SGC7901, and to investigate the relationship between cell proliferation and the expression of extracellular signal-regulated kinase 2 (ERK2).
METHODS: The cell proliferation of SGC7901 cells was measured by MTT assay, and the apoptoticsis rate of the cells was examined by TUNEL staining method and flow cytometry. The expression of phosphated ERK2 was detected by immunohistochemistry and quantified measured by image analysis systems.
RESULTS: Celecoxib inhibited the proliferation of SGC7901 gastric carcinoma cells and the expression of phosphated ERK2, as well as induced the apoptosis of the SGC-7901 cells in a dose-dependent manner. The growth inhibitory rates of SGC-7901 cells treated with 10, 20, 40, 80 and, 160 μmol/L celecoxib for 48 h were 9.8%, 30%, 58.9%, 76.3%, and 88.3%, respectively. The SGC7901 cells presented typical morphological features of apoptosis by TUNEL staining. By flow cytometry, the apoptosistic rates of SGC-7901 cells (4.23 ± 0.81%, 15.50 ± 2.10%, 24.35 ± 2.32%, 31.52±3.64%, and 45.82±5.92% for 10, 20, 40, 80 and 160 μmol/L celecoxib, respectively) were significantly higher than those inof the control group(1.85 ±0.31%, P < 0.01). The optical density value of ERK2 of SGC7901 cells (10, 20, 40, 80 and 160 μmol/L celecoxib: 2.96 ± 0.24, P >0.05; 2.56 ± 0.24, P<0.05; 2.04 ± 0.20, P < 0.01; 1.68 ± 0.16, P < 0.01; 1.52 ± 0.09, P < 0.01) were significantly lower than that ofin the control group (3.32 ± 0.28).
CONCLUSION: Celecoxib can inhibit the growth and induce the apoptosis of SGC7901 cells, and its mechanism may and induce the cell apoptosis. This effect may relate to the inhibition of ERK2 kinase signaling pathway.
- Citation: Zhang Y, Jiang MD, Zeng WZ, Hui-Xu, Xiong BJ, Min-Weng. Effects of celecoxib on cell proliferation and ERK2 expression of ERK2 in gastric cancer cells. Shijie Huaren Xiaohua Zazhi 2005; 13(18): 2213-2216
- URL: https://www.wjgnet.com/1009-3079/full/v13/i18/2213.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i18.2213
非甾体类抗炎药(NSAID)可降低胃肠道肿瘤的发生率[1]. NSAID抗肿瘤作用的机制除了与其抑制环氧合酶-2(cyclooxygenase-2, COX-2)活性有关外, 还通过其它途径发挥抗肿瘤作用[2-5]. 细胞外刺激引起细胞反应是通过细胞内信号传导途径来实现的, 细胞外信号调节激酶(extracellular signal-regulated kinase, ERK)是分裂原活化蛋白激酶(mitogen activated protein kinases, MAPK) 信号传导通路家族成员之一, 参与细胞外信号传递至细胞核的过程, 与细胞的增殖、分化及肿瘤的发生有关. 关于NSAID对胃癌细胞ERK信号传导通路的影响目前报道很少. 为此, 我们观察了选择性COX-2抑制剂塞来昔布(celecoxib)对人胃癌细胞株SGC7901的增殖、凋亡作用及其对磷酸化ERK2表达的影响如下.
人胃腺癌细胞株SGC7901由第三军医大学西南医院消化实验室惠赠. RPMI1640培养液、SP试剂盒购自北京华美公司; 小牛血清, MTT购自Gibco公司, 二甲基亚砜(DMSO)购自Sigma公司, 塞来昔布购自安徽合肥森瑞公司, TUNEL试剂盒购自武汉博士德公司, 美国Image-Pro Plus专业图像软件分析系统. 胃癌细胞株SGC7901常规培养于含100 mL/L小牛血清, 1×105 U/L青霉素及链霉素的RPMI1640培养液中, 置于37 ℃, 50 mL/L CO2培养箱内培养.
取对数生长期的SGC7901细胞按每孔4×103个细胞接种于96孔板中, 24 h后换液, 加入不同浓度的塞来昔布, 终浓度分别为10, 20, 40, 80, 160 μmol/L; 继续培养48 h, 每一浓度点重复3孔, 每孔加入5 g/L MTT液20 μL, 37 ℃孵育4 h后弃去上清液, 每孔加入DMSO 150 μL, 轻轻振荡10 min, 使结晶物充分溶解, 在570 nm波长酶联免疫检测仪上测定各孔吸光度A值, 并计算细胞增殖抑制率(%)=(1-实验组平均A值/对照组平均A值)×100%. 设只加等体积溶剂DMSO的对照组. 另取对数生长期细胞以1×108/L密度按种于6孔板中, 掊养24 h后加入不同浓度塞来昔布(剂量同MTT法), 48 h后按TUNEL试剂盒说明操作. 凋亡指数计算方法: 在高倍视野(400×)下, 随机选5个视野, 分别计数200个细胞及其中的凋亡细胞数. 凋亡指数=凋亡细胞数/总细胞数×100%. 以上试验重复3次. 再取不同浓度塞来昔布作用SGC7901细胞48 h后的各组细胞, 用700 mL/L冷乙醇1.5 mL固定1 h, PBS漂洗混匀细胞, 加入含RNA酶A的碘化吡啶染料, 避光1 h, 上机检测细胞周期. 以上实验重复3次, 取其平均值. 再取不同浓度塞来塞布作用SGC7901细胞48 h后的各组细胞(细胞数≥1×106)细胞爬片, PBS洗后950 mL/L乙醇固定30 min, 采用常规SP法, 细胞爬片依次用5 mL/L H2O2/甲醇 30 min, 正常羊血清室温封闭15 min, 滴加一抗, 37 ℃孵育2 h, 滴加生物素化二抗37 ℃孵育15 min, 滴加SP复合物37 ℃ 15 min, DAB显色, 苏木精复染、脱水、透明、封片, 光镜下观察, 图像分析仪分析, 用平均吸光度表示P-ERK相对表达量. 以上实验重复3次, 取其均值.
统计学处理 实验数据采用SPSS10.0统计分析软件进行t检验, 所有数值以mean±SD表示, P<0.05为有统计学意义.
塞来昔布能明显抑制胃癌细胞增殖, 呈剂量依赖性. 0, 10, 20, 40, 80, 160 μmol/L塞来昔布对胃癌细胞的抑制率分别为0%, 9.8%, 30%, 58.9%, 76.3%, 88.3%.
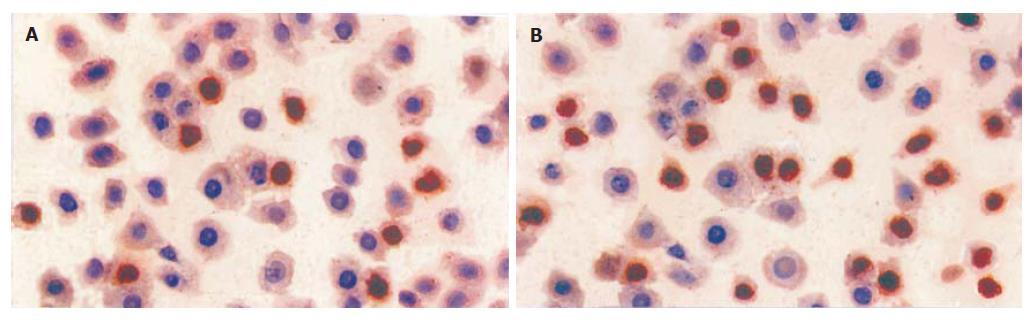
TUNEL染色显示塞来昔布作用于胃癌细胞48 h后, 见典型的凋亡细胞, 现为细胞体积缩小、核固缩深染、凋亡小体形成(图1). 10, 20, 40, 80, 160 μmol/L塞来昔布作用48 h后胃癌细胞凋亡指数分别为(3.2±0.8)%, (13.0±2.1)%, (21.5±2.2)%, (29.2±3.8)%, (45.8±4.7)%, 较对照组(0.5±0.3)%显著增加(P<0.01), 提示塞来昔布呈剂量依赖方式诱导胃癌细胞SGC7901凋亡.
随药物剂量的增加, 细胞凋亡率明显增加, 与TUNEL染色结果相近; 对细胞周期分布的表现为G0/G1期细胞比例增高, S期细胞比例降低(表1).
| μmol/L | 凋亡率 | G0/G1 | S | G2/M |
| 0 | 1.85 ± 0.31 | 45.43 ± 2.81 | 43.51 ± 5.35 | 11.06 ± 1.02 |
| 10 | 4.23 ± 0.81b | 48.53 ± 2.79 | 41.56 ± 4.76 | 9.91 ± 0.95 |
| 20 | 15.50 ± 2.10b | 56.68 ± 3.84a | 35.48 ± 3.96 | 7.84 ± 1.01 |
| 40 | 24.35 ± 2.32b | 65.40 ± 5.53b | 28.23 ± 3.67a | 6.37 ± 0.89 |
| 80 | 31.52 ± 3.64b | 70.98 ± 5.58b | 24.16 ± 2.45b | 4.86 ± 0.75 |
| 160 | 45.82 ± 5.92b | 74.90 ± 6.65b | 21.95 ± 1.82b | 3.15 ± 0.82 |
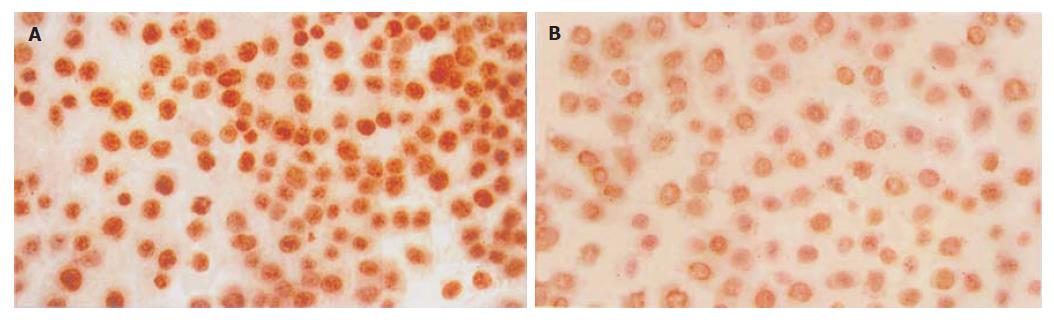
胃癌细胞磷酸化ERK2表达主要定位于细胞核内, 细胞质有少量表达, 染成棕黄色(图2). 10, 20, 40, 80, 160 μmol/L塞来昔布作用48 h后其胃癌细胞磷酸化ERK2的平均吸光度分别为2.96±0.24(P>0.05), 2.56±0.24(P<0.05), 2.04±0.20 (P<0.01), 1.68±0.16(P<0.01), 1.52±0.09(P<0.01), 显著低于对照组3.32±0.28, 随着塞来昔布剂量增加, 其核内磷酸化ERK2表达逐渐减少. 说明塞来昔布能抑制胃癌细胞ERK2磷酸化, 阻止其向核内移位.
NSAID能使结肠癌的发生率降低40-50%[1].NSAID的作用靶点-COX-2在胃癌有较高表达[6-10], NSAID能抑制体外培养的胃癌细胞COX-2的表达及细胞增殖[11,12], 抑制裸鼠胃癌原位移植瘤的生长[13,14], 对化学致癌剂诱导的大鼠胃癌具有化学预防作用[15,16]. NSAID可能通过抑制COX-2活性的途径发挥抗肿瘤作用. 塞来昔布是一种高选择性COX-2抑制剂. NSAID抑制胃癌细胞生长与其诱导细胞凋亡有关[17-20]. 我们首先用TUNEL法显示塞来昔布作用胃癌细胞后可见大量凋亡细胞出现, 流式细胞仪分析显示塞来昔布可将细胞阻滞在G0/G1期, 并诱导细胞凋亡, 10, 20, 40, 80, 160 μmol/L塞来昔布作用于胃癌细胞24 h后, 凋亡率分别为4.23%, 15.50%, 24.35%, 31.52%, 45.82%, 呈剂量依赖性. 因此, 塞来昔布对胃癌细胞的抑制作用可能与其诱导细胞凋亡有关. 郑文斌et al[21]还发现高浓度塞来昔布对胃癌多药耐药细胞亦有明显抑制生长的作用.
然而NSAID类药物可以在完全缺乏COX活性的细胞中发挥抗增殖作用, 说明NSAID尚有独立于环氧合酶的其它途径参与对胃癌细胞的抑制作用[22,23]. ERK位于胞质, 激活后发生磷酸化转位至胞核[24,25], 调节转录因子如NF-κB, AP-1的活性, 产生细胞效应. ERK通路上调与胃黏膜细胞的增殖和转化有关, 与胃癌的发生、TMN分期、淋巴结转移相关[26-29]. NSAID抑制胃癌细胞生长作用是否与ERK途径有关的问题值得探讨. 我们采用免疫组化方法显示磷酸化ERK2主要分布在细胞核内, 胞质有少量表达. 定量分析显示塞来昔布能使胃癌细胞磷酸化ERK2表达减少, 呈剂量依赖性. 提示塞来昔布能阻断ERK2磷酸化, 从而阻断细胞外信号向细胞内传递, 达到抑制胃癌细胞增殖的作用. Husain et al[30]发现两类NSAID都明显抑制ERK2活化. 因此我们认为塞来昔布可能通过抑制MAPK(ERK2)途径来抑制人胃癌细胞株SGC7901的增殖和生长, 发挥其防癌、抗癌作用. 陈波 et al[31]发现PD098059可能通过抑制MAPK的活性, 抑制胃癌细胞增殖, 对长春新碱诱导MGC803细胞的凋亡具有协同作用. 因此, MAPK(ERK)可能成为胃癌治疗的一个新的靶点, 塞来昔布对COX-2有很高的选择性, 其COX-1 IC50与COX-2 IC50的比值为375, 在临床应用有较高的安全性. 塞来昔布于2000年被美国食品药品管理局首次批准用于家族性息肉病的治疗. 国内已有塞来昔布用于预防胃癌的实验研究报道[15-16]. 进一步深入研究COX-2抑制剂抗肿瘤的机制, 可为临床开辟新的抗癌手段提供理论依据.
电编: 李琪 编辑:潘伯荣 审读:张海宁
| 1. | Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891-899. [PubMed] [DOI] |
| 2. | Soh JW, Mao Y, Kim MG, Pamukcu R, Li H, Piazza GA, Thompson WJ, Weinstein IB. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2-terminal kinase 1. Clin Cancer Res. 2000;6:4136-4141. [PubMed] |
| 3. | Sánchez-Alcázar JA, Bradbury DA, Pang L, Knox AJ. Cyclooxygenase (COX) inhibitors induce apoptosis in non-small cell lung cancer through cyclooxygenase independent pathways. Lung Cancer. 2003;40:33-44. [PubMed] [DOI] |
| 4. | Grösch S, Tegeder I, Niederberger E, Bräutigam L, Geisslinger G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001;15:2742-2744. [PubMed] |
| 5. | Nagatsuka I, Yamada N, Shimizu S, Ohira M, Nishino H, Seki S, Hirakawa K. Inhibitory effect of a selective cyclooxygenase-2 inhibitor on liver metastasis of colon cancer. Int J Cancer. 2002;100:515-519. [PubMed] [DOI] |
| 6. | Lim HY, Joo HJ, Choi JH, Yi JW, Yang MS, Cho DY, Kim HS, Nam DK, Lee KB, Kim HC. Increased expression of cyclooxygenase-2 protein in human gastric carcinoma. Clin Cancer Res. 2000;6:519-525. [PubMed] |
| 7. | Yamagata R, Shimoyama T, Fukuda S, Yoshimura T, Tanaka M, Munakata A. Cyclooxygenase-2 expression is increased in early intestinal-type gastric cancer and gastric mucosa with intestinal metaplasia. Eur J Gastroenterol Hepatol. 2002;14:359-363. [PubMed] [DOI] |
| 8. | Wang BC, Guo CQ, Sun C, Sun QL, Liu GY, Li DG. Mechanism and clinical significance of cyclooxygenase-2 expression in gastric cancer. World J Gastroenterol. 2005;11:3240-3244. [PubMed] [DOI] |
| 9. | Wang BC, Guo CQ, Sun C, Sun QL, Liu GY, Li DG. Mechanism and clinical significance of cyclooxygenase-2 expression in gastric cancer. World J Gastroenterol. 2005;11:3240-3244. [PubMed] |
| 10. | Saukkonen K, Nieminen O, van Rees B, Vilkki S, Härkönen M, Juhola M, Mecklin JP, Sipponen P, Ristimäki A. Expression of cyclooxygenase-2 in dysplasia of the stomach and in intestinal-type gastric adenocarcinoma. Clin Cancer Res. 2001;7:1923-1931. [PubMed] |
| 12. | 孙 波, 吴 云林, 张 学军, 王 升年, 贺 恒益, 乔 敏敏, 章 永平, 钟 捷. 舒林酸抑制人胃腺癌细胞增殖及诱导凋亡. 世界华人消化杂志. 2001;9:997-1002. [DOI] |
| 14. | 刘 纯伦, 唐 承薇, 万 学红. 选择性环氧合酶-2抑制剂对胃癌细胞生长的影响. 四川大学学报(医学版). 2003;34:480-483. |
| 15. | 兰 春慧, 房 殿春, 向 德兵, 贺 志高, 陈 东风, 樊 丽琳. 环氧合酶-2抑制剂celecoxib抑制胃癌生长的实验研究. 胃肠病学和肝病学杂志. 2005;14:134-136. |
| 17. | Uefuji K, Ichikura T, Shinomiya N, Mochizuki H. Induction of apoptosis by JTE-522, a specific cyclooxygenase-2 inhibitor, in human gastric cancer cell lines. Anticancer Res. 2000;20:4279-4284. [PubMed] |
| 19. | Ma L, Xie YL, Yu Y, Zhang QN. Apoptosis of human gastric cancer SGC-7901 cells induced by mitomycin combined with sulindac. World J Gastroenterol. 2005;11:1829-1832. [PubMed] [DOI] |
| 23. | Honjo S, Osaki M, Ardyanto TD, Hiramatsu T, Maeta N, Ito H. COX-2 inhibitor, NS398, enhances Fas-mediated apoptosis via modulation of the PTEN-Akt pathway in human gastric carcinoma cell lines. DNA Cell Biol. 2005;24:141-147. [PubMed] [DOI] |
| 24. | Aplin AE, Stewart SA, Assoian RK, Juliano RL. Integrin-mediated adhesion regulates ERK nuclear translocation and phosphorylation of Elk-1. J Cell Biol. 2001;153:273-282. [PubMed] [DOI] |
| 25. | Cyert MS. Regulation of nuclear localization during signaling. J Biol Chem. 2001;276:20805-20808. [PubMed] [DOI] |
| 28. | Liang B, Wang S, Zhu XG, Yu YX, Cui ZR, Yu YZ. Increased expression of mitogen-activated protein kinase and its upstream regulating signal in human gastric cancer. World J Gastroenterol. 2005;11:623-628. [PubMed] [DOI] |
| 29. | Shin EY, Ma EK, Kim CK, Kwak SJ, Kim EG. Src/ERK but not phospholipase D is involved in keratinocyte growth factor-stimulated secretion of matrix metalloprotease-9 and urokinase-type plasminogen activator in SNU-16 human stomach cancer cell. J Cancer Res Clin Oncol. 2002;128:596-602. [PubMed] [DOI] |