修回日期: 2005-07-10
接受日期: 2005-07-15
在线出版日期: 2005-09-15
目的: 探讨核因子-κB(nuclear factor-Kappa B, NF-κB)在癌前病变及胃癌组织中的表达及其与细胞毒素相关抗原A幽门螺杆菌(CagA+H. pylori)感染之间的关系.
方法: 慢性浅表性胃炎34例, 肠腺化生31例, 不典型增生34例, 胃癌55例, 应用免疫组化方法(SABC法)检测NF-κB p65的表达, 以14C-呼气试验、快速尿素酶试验和Warthin-Starry银染色检测H. pylori, 采用斑点金免疫渗滤法检测患者血清抗H. pylori CagA IgG抗体, 分析NF-κB p65表达与CagA+H. pylori感染之间、以及与胃癌组织学分型、临床病理分期、淋巴结转移的关系.
结果: 在慢性浅表性胃炎、肠腺化生、不典型增生和胃癌组中, NF-κB p65阳性表达率分别为15.0%, 41.9%, 64.7%和78.2%, 呈逐渐增高趋势, 各组间有显著性差异(χ2 = 43.98, P<0.01); H. pylori感染率分别为70.0%, 67.7%, 73.5%和54.5%, 各组间无显著性差异(P>0.05). CagA+H. pylori构成比分别为53.6%, 61.9%, 68.0%和73.3%, 各组间无显著性差异(P>0.05). 在肠化组中, H. pylori 阳性和CagA+H. pylori感染的患者NF-κB p65阳性表达率分别为57.1%和76.9%, 显著高于同组无H. pylori感染的10.0%(χ2 = 6.18, P<0.05)和CagA- H. pylori感染者的25.0%(χ2 = 5.45, P<0.05). 在胃癌组中, NF-κB p65阳性表达与T分期(χ2 = 5.91, P<0.05)及淋巴结转移有关(χ2 = 7.47, P<0.05), 但与胃癌组织学分型无关(P>0.05).
结论: NF-κB的异常活化在胃癌前病变及癌变过程中起作用, 早期的活化与CagA+H. pylori感染有关.
引文著录: 李国平, 吴灵飞, 蒲泽锦, 冯家琳, 郑宗茂, 王炳周. CagA+幽门螺杆菌胃癌前及癌变中NF-κB的表达增强. 世界华人消化杂志 2005; 13(17): 2064-2068
Revised: July 10, 2005
Accepted: July 15, 2005
Published online: September 15, 2005
AIM: To determine the expression of nuclear factor kappa B (NF-κB) in human gastric precancerous lesions and carcinoma and its correlation with CagA+H. pylori infection.
METHODS: The expression of NF-κB p65 was detected by immunohistochemistry (SABC assay), and H. pylori were examined using 14C-breath test, rapid urease test and Warthin-Starry staining in patients with chronic superficial gastritis (CSG: n = 34), intestinal metaplasia (IM: n = 31), atypical dysplasia (AD: n = 34) and gastric cancer (GC: n = 55). Serum CagA IgG antibody was detected by dot immunogold filtration assay. The correlations of NF-κB p65 expression with CagA+H. pylori infection as well as the histological types, clinicopathological stages and lymph node metastasis were analyzed.
RESULTS: The expression of NF-κB p65 in CSG, IM, AD, GC was 15.0%, 41.9%, 64.7%, and 78.2%, respectively, and there were significant differences between them (χ2 = 43.98, P <0.01). The rates of H. pylori infection were 70.0%, 67.7%, 73.5%, and 54.5%, respectively, and there were no significant differences between them (P > 0.05). The percentage of CagA+H. pylori infection were 53.6%, 61.9%, 68.0%, and 73.3%, respectively, and there were no significantdifferences (P > 0.05). In IM, the positive rate of NF-κB p65 expression in H. pylori or CagA+H. pylori positive patients were significantly higher than that in patients without H. pylori infection or with CagA- H. pylori infection (57.1%, 76.9% vs 10%, 25.0%, P <0.05). In GC patients, the positive expression of NF-κB p65 was correlation with the T stages(χ2 = 5.91, P <0.05)and lymph node metastasis (χ2 = 7.47, P <0.01), but not with the pathohistological types (P > 0.05).
CONCLUSION: NF-κB is constitutively activated in human gastric precancerous lesions and carcinoma tissue and correlates with tumor progression. The early activation may be related to CagA+H. pylori infection.
- Citation: Li GP, Wu LF, Pu ZJ, Feng JL, Zheng ZM, Wang BZ. Increased expression of NF-κB p65 in CagA+ H. pylori-related gastric precancerous lesions and carcinoma. Shijie Huaren Xiaohua Zazhi 2005; 13(17): 2064-2068
- URL: https://www.wjgnet.com/1009-3079/full/v13/i17/2064.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i17.2064
幽门螺杆菌(H. pylori)感染是慢性活动性胃炎的主要病因, 是萎缩性胃炎肠上皮化生和不典型增生等癌前病变的促进因素[1-5].流行病学调查资料表明,H. pylori感染者发生胃癌的危险性较非感染者6倍[6],但确切机制仍不清楚. 核因子-κB(nuclear factor kappa B, NF-κB)作为一种多向转录调节因子[7], 广泛参与多种生理和病理过程的基因调控[8-11], 最近发现它在肿瘤发生发展中亦起重要作用[12-15], 但有关在胃癌发病中的作用报道尚少. 有文献报道, 表达细胞毒素相关抗原A的H. pylori(CagA+H. pylori) 可上调NF-κB的表达[16]. 我们研究胃癌及癌前病变中NF-κB的表达以及H. pylori, 尤其是CagA+H. pylori的感染状态,旨在探讨二者之间的相互作用关系及其对胃癌发生发展的影响.
2002-5/2004-12我院胃癌患者55例, 男30例, 女25例, 年龄32-77(平均62.4)岁. 高分化癌25例, 中分化癌13例, 低分化癌11例, 黏液腺癌6例.T1期2例, T2期11例, T3期29例, T4期13例.淋巴结转移40例, 无淋巴结转移15例.所有患者术前均未行化疗或放疗.胃镜取材活检标本中慢性浅表性胃炎40例,慢性萎缩性胃炎伴肠化31例, 不典型增生34例,患者均经病理组织学诊断证实.胃黏膜活检标本取自胃窦或病变区, 手术标本均取癌灶及癌旁2 cm组织, 采用40 g/L多聚甲醛固定, 常规脱水、石蜡包埋, 备测.取胃窦部黏膜作快速尿素酶试验、Warthin-starry 银染色结合14C-呼气试验, 其中2项阳性定为H. pylori感染.微量胶囊法14C-呼气试验所需试剂由深圳养和生物科技公司提供, 按说明书检测样品每分钟衰变数(dmp), ≥200 dmp为(+), ≤150 dmp为(-); Warthin-Starry银染色按常规组织病理学技术进行, 深棕色细长弯曲为H. pylori染色.CagA+H. pylori 检测 采用斑点金免疫渗滤法.试剂盒购自西安联尔生物技术公司, 仅对H. pylori阳性者检测,反应完成后有1个红色斑点为阴性, 有2个红色斑点为阳性, 无红色斑点为无效.阳性者被视为CagA+H. pylori感染.
采用链霉亲和素-生物素-过氧化物酶复合物(SABC)法 兔抗人NF-κB p65及SP试剂盒购自北京中山生物公司.4μm厚连续切片, 二甲苯脱蜡、酒精水化后, 浸入30 mL/L的双氧水中30 min以阻断内源性过氧化物酶的活性, 微波抗原修复, 山羊血清封闭, 分别依次滴加抗NF-κB p65抗体(工作浓度1: 100), 4℃过夜, PBS液洗涤3次.生物素化二抗, 加辣根过氧化物酶标记的链霉卵白素工作液, 室温﹑湿盒中作用30 min, PBS液洗涤3次, DAB液显色, 苏木精轻度复染后脱水、透明、封片.以PBS代替一抗作阴性对照, 用已知胃癌阳性切片作阳性对照.在组织切片中显示胞质或胞核染为淡黄至棕黄色者为阳性细胞.先在低倍镜下选择阳性细胞最集中的5个视野,每个视野观察100个细胞,最后取5个视野中阳性细胞平均百分数作为每张切片的观察结果.根据Handel et al[17]的分级标准采用双盲法记分, 根据阳性细胞所占比例判断结果: <5%为阴性(-),5%-24%为弱阳性(+),25%-50%为中度阳性(++), >50%为强阳性(+++).根据染色强度将阳性信号分为3类: 染色强度弱(+)-淡黄色或仅个别细胞呈黄至棕黄色染色, 染色强度强(+++)-呈棕黄至棕褐色染色, 中等染色强度(++)-染色强度介于弱阳性与强阳性之间. 染色强度×阳性细胞百分数为每例组织染色的综合记分.综合记分≥1判为表达阳性, <1则为阴性.
统计学处理 采用χ2检验和Spearman等级相关分析, 应用SPSS10.0软件包, 检验水准α = 0.05.
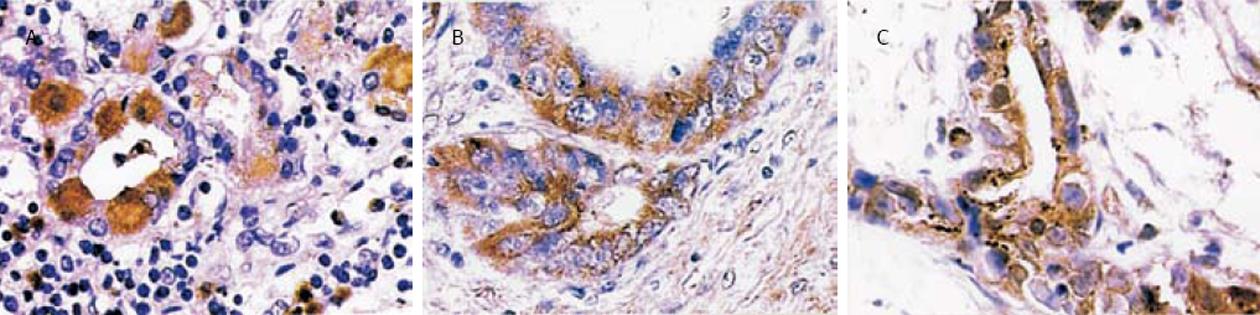
慢性浅表性胃炎40例中,H. pylori 阳性28例(CagA+H. pylori 15例,占53.6%),H. pylori阴性12例, H. pylori感染率为70.0%.慢性萎缩性胃炎伴肠化31例中, H. pylori 阳性21例(CagA+H. pylori 13例, 占61.9%), H. pylori 阴性10 例, H. pylori感染率为 67.7%.不典型增生34例中, H. pylori阳性25例(CagA+H. pylori 17 例,占68.0%), H. pylori 阴性 9 例, H. pylori感染率为73.5%.55例胃癌组织中, H. pylori 阳性30例(CagA+H. pylori22例, 占73.3%), H. pylori 阴性25例, H. pylori 感染率为 54.5%.各组H. pylori感染率无显著性差异(χ2 = 4.32, P>0.05).CagA+H. pylori感染的比例在胃炎、胃癌前病变至胃癌组中有逐渐增高趋势, 但无统计学意义(χ2 = 2.69,P>0.05).NF-κB p65蛋白表达呈棕黄色颗粒, 定位于细胞质, 部分亦可见于细胞核.慢性浅表性胃炎组织中胞质、胞核中很少呈现表达.癌前病变组织中其表达信号逐渐增强, 在不典型增生中炎性细胞浸润明显, 腺体结构破坏, 异型细胞胞质中可见其表达, 为棕黄颗粒(图1A).胃癌细胞中则常见其表达,呈粗细不等的棕黄或棕褐色颗粒, 胞质、胞核同时着色,表达强度为中等或强阳性(图1B-C).慢性浅表性胃炎﹑慢性萎缩性胃炎伴肠化﹑不典型增生和胃癌组织中NF-κB p65的表达率分别为15.0%, 41.9%, 64.7%和78.2%, 呈递增趋势, 经统计学处理, 差异有显著性(χ2 = 43.98, P<0.005).
按照H. pylori感染情况分组, 浅表性胃炎中H. pylori (+)组NF-κB p65表达率高于H. pylori(-)组, 但无显著性差异(P>0.05). 肠化患者中H. pylori(+)组NF-κB p65表达率显著高于H. pylori(-)组, 差异有统计学意义(χ2 = 6.18, P<0.05).不典型增生和胃癌组中H.pylori(+)与H. pylori(-)患者NF-κB p65表达阳性率相近, 经统计学处理, 均无显著性差异(P>0.05)(表1).按照CagA情况分组, 慢性萎缩性胃炎伴肠化组中CagA+H. pylori患者NF-κB p65阳性率为76.9%, CagA- H. pylori患者的阳性率为25.0%,二者相比, 差异有统计学意义(χ2 = 5.45,P<0.05).其余各组组内比较均无显著性差异(P>0.05)(表2).
流行病学调查资料表明, 胃癌发生率与当地H. pylori感染率呈正相关, 然而, H. pylori如何引起胃癌的机制并不清楚.一般认为, 胃癌的发生是一个有多因素参与的复杂而漫长的过程.大多从慢性浅表性胃炎至萎缩性胃炎, 逐渐出现肠上皮化生和不典型增生等癌前病变, 最终才转化成癌.本结果表明, 在有胃病症状的人群中H. pylori的感染率为54.5%-73.5%, 与文献报道相同[18,19].胃癌组患者的感染率较低(54.5%), 我们认为可能与研究方法有关.通过血清学方法, 我们发现在胃炎、胃癌前病变至胃癌组中CagA+H. pylori感染比例有逐渐增高趋势, 显示检测抗体的方法可能比实时检测细菌更能反映H. pylori对胃部疾病的长期影响, 新近Storskrubb et al在H. pylori对胃癌前病变的一组流行病学调查报告中亦支持此观点[20].慢性萎缩性胃炎伴肠化H. pylori阳性或CagA+H. pylori患者NF-κB p65的阳性表达率显著高于H. pylori阴性或CagA-H. pylori 患者, 慢性浅表性胃炎组中H. pylori阳性NF-κB p65表达虽高于H. pylori阴性患者, 但未显出差异且均在较低的水平, 提示H. pylori感染引起NF-κB p65表达上调需要一定的时间.不典型增生及胃癌组NF-κB p65的表达均明显增高, 且与H. pylori或CagA+H. pylori感染无关, 说明一旦进入病变严重或不可逆阶段, 由于涉及多种遗传物质的改变, H. pylori的作用已不再易于显示出来, NF-κB p65的激活是多因素作用长期累积的结果[21,22].CagA+H. pylori感染的胃炎向胃癌转变过程中NF-κB p65可能是重要的分子生物学环节.H. pylori, 特别是CagA+H. pylori引起NF-κB p65活化, 并与其它因素一起启动靶基因c-myc,Cyclin D 和bcl-2 的转录[23-27],破坏正常细胞的分化过程, 最终导致胃上皮细胞向恶性方向转化[28], 可能是其参与胃癌发生的机制之一. 最近研究发现, NF-κB在肿瘤的发生、发展中起重要作用, 可调控细胞的增殖﹑分化、凋亡及恶性转化, 其活性失控常与哺乳动物肿瘤发生有关[29-31].有证据表明, NF-κB的持续活化可作为结肠癌、胰腺癌、乳腺癌、前列腺癌等多种实体肿瘤的标志[7-11,22,29]. Wang et al[32]检测胰腺组织中NF-κB p65的表达, 结果胰腺癌的表达阳性率为67%, 而正常胰腺组织表达罕见.在前列腺癌的研究中, Huang et al[33]报道NF-κB活化后可抑制细胞凋亡, 促进其增殖. Sasaki et al[15]应用免疫组织化学方法检测 NF-κB p65 在胃癌细胞胞质和胞核中的表达情况, 结果肿瘤组织中NF-κB p65的表达明显高于癌旁正常上皮细胞; 电泳迁移率改变分析显示胃癌组织中 NF-κB的 DNA 结合活性与核转位的活性均增加. 本研究中NF-κB p65在慢性浅表性胃炎、慢性萎缩性胃炎伴肠化、不典型增生及胃癌组织中的表达阳性率呈递增趋势, 各组之间差异有统计学意义,且随着组织学病变程度的加重, 阳性细胞数量逐渐增多,分布范围也逐渐扩大, 与Sasaki et al[15]结果一致.NF-κB p65在癌前病变中有较高的阳性率,表明NF-κB p65蛋白表达参与了胃癌前病变的进展, 为胃癌癌变的早期事件, 有可能成为检测早期癌变的标志物.本组55例胃癌中, NF-κB p65表达阳性率在高分化腺癌中较高, 但与中分化腺癌、低分化或黏液癌无显著性差异, 提示NF-κB的表达与肿瘤发生的组织学类型无关.比较不同肿瘤分期中NF-κB p65的表达, T3, T4期的胃癌此蛋白的表达显著高于T1, T2期, 而且伴淋巴结转移者显著高于无淋巴结转移者, 显示NF-κB p65表达与胃癌的分期、浸润深度及淋巴结转移等病理学特性关系密切, 越近晚期的癌肿表达阳性率相对也越高, 与史朝晖et al[34]、王维et al[35]的报道相似, 提示NF-κB p65的异常活化与胃癌的发病密切相关外, 还可能对胃癌侵袭生长施加影响, 并可增加其转移潜能[13,14].从另一角度分析, 此蛋白表达与胃癌侵润及转移有关, 则有可能作为判断胃癌预后的指标[36].
编辑: 潘伯荣 审读: 张海宁
| 2. | Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477-484. [PubMed] [DOI] |
| 3. | Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301-310. [PubMed] [DOI] |
| 4. | Ma XT, Wang S, Ye YJ, Du RY, Cui ZR, Somsouk M. Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma. World J Gastroenterol. 2004;10:1569-1573. [PubMed] [DOI] |
| 6. | Wang TC, Dangler CA, Chen D, Goldenring JR, Koh T, Raychowdhury R, Coffey RJ, Ito S, Varro A, Dockray GJ. Synergistic interaction between hypergastrinemia and Helicobacter pylori infection in a mouse model of gastric cancer. Gasteroenterology. 2000;118:36-47. [PubMed] [DOI] |
| 7. | Lin A, Κarin M. NF-kappa B in cancer: a marked target. Semin Cancer Biol. 2003;139:107-114. [PubMed] [DOI] |
| 8. | Yu LL, Yu HG, Yu JP, Luo HS. Nuclear factor-kappa B regulates cyclooxygenase-2 expression and cell proliferation in human colorectal carcinoma tissue. Eksp Onkol. 2004;26:40-47. [PubMed] |
| 9. | Ross JS, Kallakury BV, Sheehan CE, Fisher HA, Kaufman RP Jr, Kaur P, Gray K, Stringer B. Expression of nuclear factor- kappa B and I kappa B alpha proteins in prostatic adenocarcinomas: correlation of nuclear factor-kappa B immunoreactivity with disease recurrence. Clin Cancer Res. 2004;10:2466-2472. [PubMed] [DOI] |
| 10. | Oya M, Takayanagi A, Horiguchi A, Mizuno R, Ohtsubo M, Marumo K, Shimizu N, Murai M. Increased nuclear factor-kappa B activation is related to the tumor development of renal cell carcinoma. Carcinogenesis. 2003;24:377-384. [PubMed] [DOI] |
| 11. | Ghosh S, Κarin M. Missing pieces in the NF-kappa B puzzle. Cell. 2002;109 Suppl:S81-S96. [PubMed] |
| 12. | Yu LL, Yu HG, YU JP, Luo HS, Xu XM, Li JH. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human colorectal carcinoma tissue. World J Gastroenterol. 2004;10:3255-3260. [PubMed] [DOI] |
| 13. | Yu HG, Zhong X, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Increased expression of nuclear factor-kappaB/RelA is correlated with tumor angiogenesis in human colorectal cancer. Int J Colorectal Dis. 2004;19:18-22. [PubMed] [DOI] |
| 14. | Cao HJ, Fang Y, Zhang X, Chen WJ, Zhou WP, Wang H, Wang LB, Wu JM. Tumor metastasis and the reciprocal regulation of heparanase gene expression by nuclear factor kappa B in human gastric carcinoma tissue. World J Gastroenterol. 2005;11:903-907. [PubMed] [DOI] |
| 15. | Sasaki N, Morisaki T, Hashizume K, Yao T, Tsuneyoshi M, Noshiro H, Nakamura K, Yamanaka T, Uchiyama A, Tanaka M. Nuclear factor-kappaB p65 (RelA) transcription factor is constitutively activated in human gastric carcinoma tissue. Clin Cancer Res. 2001;7:4136-4142. [PubMed] |
| 16. | Lawniczak M, Starzynska T. Helicobacter pylori CagA(+) infection in gastric cancer patients. Pol Merkuriusz Lek. 2002;13:216-220. [PubMed] |
| 17. | Handel ML, Mcmorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762-1770. [PubMed] [DOI] |
| 18. | Whary MT, Sundina N, Bravo LE, Correa P, Quinones F, Caro F, Fox JG. Intestinal helminthiasis in colombian children promotes a Th2 response to Helicobacter pylori: possible implications for gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2005;14:1464-1469. [PubMed] [DOI] |
| 19. | Ye W, Held M, Enroth H, Kraaz W, Engstrand L, Nyren O. Histology and culture results among subjects with antibodies to CagA but no evidence of Helicobacter pylori infection with IgG ELISA. Scand J Gastroenterol. 2005;40:312-318. [PubMed] [DOI] |
| 20. | Storskrubb T, Aro P, Ronkainen J, Vieth M, Stolte M, Wreiber K, Engstrand L, Nyhlin H, Bolling-Sternevald E, Talley NJ. A negative Helicobacter pylori serology test is more reliable for exclusion of premalignant gastric conditions than a negative test for current H. pylori infection: a report on histology and H. pylori detection in the general adult population. Scand J Gastroenterol. 2005;40:302-311. [PubMed] [DOI] |
| 21. | Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor-κB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675-681. [PubMed] |
| 22. | Sovak MA, Bellas RE, Kin DW, Zanieski DJ, Rogers AE, Traish AM, Sonenshein GE. Aberrant nuclear factor-kappaB/Rel expression and the pathogenesis of breast cancer. J Clin Invest. 1997;100:2952-2960. [PubMed] [DOI] |
| 23. | Wang HT, Li ZH, Yuan JP, Zhao W, Shi XD, Tong SQ, Guo XK. Effect of Helicobacter pylori VacA on gene expression of gastric cancer cells. World J Gastroenterol. 2005;11:109-113. [PubMed] [DOI] |
| 24. | Navaglia F, Basso D, Zambon CF, Ponzano E, Caenazzo L, Gallo N, Falda A, Belluco C, Fogar P, Greco E. Interleukin 12 gene polymorphisms enhance gastric cancer risk in H. pylori infected individuals. J Med Genet. 2005;42:503-510. [PubMed] [DOI] |
| 25. | Kawabe A, Shimada Y, Uchida S, Maeda M, Yamasaki S, Kato M, Hashimoto Y, Ohshio G, Matsumoto M, Imamura M. Expression of cyclooxygenase-2 in primary and remnant gastric carcinoma: comparing it with p53 accumulation, Helicobacter pylori infection, and vascular endothelial growth factor expression. J Surg Oncol. 2002;80:79-88. [PubMed] [DOI] |
| 26. | Papa A, Danese S, Sgambato A, Ardito R, Zannoni G, Rinelli A, Vecchio FM, Gentiloni-Silveri N, Cittadini A, Gasbarrini G. Role of Helicobacter pylori CagA+ infection in determining oxidative DNA damage in gastric mucosa. Scand J Gastroenterol. 2002;37:409-413. [PubMed] [DOI] |
| 27. | Maihofner C, Charalambous MP, Bhambra U, Lightfoot T, Geisslinger G, Gooderham NJ; Colorectal Cancer Group. Expression of cyclooxygenase-2 parallels expression of interleukin-1beta, interleukin-6 and NF-kappaB in human colorectal cancer. Carcinogenesis. 2003;24:665-671. [PubMed] [DOI] |
| 28. | Ricca A, Biroccio A, Trisciuoglio D, Cippitelli M, Zupi G, Del Bufalo D. relA over-expression reduces tumorigenicity and activates apoptosis in human cancer cells. Br J Cancer. 2001;85:1914-1921. [PubMed] [DOI] |
| 29. | Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-kappaB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501-5507. [PubMed] [DOI] |
| 30. | Bueso-Ramos CE, Rocha FC, Shishodia S, Medeiros LJ, Kantarjian HM, Vadhan-Raj S, Estrov Z, Smith TL, Nguyen MH, Aggarwal BB. Expression of constitutively active nuclear-kappa B RelA transcription factor in blasts of acute myeloid leukemia. Hum Pathol. 2004;35:246-253. [PubMed] [DOI] |
| 31. | Tai DI, Tsai SL, Chang YH, Huang SN, Chen TC, Chang KS, Liaw YF. Constitutive activation of nuclear factor kappaB in hepatocellular carcinoma. Cancer. 2000;89:2274-2281. [PubMed] [DOI] |
| 32. | Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119-127. [PubMed] |
| 33. | Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188-4197. [PubMed] [DOI] |
| 34. | 史 朝晖, 常 新忠, 姜 希宏, 李 兆亭. 胃癌组织中核因子-κB p65的表达及其与血管内皮生长因子的关系. 中国普外 基础与临床杂志. 2004;11:113-115. |
| 36. | Yamamoto S, Tomita Y, Hoshida Y, Sakon M, Kameyama M, Imaoka S, Sekimoto M, Nakamori S, Monden M, Aozasa K. Expression of valosin-containing protein in colorectal carcinomas as a predictor for disease recurrence and prognosis. Clin Cancer Res. 2004;10:651-657. [PubMed] [DOI] |