修回日期: 2005-05-04
接受日期: 2005-05-14
在线出版日期: 2005-07-28
目的: 探讨人胃癌细胞系AGS中12-脂肪氧化酶(12-lipoxygenase, 12-LOX)的表达情况及其抑制剂黄芩甙元(baicalein)对AGS细胞增殖的影响.
方法: AGS在含100 mL/L小牛血清+100 kU/L青、链霉素的RPMI1640培养基中常规培养. 用RT-PCR检测12-LOX在AGS中的表达情况, 分别以20, 40, 80 mmol/L baicalein干预细胞, 用四氮唑蓝(thiazolyl blue tetrazolium bromide, MTT)法在24, 48, 72 h测量细胞吸光度, 检测baicalein对细胞增殖的影响; 用倒置显微镜和电子显微镜进行形态观察; 通过脱氧核糖核酸末端转移酶介导的dUTP缺口末端标记法(TUNEL)和吖啶橙-溴化乙锭(AO-EB)染色法检测细胞凋亡指数.
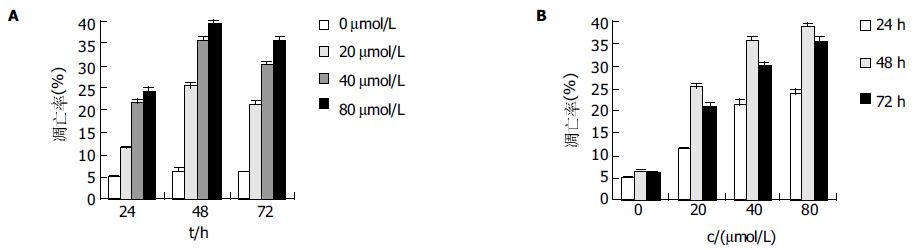
结果: AGS细胞中存在12-LOXmRNA表达;MTT显示baicalein大致呈时间、剂量依赖性抑制细胞增殖(各组间P<0.01, 除外40mmol/L 24 h组与48 h组);AGS细胞存活率最高为82.1%, 最低为38.4%. 倒置显微镜下可见baicalein干预后的细胞皱缩, 部分细胞胞体变圆, 脱离贴壁状态, 成为悬浮细胞; 电镜下可见baicalein干预后的细胞出现了凋亡的特征性改变: 核染色质边集及凋亡小体形成; AO-EB染色法显示除0 mmol/L组不同时间点细胞凋亡指数无显著差异外(P>0.05), 其余各干预组细胞凋亡指数均有显著差异(P<0.01); TUNEL染色法显示48 h时, baicalein 80 mmol/L细胞调亡指数为38.37±0.36%而0 mmol/L组细胞调亡指数为7.27±0.21%, 两组间存在显著差异(P<0.01).
结论: 人胃癌细胞AGS中存在12-LOXmRNA表达, 其特异性抑制剂baicalein能抑制AGS细胞增殖并促进其凋亡.
引文著录: 黄彩云, 陈丰霖, 李建英, 陈治新, 王小众. AGS细胞系中12-LOX的表达及其抑制剂对细胞增殖的影响. 世界华人消化杂志 2005; 13(14): 1652-1657
Revised: May 4, 2005
Accepted: May 14, 2005
Published online: July 28, 2005
AIM: To investigate the expression of 12-lipoxygenase (12-LOX) and the possible effect of its inhibitor on proliferation of human gastric cancer cell line AGS.
METHODS: AGS cells were cultured in RPMI-1640 medium with 100 mL/L fetal bovine serum (FBS), 100 kU/L penicillin and 100 kU/L streptomycin. 12-LOX mRNA was analyzed by reverse transcription polymerase chain reaction (RT-PCR). The effect ofbaicalein on the proliferation of the cells was detected by thiazolyl blue tetrazolium bromide (MTT) method. The morphological changes of cellular and subcellullar structures were observed under inverted microscope and electron microscopy. AO-EB double and TUNEL staining were used to evaluate the apoptotic index of AGS cells.
RESULTS: 12-LOX mRNA was expressed in human AGS cells. At concentrations from 20 to 80 mmol/L, baicalein inhibited the proliferation of AGS cells in a concentration- and time-dependent manner from 24 to 72 hours. There were significant differences between any other groups (P < 0.01) except 40 mmol/L group at 24 h and 48 h. The maximum survival rate of the cells was 82.1% and the minimum one was 38.4%. Morphological changes such as chromatin condensatio, apoptotic bodies were observed after treated with baicalein, and some cells become round and suspended. Significant apoptosis was also induced by baicalein, and the apoptotic indexes detected by AO-EB staining showed marked differences between any other groups (P < 0.01) except in 0 mmol/L group. The apoptotic index detected by TUNEL staining showed marked difference between 80 and 0 mmol/L at 48 h (38.37±0.36% vs 7.27±0.21%, P < 0.01 ).
CONCLUSION: 12-LOX mRNA is expressed in AGS cell line. Baicalein not only promotes proliferation, but also induces apoptosis of human gastric cancer cells in vitro.
- Citation: Huang CY, Chen FL, Li JY, Chen ZX, Wang XZ. Expression of 12-lipoxygenase and its inhibitor's effect on proliferation of human AGS cell line. Shijie Huaren Xiaohua Zazhi 2005; 13(14): 1652-1657
- URL: https://www.wjgnet.com/1009-3079/full/v13/i14/1652.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i14.1652
12-LOX是脂肪氧化酶家族(Lipoxygenases:5, 8, 12, 15-LOX)中的成员之一[1-4], 他是催化花生四烯酸(arachidonic acid, AA)转化为12-羟基廿碳四烯酸(12-hydroxyeicosatetraenoic acid, 12-HETE)的关键酶[5], 其与肿瘤的关系已成为近年来的研究热点. 尽管12-LOX已被证实在多种肿瘤组织及细胞系中表达[6-10], 但其在胃癌细胞系AGS中的表达情况及其确切作用却少有报道. baicalein是从中草药黄芪中提取的一种具有抗炎、抗氧化和抗癌活性的黄酮类化合物, 作为12-LOX的特异性抑制剂, 他在体和离体实验均显示有较强的抑制生长和诱导凋亡的作用[11-16]. 我们用RT-PCR检测12-LOX mRNA在AGS中的表达情况, 应用12-LOX的特异性抑制剂baicalein对体外培养的AGS细胞进行干预, 分别采用MTT、电镜、AO-EB和TUNEL对AGS细胞增殖与凋亡进行研究, 试图探讨12-LOX的特异性抑制剂baicalein对胃癌AGS细胞增殖与凋亡的影响.
人胃癌细胞系AGS(中分化胃腺癌细胞系)购自中国科学院上海细胞生物研究所, baicalein购自Alexis Biochemicals公司, 四甲基偶氮唑盐(MTT)购自Fluka公司, RPMI1640购自Gibco公司, 胰蛋白酶购自Sigma公司, 小牛血清购自杭州四季青生物工程材料有限公司, TUNEL试剂购自北京中山公司, RT-PCR体系购自深圳晶美公司, RNA提取试剂(RNA isolation kit)购自Gentra Systems Inc, 其余试剂均为国产生化试剂.
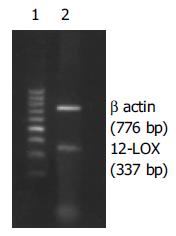
AGS细胞在含100 mL/L小牛血清+100 Uk/L青、链霉素的RPMI1640培养基中, 于37 ℃, 50 mL/L CO2培养箱中常规培养. 收集未经药物干预的AGS细胞 2×106, PBS洗涤2遍后, 按RNA提取试剂盒说明书提取细胞总RNA, 测其A260为0.1880(A260/A280 = 1.8970), 取RNA样品4 mg, 42 ℃, 60 min, 95 ℃, 5 min合成cDNA, 12-LOX基因上游引物:5'-CTTCCCGTGCTACCGCTG-3', 下游引物:5'-TGGGGTT GGCACCATTGAG-3, 337 bp; 内参β-actin基因上游引物:5'-CTATTGGCAACGAGCGGTTC-3', 下游引物:5'-CTTAGGAGTGGGGGTGGCTT-3', 776 bp. 取RT反应产物2.0 mL进行PCR扩增(反应体系25 mL), 扩增条件: 94 ℃, 5 min; 94 ℃, 45 s; 50 ℃, 30 s; 72 ℃, 60 s; 35个循环; 72 ℃, 7 min. PCR产物10 mL在17 g/L琼脂糖凝胶上(含0.5 g/L溴化乙锭)进行电泳分离, 在紫外光下自显影, 经计算机扫描成像. 取对数生长期的AGS细胞, 经2.5 g/L胰蛋白酶消化后, 以3×104/孔接种于6孔培养板中, 常规培养24 h后, 换成无血清的RPMI1640培养液同步化24 h后进行分组, 即: 对照组(含等体积无水乙醇), baicalein(baicalein溶于无水乙醇中, 用含10 mL/L小牛血清的RPMI1640稀释至baicalein终浓度, 使乙醇浓度<1 mL/L)20 mmol/L组、40 mmol/L组、80 mmol/L组, 加试剂后继续培养72 h, 分别于加试剂后24, 48和72 h取各浓度实验组和对照组细胞, 倒置显微镜下观察细胞形态. 所有实验均独立重复3遍. 取未干预细胞和经80 mmol/L baicalein干预48 h的细胞经30 g/L戊二醛-15 g/L多聚甲醛前固定2 d(4 ℃), 10 g/L锇酸-15 g/L亚铁氰化钾后固定1.5 h, PBS漂洗; 700 mL/L乙醇饱和醋酸铀块染, 乙醇-丙酮梯度脱水, 环氧树脂618包埋, 超薄切片80 nm, 醋酸铀、柠檬酸铅染色各5 min; 在日立Hu-12A型透射电镜下观察、摄影.
1.2.1 MTT法检测AGS增殖情况: 以5×103/孔接种AGS细胞于96孔培养板中, 药物干预同前, 于加试剂后的24, 48和72 h每组各取7孔用0.01 mol/L PBS冲洗两遍后加入MTT溶液(5 g/L)20 mL, 37 ℃孵育4 h, 吸去孔内培养液, 每孔加入DMSO溶液150 mL于振荡器上振荡10 min, 以空白孔调零, 在酶联免疫检测仪上测490 nm波长每孔的吸光度值(absorbance), 求其平均值. 细胞存活率 = (实验组吸光度值/对照组吸光度值)×100%(假定加药时细胞的存活率为100%, 此时记为0 h).
1.2.2 AGS凋亡情况的检测: TUNEL染色法按TUNEL试剂盒的产品说明书, 以PBS代替TdT溶液作阴性对照, DNAaseⅠ处理的切片作阳性对照, 具体步骤如下: 制备好的含有细胞的盖玻片干燥后加40 g/L多聚甲醛50 mL于室温固定1 h, PBS漂洗3×5 min; 加3 mL/L过氧化氢甲醇溶液50 mL于室温孵育30 min, PBS漂洗3×5 min; 加50 mL含1 g/L TritonX-100的枸橼酸钠溶液4 ℃通透3 min, PBS漂洗3×5 min; 加50 mL TUNEL反应液于37 ℃湿盒中孵育1 h, PBS漂洗3×5 min; 加50 mL Converter-POD于37 ℃湿盒中孵育30 min, PBS漂洗3×5 min; 加50 mL DAB底物显色液室温孵育10 min, 双蒸水洗涤10 min, 苏木素复染10 s, 中性树胶封片, 光镜下观察、计数并拍照, 每片计数500个细胞, 计算细胞凋亡指数(即每100个细胞中TUNEL阳性细胞所占的百分比).AO-EB双染色法 细胞培养于培养瓶中, 药物干预同前, 于规定时间分别取各浓度干预细胞和未干预细胞, 常规胰蛋白酶消化并制成单个细胞悬液, 取细胞悬液90 mL, 加AO染液(acridine orange, AO, 100 mg/L溶于PBS)和EB染液(ethidium bromide, EB, 100 mg/L溶于PBS)各5 mL, 用前混合, 盖玻片封片后立即于490 nm蓝色荧光下观察、计数并拍照. 每片计数500个细胞, 计算细胞凋亡指数, 凋亡指数 = (早期凋亡细胞+晚期凋亡细胞)/总细胞数×100%.
统计学处理 数据以平均数±标准差(mean±SD)表示, 采用SPSS10.0 for windows软件包进行分析, 均数间两两比较用方差分析, 率间比较用χ2检验.
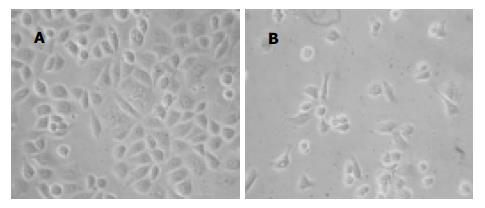
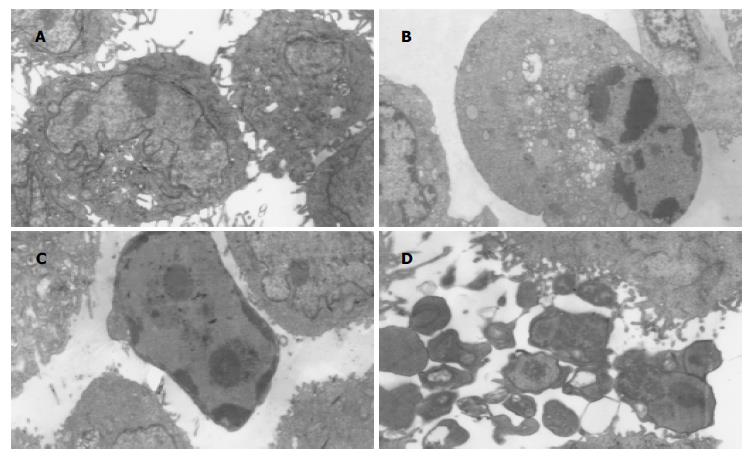
AGS细胞RT-PCR产物经电泳分析, 可见1条约776 bp的内参β-actin基因扩增带和1条约337 bp的12-LOX基因扩增带(图1). 倒置显微镜下干预组细胞皱缩, 部分细胞肿胀明显, 细胞质内可见泡状结构, 部分细胞细胞体变圆, 脱离贴壁状态, 成为悬浮细胞, baicalein对AGS细胞形态变化的影响与时间和剂量有关, 随着时间的延长, 药物浓度的加大, 正常形态细胞数明显减少, 异常形态细胞数逐渐增多(图2B); 未干预组细胞则呈多边形贴壁生长, 悬浮细胞很少(图2A). 透射电镜下未经baicalein干预的细胞表面存在丰富的微绒毛, 胞膜完整, 各细胞器形态结构无异常改变, 核染色质分布均匀(图3A); 经baicalein干预的细胞表面微绒毛减少甚至消失, 胞膜完整, 细胞皱缩, 核染色质固缩, 边集于核膜下, 部分凋亡细胞可见核碎裂及凋亡小体形成(图3B-D).
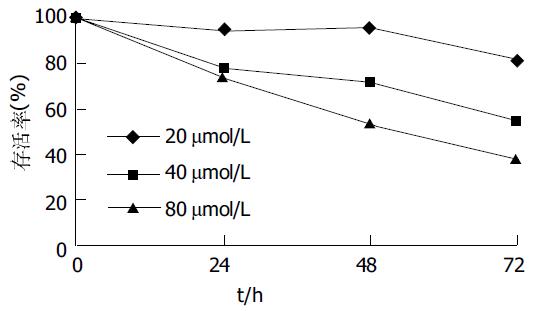
不同浓度baicalein在72 h内大致呈时间、剂量依赖性抑制AGS增殖(除40 mmol/L48 h组与24 h组P = 0.122外, 其余各组间两两比较P<0.01), 细胞存活率呈下降趋势, baicalein干预72 h, AGS细胞存活率最高为82.1%, 最低为38.4%(图4).
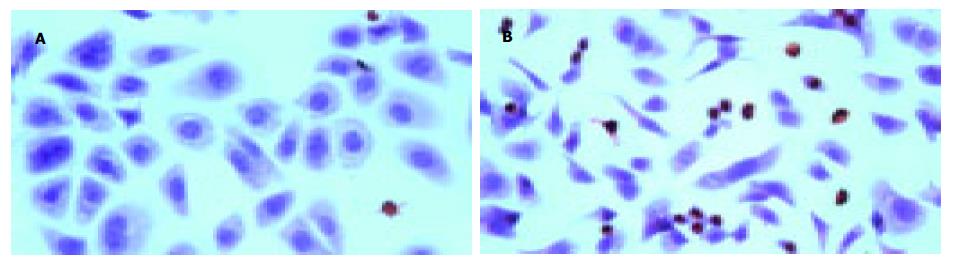
AO-EB双染色法: 正常活细胞核染色质被AO染成均匀一致的绿色或黄绿色, 核染色质结构正常(图6A或图6B中1所示); 早期凋亡细胞核染色质也被AO染成绿色或黄绿色, 但染色不均匀, 荧光增强, 核染色质呈固缩状或圆株状(图6B中2所示); 晚期凋亡细胞核染色质被EB染成橙色呈固缩状或圆株状(图6B中3所示); 死细胞核染色质也被EB染成橙色或红色, 但细胞核染色质结构尚正常(图6B中4所示). 同一时间点不同浓度的细胞凋亡指数均显著高于对照组(P<0.01)(图5A); 实验组48 h细胞凋亡指数比24 h明显增多(P<0.01), 但72 h细胞凋亡指数却比48 h明显减少(P<0.01), 而对照组3个时间点细胞凋亡指数均无显著差异(P>0.05)(图5B), 各实验组中以80 mmol/L组作用48 h引起AGS凋亡的作用最明显, baicalein诱导AGS细胞凋亡呈现剂量依赖性. 经TUNEL染色与苏木素复染后, 80 mmol/L baicalein干预48 h的AGS非凋亡细胞, 细胞核染成蓝色, 但细胞大小不一, 形态各异; 凋亡细胞皱缩, 细胞核染成棕色(图7B); 对照组凋亡细胞少见(图7A), 细胞大小一致, 在不同视野下进行细胞计数, 测得其凋亡指数为7.27±0.21%(n = 3), 而干预组AGS细胞凋亡指数明显高于未干预组, 凋亡指数为38.37±0.36%(n = 3), 二者之间有显著性差异(P<0.01).
胃癌是消化系统常见的恶性肿瘤, 其机制迄今未明, 但胃黏膜上皮细胞增殖过度及凋亡受抑是胃癌发生发展的重要原因. 随着对胃癌发病机制研究的进一步深入, 花生四烯酸代谢与胃癌的关系已越来越受到人们的关注. 花生四烯酸代谢至少有两条途径, 即: 环氧化酶途径和脂肪氧化酶途径. 其中环氧化酶代谢途径在胃癌的发生发展中起重要作用已有大量文献报道[17-20], 而脂肪氧化酶代谢途径与胃癌形成的关系国内外报道较少, 尤其是12-LOX与胃癌的研究更是罕见报道. 在本次研究中我们发现, 12-LOX参与了胃癌细胞AGS增殖与凋亡之间的调控. 12-LOX有3个亚型, 即: 白细胞型、表皮型和血小板型[21], 其中, 血小板型12-LOX及其代谢产物12-HETE被认为具有刺激肿瘤细胞生长与增殖, 提高肿瘤细胞侵袭能力及诱导肿瘤血管形成等作用, 而这些作用又均可被12-LOX特异性抑制剂baicalein所抑制[22-25].Baicalein作为12-LOX的特异性抑制剂, 其作用机制仍在探讨中, 但目前比较一致的观点认为baicalein是通过阻断细胞周期, 启动线粒体凋亡途径而发挥其生物学作用的[13,26-29].12-LOX在正常组织中低表达或不表达, 在肿瘤组织中却过度表达[8,10], 因此, 12-LOX过度表达可能是其促进肿瘤发生发展的原因之一.
我们在体外培养AGS细胞的基础上, 使用RT-PCR技术从mRNA水平证实了12-LOX在胃癌细胞系AGS中存在表达. 实验还从细胞水平证明了baicalein可能具有抑制AGS增殖和促进AGS凋亡的作用. 在研究baicalein对AGS增殖的影响中, 我们发现, baicalein大致呈时间、剂量依赖性的抑制AGS细胞增殖, 这点与文献[30]报道一致, 但baicalein只有达到较高浓度并且作用足够的时间方能起到显著抑制作用, 与文献[30]报道的低浓度作用较短时间即可起到显著抑制作用略有不同; 在研究baicalein对AGS凋亡的影响中, 倒置显微镜下可见baicalein干预后, 细胞形态发生了显著变化, 透射电镜下可以见到典型的凋亡细胞. TUNEL结果显示, 实验组细胞的凋亡指数与对照组存在显著性差异, 在用AO-EB双染色法计算细胞凋亡指数时我们发现, 细胞凋亡指数与药物浓度成正比, 但和时间却没有明显的线性关系, 细胞的凋亡指数至72 h反而呈现下降趋势, 这可能与72 h细胞死亡率相应增加有关, 此现象提示, baicalein抑制细胞生长除与诱导凋亡有关可能还存在其他机制, 因为在实验中我们还发现, 随着时间的推移, 实验组的死亡细胞数也随之增加, 尤以药物作用72 h最为明显, 而此现象在对照组却不明显. 总之, 胃癌细胞AGS中存在12-LOXmRNA表达, 且12-LOX还参与了胃癌细胞AGS增殖与凋亡的调控, 12-LOX具有促进胃癌细胞增殖并抑制其凋亡的作用, 至于12-LOX是如何促进胃癌细胞增殖并抑制其凋亡, 其机制有待进一步探索.
福建医科大学电镜室.
编辑: 潘伯荣 审读:张海宁
| 1. | Smith WL. The eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315-324. [PubMed] [DOI] |
| 2. | Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1-15. [PubMed] [DOI] |
| 3. | Kuhn H, Thiele BJ. The diversity of the lipoxygenase family. Many sequence data but little information onbiological significance. FEBS Lett. 1999;449:7-11. [PubMed] [DOI] |
| 4. | Brash AR. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J Biol Chem. 1999;274:23679-23682. [PubMed] [DOI] |
| 5. | Hamberg M, Samuelsson B. Prostaglandin endoperoxides. novel transformations of arachidonic acid in humanplatelets. Proc Natl Acad Sci USA. 1974;71:3400-3404. [PubMed] [DOI] |
| 6. | Yoshimura R, Inoue K, Kawahito Y, Mitsuhashi M, Tsuchida K, Matsuyama M, Sano H, Nakatani T. Expression of12-lipoxygenase in human renal cell carcinoma and growth prevention by its inhibitor. Int J Mol Med. 2004;13:41-46. [PubMed] |
| 7. | Yoshimura R, Matsuyama M, Tsuchida K, Kawahito Y, Sano H, Nakatani T. Expression of lipoxygenase in humanbladder carcinoma and growth inhibition by its inhibitors. J Urol. 2003;170:1994-1999. [PubMed] [DOI] |
| 8. | Yoshimura R, Matsuyama M, Mitsuhashi M, Takemoto Y, Tsuchida K, Kawahito Y, Sano H, Nakatani T. Relationship between lipoxygenase and human testicular cancer. Int J Mol Med. 2004;13:389-393. [PubMed] [DOI] |
| 9. | Jiang WG, Douglas-Jones A, Mansel RE. Levels of expression of lipoxygenases and cyclooxygenase-2 in humanbreast cancer. Prostaglandins Leukot Essent Fatty Acids. 2003;69:275-281. [PubMed] [DOI] |
| 10. | Matsuyama M, Yoshimura R, Mitsuhashi M, Hase T, Tsuchida K, Takemoto Y, Kawahito Y, Sano H, Nakatani T. Expression of lipoxygenase in human prostate cancer and growth reduction by its inhibitors. Int J Oncol. 2004;24:821-827. [PubMed] [DOI] |
| 11. | Ikemoto S, Sugimura K, Kuratukuri K, Nakatani T. Antitumor effects of lipoxygenase inhibitors on murine bladder cancer cell line (MBT-2). Anticancer Res. 2004;24:733-736. [PubMed] |
| 12. | Chang WH, Chen CH, Gau RJ, Lin CC, Tsai CL, Tsai K, Lu FJ. Effect of baicalein on apoptosis of the human HepG2 cell line was induced by mitochondrial dysfunction. Planta Med. 2002;68:302-306. [PubMed] [DOI] |
| 13. | Pidgeon GP, Kandouz M, Meram A, Honn KV. Mechanisms controlling cell cycle arrest and induction of apoptosis after 12-lipoxygenase inhibition in prostate cancer cells. Cancer Res. 2002;62:2721-2727. [PubMed] |
| 14. | Tong WG, Ding XZ, Adrian TE. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2002;296:942-948. [PubMed] [DOI] |
| 15. | Ikemoto S, Sugimura K, Yoshida N, Yasumoto R, Wada S, Yamamoto K, Kishimoto T. Antitumor effects of Scutellariae radix and its components baicalein, baicalin, and wogonin on bladder cancer cell lines. Urology. 2000;55:951-955. [PubMed] [DOI] |
| 16. | Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2002;1:929-935. [PubMed] |
| 17. | Fan XM, Zheng FS, Liu HY, Ma YH, Wong B. Mechanism of apoptosis induced by specific COX-2 inhibitor SC236 in gastric cancer cells. Zhonghua Zhongliu Zazhi. 2005;27:145-147. [PubMed] |
| 18. | Wu CY, Wang CJ, Tseng CC, Chen HP, Wu MS, Lin JT, Inoue H, Chen GH. Helicobacter pylori promote gastric cancer cells invasion through a NF-kappa B and COX-2-mediated pathway. World J Gastroenterol. 2005;11:3197-3203. [PubMed] [DOI] |
| 19. | Fu YG, Sung JJ, Wu KC, Wu HP, Yu J, Chan M, Chan VY, Chan KK, Fan DM, Leung WK. Inhibition of gastric cancer-associated angiogenesis by antisense COX-2 transfectants. Cancer Lett. 2005;224:243-252. [PubMed] [DOI] |
| 20. | Chen XL, Su BS, Sun RQ, Zhang J, Wang YL. Relationship between expression and distribution of cyclooxygenase-2 and bcl-2 in human gastric adenocarcinoma. World J Gastroenterol. 2005;11:1228-1231. [PubMed] [DOI] |
| 21. | Yamamoto S, Suzuki H, Ueda N. Arachidonate 12-lipoxygenases. Prog Lipid Res. 1997;36:23-41. [PubMed] [DOI] |
| 22. | Pidgeon GP, Tang K, Cai YL, Piasentin E, Honn KV. Overexpression of platelet-type 12-lipoxygenase promotes tumor cell survival by enhancing alpha (v) beta (3) and alpha (v) beta (5) integrin expression. Cancer Res. 2003;63:4258-4267. [PubMed] |
| 23. | Nie D, Nemeth J, Qiao Y, Zacharek A, Li L, Hanna K, Tang K, Hillman GG, Cher ML, Grignon DJ. Increased metastatic potential in human prostate carcinoma cells by overexpression of arachidonate 12-lipoxygenase. Clin Exp Metastasis. 2003;20:657-663. [PubMed] [DOI] |
| 24. | Nie D, Hillman GG, Geddes T, Tang K, Pierson C, Grignon DJ, Honn KV. Platelet-type 12-lipoxygenase in a human prostate carcinoma stimulates angiogenesis and tumor growth. Cancer Res. 1998;58:4047-4051. [PubMed] |
| 25. | Timar J, Raso E, Dome B, Li L, Grignon D, Nie D, Honn KV, Hagmann W. Expression, subcellular localization and putative function of platelet-type 12-lipoxygenase in human prostate cancer cell lines of different metastatic potential. Int J Cancer. 2000;87:37-43. [PubMed] [DOI] |
| 26. | Lee HZ, Leung HW, Lai MY, Wu CH. Baicalein induced cell cycle arrest and apoptosis in human lung squamous carcinoma CH27 cells. Anticancer Res. 2005;25:959-964. [PubMed] |
| 27. | Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2002;1:929-935. [PubMed] |
| 28. | Tong WG, Ding XZ, Adrian TE. The mechanisms of lipoxygenase inhibitor-induced apoptosis in human breast cancer cells. Biochem Biophys Res Commun. 2002;296:942-948. [PubMed] [DOI] |
| 29. | Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Mol Cancer Ther. 2002;1:929-935. [PubMed] |
| 30. | Wong BC, Wang WP, Cho CH, Fan XM, Lin MC, Kung HF, Lam SK. 12-Lipoxygenase inhibition induced apoptosis in human gastric cancer cells. Carcinogenesis. 2001;22:1349-1354. [PubMed] [DOI] |