修回日期: 2005-04-10
接受日期: 2005-04-20
在线出版日期: 2005-06-28
目的: 我们已经报道锰型超氧化物岐化酶(MnSOD)高表达可明显抑制SGC7901胃癌细胞的增殖, 本研究拟进一步探索其中所涉及的分子机制.
方法: 利用DCFH-DA荧光染色方法检测正义、反义及空载SGC7901胃癌细胞株内的活性氧水平.透射电镜下观察肿瘤细胞形态及细胞器结构改变.RT-PCR方法检测三者缺氧诱导因子HIF-1a的表达.
结果: 正义MnSOD-7901胃癌细胞胞内ROS下降, HIF-1a表达下调.反义组电镜下细胞形态明显小于正义组, 正义组线粒体明显较反义组丰富, 但两组线粒体均呈空泡状, 缺少线粒体嵴.反义组核糖体明显较正义组丰富, 内质网也略多.
结论: MnSOD依赖的抑制胃癌细胞增殖途径与下调肿瘤的胞内ROS水平, 下调HIF-1a的表达及改变细胞器的超微结构有关.
引文著录: 陈坚, 林庚金, 程建, 唐峰, 朱虹光, 刘珊林. 锰型超氧化物岐化酶基因转染抑制SGC7901胃癌细胞增殖的机制. 世界华人消化杂志 2005; 13(12): 1386-1389
Revised: April 10, 2005
Accepted: April 20, 2005
Published online: June 28, 2005
AIM: To investigate the molecular mechanisms involved in maganese superoxide dismutase (MnSOD) mediated growth inhibition of gastric cancer cell line SGC7901.
METHODS: Sense and antisense MnSOD were stably tranfected into gastric cancer cell line SGC7901 to establish the sublines of MnSOD-7901 and MnSOD-AS7901. The levels of endogenous reactive oxygen species (ROS) in MnSOD-7901 and MnSOD-AS7901 cells were evaluated by DCFH-DA fluorescence staining assay, and then were compared with that in the mock transfectant (vector-7901). The ultrastructures of the cells were observed by transmission electron microscopy. The transcription of hypoxia inducible factor 1a (HIF-1a) in the cells was detected by reverse transcription polymerase chain reaction (RT-PCR).
RESULTS: The level of ROS was decreased, and the expression of HIF-1a was down-regulated in MnSOD-7901 cells as compared with that in vector-7901. MnSOD-AS7901 cells were much smaller in size and had few mitochondrions as compared with MnSOD-7901 cells, but vacuole-like mitochondrions and incomplete mitochondrial cristaes were observed in both kinds of cells. In addition, the endoplasmic reticula and ribosomes were also more plentiful in MnSOD-AS7901 cells.
CONCLUSION: The MnSOD-dependent growth inhibition of gastric cancer cells is associated with the reduction of endogenous ROS and down-regulation of HIF-1a expression. In addition, the organelle alteration is also involved in this process.
- Citation: Chen J, Lin GJ, Cheng J, Tang F, Zhu HG, Liu SL. Mechanism of maganese superoxide dismutase mediated growth inhibition of gastric cancer cell line SGC7901. Shijie Huaren Xiaohua Zazhi 2005; 13(12): 1386-1389
- URL: https://www.wjgnet.com/1009-3079/full/v13/i12/1386.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v13.i12.1386
活性氧(reactive oxygen species, ROS)是需氧生物机体代谢过程中产生的一类性质活泼的代谢产物, 主要包括超氧阴离子O2-, 羟基自由基-OH, 和过氧化氢(H2O2)等.线粒体内的氧化呼吸链是ROS产生的主要场所, 锰型超氧化物歧化酶(MnSOD)是线粒体内的主要酶性自由基清除剂, 能将超氧阴离子岐化为过氧化氢和氧, 对维持胞内氧化还原平衡至关重要.目前已有许多学者报道恶性肿瘤内MnSOD活性明显降低[1-3], MnSOD的表达和肿瘤的分化程度及恶性表型均密切相关[4].本课题组[5]已利用电穿孔方法将人反义和有义MnSOD cDNA真核表达载体pHbA-SOD(-)/pHbA-SOD(+)成功转染SGC7901胃癌细胞, 并用G418选择性培养液筛选出稳定表达的克隆.与空载质粒株(Vector-7901细胞)相比, 稳定转染正义MnSOD cDNA 表达载体的SGC7901胃癌细胞(下称MnSOD-7901细胞)细胞增殖受抑, 反义MnSOD-7901亚系(下称MnSOD-AS7901细胞)则增殖加速, 但机制仍有待阐明.本文拟从胞内活性氧水平、细胞器超微结构改变及缺氧诱导因子(hypoxia inducible factor, HIF)表达方面进一步探讨其分子机制.
SGC7901人反义和有义MnSOD cDNA真核表达载体pHbA-SOD(-)/pHbA-SOD(+)[6]由日本Hirose教授惠赠, 稳定转染反义和正义MnSOD基因的SGC7901人胃癌细胞株由本实验室自行筛选培育.RPMI1640培养基和Trizol为Gibcol公司产品, 小牛血清为上海市放射医学研究所监制产品; 2',7'-二氯氢化荧光素二酯(DCF-DA)为Calbiochem公司产品; 逆转录所需试剂购自Promega公司; PCR所需试剂购自生工公司; 其他试剂均为市售产品.相差倒置显微镜、Hitachi F-3000荧光检测仪、M750UVIS-A型分光光度计、Biometra热循环仪、核酸电泳系统、凝胶成像扫描仪均为Pharmacia公司产品.
1.2.1 细胞培养: 三种7901细胞以含有100 mL/L小牛血清的RPMI 1640完全培养基于37℃, 50 mL/L CO2的培养箱中培养, 培养基中补充100 kU/L青霉素和100 mg/L链霉素.
1.2.2 细胞内ROS水平测定: 细胞内ROS水平按Jakubowski et al[7]建立的方法检测.DCF-DA进入细胞后, 经酯酶作用脱去二酯生成2',7'-二氯氢化荧光素(DCFH), DCFH被超氧阴离子和过氧化氢等ROS氧化, 生成发荧光的2',7'-二氯荧光素(DCF)[7], 通过DCF水平的变化即可反映ROS水平的变化.该方法很灵敏, 检测的ROS最低极限为25 pmol.细胞用2.5 g/L胰蛋白酶消化后与5 mmol/L DCF-DA 37℃保温30 min, 总体积3 mL, 细胞浓度为5×108/L.采用Hitachi F-3000荧光检测仪, 以激发波长488 nm和发射波长525 nm检测DCF荧光强度.
1.2.3 透射电镜观察: 另取25 mL培养瓶中培养的生长良好的SGC7901正义、反义及空载细胞各一瓶, 弃培液后加入PBS 5 mL, 用细胞刷刮取细胞后移入离心管中, 1 500 r/min离心15 min, 弃上清.然后经细胞固定、脱水、浸透、固化、切片、染色过程, 最后由透射电镜观察、拍片.
1.2.4 RT-PCR检测HIF-1a表达: 按TRIzol试剂操作说明提取正义、反义转染细胞和亲本细胞的总RNA.紫外检测仪分别在260 nm和280 nm波长下检测RNA的光密度吸收值, 测定RNA的纯度, 并用甲醛变性电泳实验检测RNA的完整性.均取2 mg RNA进行逆转录, 然后各自取cDNA 5 mL 用于PCR反应.以b-actin为内参照引物见表1.反应程序为: 94℃变性 1 min, 62℃退火 1 min, 72℃ 延伸1.5 min, 30个循环, 最后72℃ 10 min结束反应.反应产物-20℃保存, 取10 mL用15 g/L 琼脂糖凝胶电泳30 min, 凝胶成像仪扫描摄影.
| Gene | Sense primer | Anti-sense primer | Length |
| HIF-1a | CCATTTTCTACTCAGGACACAG | TCTACATGCTAAATCAGAGGGT | 699 bp |
| b-actin | ACCTTCAACAACCCAGCCATGTACG | CTGATCCACATCTGCTGGAGGGTGG | 703 bp |
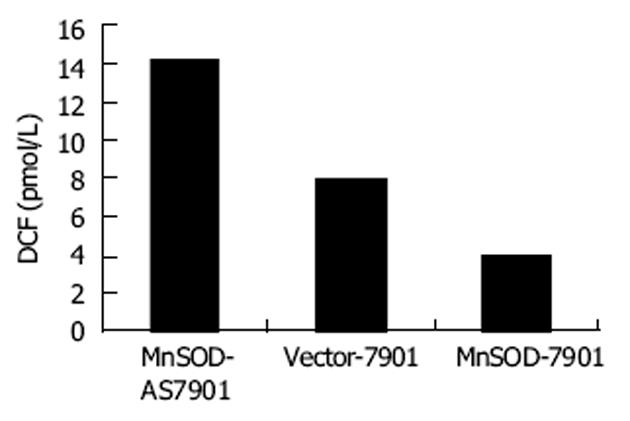
与Vector-7901细胞相比较, MnSOD-7901细胞胞内ROS水平降低到47.5%, 而MnSOD-AS7901升高到对照细胞的1.8倍(图1), 说明MnSOD高、低表达明显改变肿瘤细胞胞内氧化还原状态.
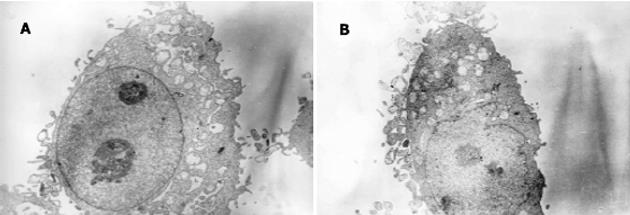
正义、反义及空载细胞均呈卵圆形, 核大, 核仁明显.细胞表面有较多微绒毛.各随机挑选3个细胞测量大小, 发现反义组细胞形态小于正义组, 二组长径分别为12.0±1.1 mm和15.3±1.3 mm(P = 0.003<0.05); 短径分别为9.3±0.9 mm和11.3±1.1 mm(P = 0.002<0.05).正义组线粒体明显较反义组丰富, 但两组线粒体均呈空泡状, 缺少线粒体嵴.反义组核糖体明显较正义组丰富, 内质网也略多(图2A-B).
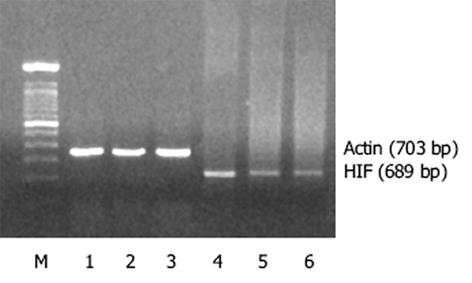
与Vector-7901的光密度比值0.16±0.01(HIF/Actin)相比, MnSOD-AS7901、MnSOD-7901的光密度比值分别为0.41±0.03(P = 0.001<0.01)、及0.11±0.01(P = 0.006<0.05).说明MnSOD高、低表达明显改变肿瘤细胞胞内HIF1的表达(图3).
MnSOD的高活性可下调多种肿瘤细胞的增殖活性, 但其中所涉及的分子机制尚未完全阐明[8-10].我们发现MnSOD-7901、Vector-7901及MnSOD-AS7901细胞均存在线粒体结构的异常, 如空泡样变及线粒体嵴缺乏等, 其中尤以MnSOD-AS7901最为明显.缺氧过程中ROS水平会上升, 提示MnSOD依赖的抑制细胞增殖的途径与ROS通路有关[11-13].我们利用DCF-DA荧光法检测发现MnSOD高、低表达可明显改变SGC7901细胞胞内ROS水平, 进一步佐证了MnSOD依赖的抑制细胞增殖途径与改变肿瘤的胞内氧化还原状态密切相关.缺氧可导致细胞肿胀及线粒体结构异常(如空泡样变及巨大线粒体).SOD可促进基础K+通道开放, 对线粒体有保护作用[14].故反义细胞中MnSOD表达缺陷为加重线粒体异常的一个重要原因.
胃癌等实体肿瘤在体内大多分化差, 生长迅速, 常常引起血供不足, 加之其内在的线粒体结构异常势必导致线粒体内氧化磷酸化功能的障碍.因此肿瘤细胞经常处于缺血缺氧的状态中[15-17].但在缺血缺氧环境下, 肿瘤组织为何仍能维持旺盛的生长.现已有许多学者报道肿瘤细胞存在着线粒体氧化磷酸化以外的能量代谢通路[18-20].鉴于MnSOD-AS7901线粒体异常更为明显, 故推测其有着线粒体氧化磷酸化以外的能量代谢通路, 即能更好地耐受正常细胞不能耐受的缺氧环境.HIF1是由缺氧诱导的、连接在EPO基因缺氧反应元件(HRE)上的一个核因子, 由a和b两个亚基组成[21-22].b亚基在细胞中呈组成性表达, a亚基在常氧下不表达或表达水平比较低, 而在缺氧时表达水平迅速升高[23-24].HIF-1a与靶基因结合后, 能促进其转录, 使机体产生一系列缺氧适应反应.其活性对维持肿瘤细胞能量代谢、新生血管形成、促进肿瘤侵袭和转移起重要作用, 并与肿瘤细胞的凋亡、增殖有关[25-28].胃癌细胞内存在HIF-1a的异常激活[29].HIF-1调控的靶基因促红细胞生成素(Epo)、血管内皮生长因子(VEGF)、内皮素-1(endothelin-1)、磷酸果糖激酶、醛缩酶、乳酸脱氢酶A、转铁因子等均与氧或能量代谢有关.Epo、VEGF等的变化使肿瘤细胞在适应缺氧微环境的同时, 增加了肿瘤的侵袭性和对放、化疗的耐受性.我们发现MnSOD高表达可下调胃癌细胞胞内HIF-1a的表达, MnSOD低表达可上调胃癌细胞内HIF-1a的表达.恶性肿瘤的失生长接触抑制势必造成局部组织及胞内的严重缺氧和代谢紊乱, 而HIF恰好能通过诱导一系列和糖酵解相关酶的表达, 增加能量供应, 调和这一矛盾.另一方面, HIF转录因子家族还通过促进VEGF、EPO的表达, 代偿性地提高了营养物质的供应, 这两方面的因素都有利于细胞的存活.故影响HIF的表达很可能是MnSOD调控肿瘤细胞增殖的又一重要机制.
此外, 电镜观察还发现MnSOD-AS7901的超微结构显示细胞体积较小, 而核糖体及内质网都较丰富, 已知核糖体是蛋白合成的场所, 内质网是细胞器装配的"工厂", 说明MnSOD-AS7901合成代谢旺盛, 这有助于从亚细胞水平解释MnSOD-AS7901细胞为何增殖较快的原因.总之, 反义MnSOD基因的转染可以改变胃癌细胞的胞内ROS水平及线粒体等细胞器的超微结构, 从而进一步触发了肿瘤细胞的缺氧状态, 后者通过活化HIF-1a诱导了一系列糖酵解酶的表达, 从提高了糖酵解水平, 代偿性地提高了营养物质的供应, 起到了促进肿瘤增殖的作用.MnSOD基因的转染正是通过抑制了以上HIF-1a的调节通路起到了抑制肿瘤增殖的效应.
编辑: 潘伯荣 审读: 张海宁
| 1. | Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12:525-535. [PubMed] |
| 2. | Toh Y, Kuninaka S, Oshiro T, Ikeda Y, Nakashima H, Baba H, Kohnoe S, Okamura T, Mori M, Sugimachi K. Overexpression of manganese superoxide dismutase mRNA may correlate with aggressiveness in gastric and colorectal adenocarcinomas. Int J Oncol. 2000;17:107-111. [PubMed] [DOI] |
| 3. | Janssen AM, Bosman CB, van Duijn W, Oostendorp-van de Ruit MM, Kubben FJ, Griffioen G, Lamers CB, van Krieken JH, van de Velde CJ, Verspaget HW. Superoxide dismutases in gastric and esophageal cancer and the prognostic impact in gastric cancer. Clin Cancer Res. 2000;6:3183-3187. [PubMed] |
| 4. | 陈 坚, 林 庚金, 钱 立平, 程 建, 施 冬云, 张 亚东, 刘 珊林. 内源性活性氧水平及锰型超氧化物歧化酶的表达与胃癌细胞分化、 增殖相关. 生物物理学报. 2003;19:133-138. |
| 6. | Hirose K, Longo DL, Oppenheim JJ, Matsushima K. Overexpression of mitochondrial manganese superoxide dismutase promotes the survival of tumor cells exposed to interleukin-1, tumor necrosis factor, selected anticancer drugs, and ionizing radiation. FASEB J. 1993;7:361-368. [PubMed] |
| 7. | Jakubowski W, Bartosz G. 2, 7-dichlorofluorescin oxidation and reactive oxygen species: what does it measure? Cell Biol Int. 2000;24:757-760. [PubMed] [DOI] |
| 8. | Kim KH, Rodriguez AM, Carrico PM, Melendez JA. Potential mechanisms for the inhibition of tumor cell growth by manganese superoxide dismutase. Antioxid Redox Signal. 2001;3:361-373. [PubMed] [DOI] |
| 9. | Li N, Oberley TD, Oberley LW, Zheng W. Overexpression of manganese superoxide dismutase in DU145 human prostate carcinoma cells has multiple effects on cell phenotype. Prostate. 1998;35:221-233. [PubMed] [DOI] |
| 10. | Li Z, Khaletskiy A, Wang J, Wong JY, Oberley LW, Li JJ. Genes regulated in human breast cancer cells overexpressing manganese-containing superoxide dismutase. Free Radic Biol Med. 2001;30:260-267. [PubMed] [DOI] |
| 11. | Melendez JA, Melathe RP, Rodriguez AM, Mazurkiewicz JE, Davies KJ. Nitric oxide enhances the manganese superoxide dismutase-dependent suppression of proliferation in HT-1080 fibrosarcoma cells. Cell Growth Differ. 1999;10:655-664. [PubMed] |
| 12. | Ranganathan AC, Nelson KK, Rodriguez AM, Kim KH, Tower GB, Rutter JL, Brinckerhoff CE, Huang TT, Epstein CJ, Jeffrey JJ. Manganese superoxide dismutase signals matrix metalloproteinase expression via H2O2-dependent ERK1/2 activation. J Biol Chem. 2001;276:14264-14270. [PubMed] |
| 13. | Rodriguez AM, Carrico PM, Mazurkiewicz JE, Melendez JA. Mitochondrial or cytosolic catalase reverse the MnSOD dependent inhibition of proliferation by enhancing respiratory chain activity, net ATP production, and decreasing the steady state level of H2O2. Free Radical Biol Med. 2000;29:801-813. [PubMed] [DOI] |
| 14. | No authors listed. Effect of KATP channels opener pinacidil on functioning of liver mitochondria of rats with different resistance to hypoxia under stress conditions. Ukr Biokhim Zh. 2004;76:56-64. [PubMed] |
| 15. | Hockel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93:266-276. [PubMed] [DOI] |
| 16. | Liu SL, Liu GZ, Cheng J, Shi DY, Chen HL, Zhang YD. Influence of PKB on ROS regulation of proliferation in human 7721 hepatoma cells. Shengwu Huaxue Yu Shengwu Wuli Xuebao. 2002;34:67-72. [PubMed] |
| 17. | Richard DE, Berra E, Pouyssegur J. Angiogenesis: how a tumor adapts to hypoxia. Biochem Biophys Res Commun. 1999;266:718-722. [PubMed] [DOI] |
| 18. | Bachtiary B, Schindl M, Potter R, Dreier B, Knocke TH, Hainfellner JA, Horvat R, Birner P. Overexpression of hypoxia-inducible factor 1alpha indicates diminished response to radiotherapy and unfavorable prognosis in patients receiving radical radiotherapy for cervical cancer. Clin Cancer Res. 2003;9:2234-2240. [PubMed] |
| 19. | Liu SL, Lin X, Shi DY, Cheng J, Wu CQ, Zhang YD. Reactive oxygen species stimulated human hepatoma cell proliferation via cross-talk between PI3-K/PKB and JNK signaling pathways. Arch Biochem Biophys. 2002;406:173-182. [PubMed] [DOI] |
| 20. | Rossignol F, Solares M, Balanza E, Coudert J, Clottes E. Expression of lactate dehydrogenase A and B genes in different tissues of rats adapted to chronic hypobaric hypoxia. J Cell Biochem. 2003;89:67-79. [PubMed] [DOI] |
| 21. | Jiang Y, Zhang W, Kondo K, Klco JM, St Martin TB, Dufault MR, Madden SL, Kaelin WG Jr, Nacht M. Gene expression profiling in a renal cell carcinoma cell line: Dissecting VHL and hypoxia-dependent pathways. Mol Cancer Res. 2003;1:453-462. [PubMed] |
| 22. | Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987-7892. [PubMed] [DOI] |
| 23. | Park JH, Kim TY, Jong HS, Kim TY, Chun YS, Park JW, Lee CT, Jung HC, Kim NK, Bang YJ. Gastric epithelial reactive oxygen species prevent normoxic degradation of hypoxia-inducible factor-1alpha in gastric cancer cells. Clin Cancer Res. 2003;9:433-440. [PubMed] |
| 24. | Michaud SE, Menard C, Guy LG, Gennaro G, Rivard A. Inhibition of hypoxia-induced angiogenesis by cigarette smoke exposure: impairment of the HIF-1alpha/VEGF pathway. FASEB J. 2003;17:1150-1152. [PubMed] [DOI] |
| 25. | Turcotte S, Desrosiers RR, Beliveau R. HIF-1alpha mRNA and protein upregulation involves Rho GTPase expression during hypoxia in renal cell carcinoma. J Cell Sci. 2003;116:2247-2260. [PubMed] [DOI] |
| 26. | Freeburg PB, Robert B, St John PL, Abrahamson DR. Podocyte expression of hypoxia-inducible factor (HIF)-1 and HIF-2 during glomerular development. J Am Soc Nephrol. 2003;14:927-938. [PubMed] [DOI] |
| 27. | Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem. 1996;271:32529-32537. [PubMed] [DOI] |