修回日期: 2004-05-09
接受日期: 2004-05-24
在线出版日期: 2004-09-15
目的: 研究大肠腺癌、腺瘤恶变、管状绒毛状腺瘤、管状腺瘤中Fas及p53的表达, 探讨Fas在大肠癌发生发展中的作用.
方法: 收集石蜡包埋的大肠腺癌组织37例, 腺瘤恶变组织26例, 管状绒毛状腺瘤30例, 管状腺瘤24例, 以6例非肿瘤大肠黏膜作为对照. 通过原位杂交, 免疫组化及TUNEL等技术, 原位观察不同病变组织大肠上皮中的Fas mRNA和p53蛋白表达及细胞凋亡的状况及相互关系.
结果: Fas mRNA 阳性细胞密度: 非肿瘤黏膜39.60±6.51, 管状腺瘤50.93±21.64, 管状绒毛状腺瘤45.91±24.15, 腺瘤非恶变区25.47±14.76, 腺瘤恶变区11.92±9.47, 腺癌5.88±5.10; Fas mRNA在腺瘤中有较高表达, 而在恶性病变中表达明显下降(P <0.001). 大肠非肿瘤黏膜、管状腺瘤、管状绒毛状腺瘤、腺瘤非恶变区、腺瘤恶变区和腺癌中p53蛋白阳性细胞密度分别为8.40±2.67, 13.19±8.95, 13.50±7.29, 12.24±7.16, 73.31±28.57和 80.99±44.54; p53蛋白在非肿瘤黏膜中表达最低, 腺瘤中略增加, 在恶性病变中明显增加(P <0.001). 上皮凋亡细胞密度: 非肿瘤黏膜15.02±11.14, 管状腺瘤46.31±18.86, 管状绒毛状腺瘤29.43±16.66, 腺瘤非恶变区68.42±19.61, 腺瘤恶变区22.01±12.07, 腺癌18.64±12.88; 上皮凋亡细胞密度在腺瘤中明显升高, 在恶性病变中较低(P <0.001). p53蛋白阳性表达细胞密度与上皮凋亡细胞密度呈负相关(r = -0.389, P <0.001), Fas mRNA阳性表达细胞密度与上皮凋亡细胞密度呈正相关(r = 0.190, P <0.05).
结论: 本文证实大肠腺癌可通过下调Fas表达, 上调p53表达, 抑制细胞凋亡, 形成"Fas抵抗".
引文著录: 孙保存, 赵秀兰, 王展宏, 刘易欣, 王欣, 古强. 大肠上皮恶性转化不同阶段中Fas, p53表达及其与凋亡的关系. 世界华人消化杂志 2004; 12(9): 2070-2073
Revised: May 9, 2004
Accepted: May 24, 2004
Published online: September 15, 2004
AIM: To explore the expression of Fas and p53 in the large intestinal adenocarcinoma, malignantly transformed adenoma, tubulo-villous adenoma, and tubular adenoma, and to assess their influence on the pathogenesis of large intestinal carcinoma.
METHODS: Using in-situ hybridization, immunohistochemistry and TUNEL techniques, we examined a number of paraffin-embedded tissues including 37 cases of large intestinal adenocarcinomas, 26 cases of malignantly transformed adenomas, 30 cases of tubulo-villous adenomas, and 24 cases of tubular adenomas for Fas mRNA, p53 protein and DNA fregment. 6 cases of non-tumor mucosa were used as control group.
RESULTS: Fas mRNA positive cell density was: 39.60±6.51 in non-tumor mucosa, 50.93 21.64 in tubular adenoma, 45.91±24.15 in tubulo-villous adenoma, 25.47±14.76 in non-malignantly transformed area of adenoma, 11.92±9.47 in malignantly transformed area of adenoma, and 5.88±5.10 in adenocarcinoma, which was significantly lower in the malignant lesions than in adenomas (P <0.001). p53 protein positive cell density was: 8.40±2.67 in non-tumor mucosa, tubular adenoma 13.19±8.95, tubulo-villous adenoma 13.50±7.29, non-malignantly transformed area of adenoma 12.24±7.16, malignantly transformed area of adenoma 73.31±28.57, adenocarcinoma 80.99±44.54, among which it was the lowest in the non-tumor mucosa, slightly increased in adenoma, and significantly increased in malignant lesions (P <0.001). Apoptotic cell density was: non-tumor mucosa 15.02±11.14, tubular adenoma 46.31±18.86, tubulo-villous adenoma 29.43±16.66, non-malignantly transformed area of adenoma 68.42±19.61, malignantly transformed area of adenoma 22.01±12.07, adenocarcinoma 18.64±12.88, which was higher in adenomas, but lower in malignant lesions (P <0.001). The positively expressed cell density of p53 protein was reversely related with that of the apoptotic epithelial cells (r = -0.389, P <0.001). The positively expressed cell density of Fas mRNA was related with that of the apoptotic epithelial cells (r = 0.190, P <0.05).
CONCLUSION: The apoptotic cell death is inhibited via downregulation of Fas expression and upregulation of p53 expression in large intestinal adenocarcinoma, and a so-called "Fas resistance" mechanism is induced.
- Citation: Sun BC, Zhao XL, Wang ZH, Liu YX, Wang X, Gu Q. Expression of Fas and p53 in different stages of large intestinal malignant transformation and their association with apoptosis. Shijie Huaren Xiaohua Zazhi 2004; 12(9): 2070-2073
- URL: https://www.wjgnet.com/1009-3079/full/v12/i9/2070.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i9.2070
肿瘤细胞可能通过表达Fas配体(FasL)攻击T细胞, 产生免疫逃逸, 国内外对该方面进行了许多研究[1-19]. 本研究采用原位杂交、免疫组化及TUNEL等技术, 观察Fas mRNA, p53 蛋白及细胞凋亡在大肠肿瘤及癌前病变中的表达, 探索大肠癌发生发展中"Fas抵抗"机制的可能作用.
天津医科大学总医院1988-01/1995-02手术切除, 石蜡包埋的大肠腺癌组织37例, 腺瘤恶变组织26例, 管状绒毛状腺瘤30例, 管状腺瘤24例, 并以同期非肿瘤大肠黏膜组织蜡块6例作为正常对照组. 4 mm厚连续切片备用, 载玻片涂布APES胶以防脱片. Fas原位杂交检测试剂盒购自武汉博士德公司. 鼠抗p53蛋白(1:500稀释, 克隆号DO-1)购自Santa Cruz Biotechnology公司, 二抗、三抗为Vector公司ABC试剂盒. 凋亡试剂盒购自德国宝灵曼公司.
常规脱蜡至水, 30 mL/L H2O2室温处理10 min, 蒸馏水洗; 滴加30 mL/L柠檬酸新鲜配制的胃蛋白酶, 37 ℃消化15 min, 0.5 mol/L PBS洗5 min 3次, 蒸馏水洗1次; 每张切片约加原位杂交液20 mL, 将原位杂交专用盖玻片盖在切片上, 37 ℃杂交过夜后洗涤, 35 ℃左右, 2×ssc洗5 min×5次;滴加封闭液, 37 ℃, 30 min, 甩去多余液体, 滴加兔抗地高辛, 37 ℃, 60 min, 0.5 mol/L PBS洗2 min×3次; 滴加生物素化羊抗兔IgG, 37 ℃, 20 min, 0.5 mol/L PBS洗2 min×3次, 滴加SABC, 37℃, 20 min, 0.5 mol/L PBS洗5 min×4次; DAB显色, 15 min, 水洗; Mayer苏木素复染细胞核, 水洗, 脱水, 透明, 封固. 免疫组化ABC法常规检测大肠上皮恶性转化不同阶段组织中p53蛋白的表达. 以TUNEL法检测大肠上皮恶性转化不同阶段细胞凋亡状况, 切片以160目镜测微网在400倍放大下(面积为0.1 024 mm2)为单位面积, 计数网格中的阳性细胞数, 每组每例计数10个网格, 取其均值作为该例病变的阳性细胞密度.
统计学处理 各组阳性细胞密度间比较及指标相关性以SPSS软件行方差分析, 组间两两比较及相关系数进行处理.
| 分组 | Fas mRNA阳性 | p53蛋白阳性 | 凋亡细胞 | |||
| n | 密度 | n | 密度 | n | 密度 | |
| 非肿瘤黏膜 | 5 | 39.60±6.51bd | 6 | 8.40±2.67fh | 6 | 15.02±11.14 |
| 管状腺瘤 | 22 | 50.93±21.64bd | 23 | 13.19±8.95fh | 24 | 46.31±18.86jl |
| 管状绒毛状腺瘤 | 29 | 45.91±24.15bd | 30 | 13.50±7.29fh | 30 | 29.43±16.66j |
| 腺瘤非恶变区 | 24 | 25.47±14.76bd | 23 | 12.24±7.16fh | 23 | 68.42±19.61jl |
| 腺瘤恶变区 | 24 | 11.92±9.47b | 23 | 73.31±28.57 | 23 | 22.01±12.07 |
| 腺癌 | 35 | 5.88±5.10 | 37 | 80.99±44.54 | 37 | 18.64±12.88 |
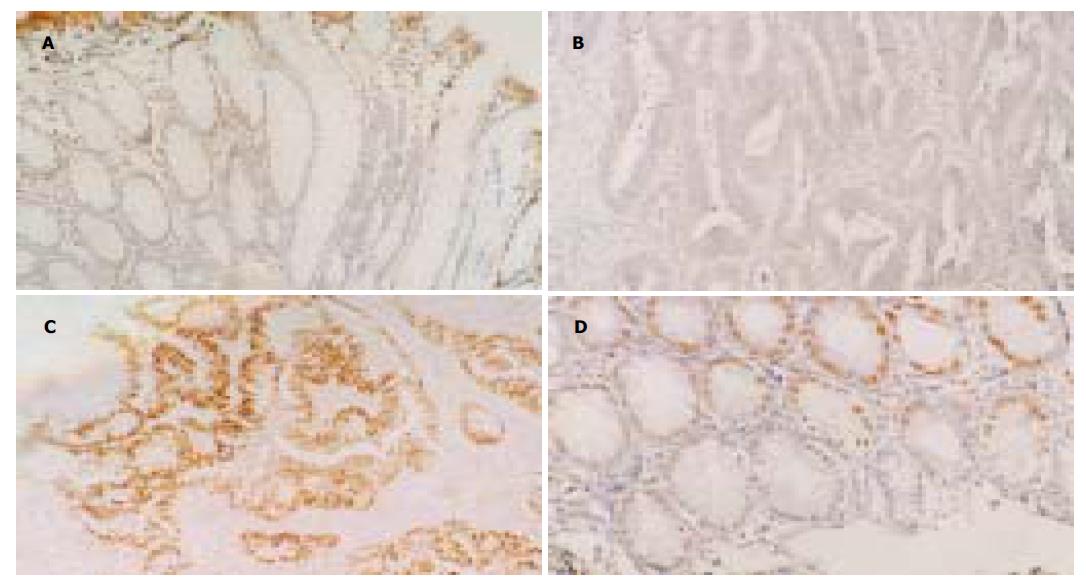
腺癌分别与非肿瘤黏膜, 管状腺瘤, 管状绒毛状腺瘤, 腺瘤非恶变区(P均<0.001)及腺瘤恶变区(P = 0.003), 腺瘤恶变区分别与非肿瘤黏膜, 管状腺瘤, 管状绒毛状腺瘤及腺瘤非恶变区(P均<0.001), 腺瘤非恶变区分别与非肿瘤黏膜(P = 0.04), 管状腺瘤, 及管状绒毛状腺瘤(P均<0.001)Fas mRNA阳性表达细胞密度之间差别有统计学意义. 余对比组间比较差别均无统计学意义. 正常非肿瘤黏膜Fas mRNA表达量中等, 良性腺瘤有轻微上升趋势, 在腺瘤非恶变区则有下降趋势, 在恶性病变中明显下降, 与腺瘤非恶变区有显著差异, 以腺癌中最低, 其与腺瘤恶变区差异亦有统计学意义(图 1A, B).
大肠腺癌分别与非肿瘤黏膜, 管状腺瘤, 管状绒毛状腺瘤, 及腺瘤非恶变区(P均<0.001), 腺瘤恶变区分别与非肿瘤黏膜, 管状腺瘤, 管状绒毛状腺瘤, 及腺瘤非恶变区(P均<0.001)p53蛋白阳性表达细胞密度之间差别有统计学意义(表1). 余对比组间比较差别均无统计学意义. p53 蛋白阳性细胞密度以非肿瘤黏膜中最低, 良性腺瘤中稍有增加, 在恶性病变中明显增高, 以腺癌中阳性表达细胞密度最高(图1C), 但与腺瘤恶变区差异无统计学意义(P = 0.784). 大肠不同病变中, p53蛋白阳性细胞有逐渐升高趋势, 而Fas mRNA阳性表达细胞密度呈略升高后明显下降. p53呈高水平表达, 而Fas mRNA呈低表达, 二者间呈负相关(r = -0.573, P <0.001).
2.3 大肠不同病变中上皮凋亡细胞与p53蛋白阳性细胞及Fas mRNA阳性细胞密度的关系 大肠腺癌分别与管状腺瘤(P <0.001), 管状绒毛状腺瘤(P = 0.002), 及腺瘤非恶变区(P <0.001), 腺瘤非恶变区分别与非肿瘤黏膜, 管状腺瘤及管状绒毛状腺瘤(P均<0.001), 腺瘤恶变区分别与管状腺瘤, 及腺瘤非恶变区(P均<0.001), 管状绒毛状腺瘤分别与管状腺瘤(P <0.003)及非肿瘤黏膜(P = 0.02), 管状腺瘤与非肿瘤黏膜(P <0.001)上皮凋亡细胞密度之间差别有统计学意义(表1). 余对比组之间比较差别均无统计学意义. 良性腺瘤中上皮凋亡细胞密度较高, 腺瘤非恶变区达高峰, 增加显著, 以非肿瘤黏膜最低, 在腺瘤-腺瘤恶变-腺癌过程中有逐渐下降趋势. 非肿瘤黏膜中p53蛋白阳性表达细胞密度与上皮细胞密度均呈最低水平, 良性腺瘤中p53蛋白阳性表达细胞密度稍增加, 但仍呈较低水平, 而上皮凋亡细胞密度则显著增加, 恶性病变中p53蛋白阳性表达细胞密度与上皮凋亡细胞密度对比明显, p53蛋白呈高水平表达而上皮凋亡则呈低水平. 二者间呈负相关(r = -0.389, P <0.001). Fas mRNA在非肿瘤黏膜中呈中等水平表达, 上皮凋亡细胞密度则最低. Fas mRNA阳性细胞密度在良性腺瘤中略有增加, 而上皮凋亡细胞密度显著增高(图1D), 在腺瘤非恶变区Fas mRNA阳性表达细胞密度与上皮凋亡细胞密度似不成比例, 但在恶性病变中, 随着Fas mRNA阳性表达细胞密度显著下降, 上皮凋亡细胞密度亦有明显下降趋势, 二者呈正相关(r = 0.190, P <0.05).
肿瘤细胞抵抗Fas介导的凋亡是Fas反击的重要前提条件[20-21]. 否则, 同一癌细胞表达Fas及FasL会发生自分泌性"自杀", 相邻癌细胞分别表达FasL和Fas则会发生旁分泌性"互相残杀". Fas介导凋亡的抑制可能涉及细胞内信号转导的多个层面的异常[22-23]. 多种癌基因和抑癌基因的突变可影响Fas的表达及其死亡信号的转导功能, 亦可造成Fas抵抗. 有证据表明c-myc突变性上调能促成Fas抵抗, 从而保护肿瘤避免凋亡, 因此有利于增生.
我们探讨了大肠不同病变中Fas表达. 在大肠不同病变中, Fas mRNA表达有轻度上升后明显下调的趋势, 以恶性病变中显著减低, 提示Fas抵抗确存在于大肠恶性病变中, 而且主要是通过Fas下调及缺失来实现的, 但鉴于Fas可存在显性负性突变, 因此可以认为阳性表达者仍有一部分为无功能性Fas, 不足以传递死亡信号. 我们还观察了p53 蛋白在大肠上皮恶性转化进程中的表达状况[24-26]. 鉴于免疫组化法检测到的p53 均为突变型p53, 结合野生型p53 能转录上调Fas表达, 诱导凋亡的概念[27], 我们初步探讨了p53蛋白在Fas抵抗中的意义. p53突变能损害Fas死亡信号的传递, 从而妨碍凋亡, 造成Fas抵抗. 本组恶性病变中p53 蛋白表达显著增加, 与其相对的是Fas mRNA显著下调及上皮凋亡明显减少, 基本符合上述观点. 腺瘤中Fas轻微上调, 可能与p53蛋白活性有关, 但必然还会与其他基因的表达有关[28-30]. 大肠癌发生中Fas抵抗的形成可能与以上提及的多种机制有关.
| 1. | Choi C, Benveniste EN. Fas ligand/Fas system in the brain: regulator of immune and apoptotic responses. Brain Res Brain Res Rev. 2004;44:65-81. [PubMed] [DOI] |
| 2. | Abrahams VM, Straszewski SL, Kamsteeg M, Hanczaruk B, Schwartz PE, Rutherford TJ, Mor G. Epithelial ovarian cancer cells secrete functional Fas ligand. Cancer Res. 2003;63:5573-5581. [PubMed] |
| 3. | Sträter J, Möller P. CD95 (Fas/APO-1)/CD95L in the gastrointestinal tract: fictions and facts. Virchows Arch. 2003;442:218-225. [PubMed] |
| 4. | Pernick NL, Sarkar FH, Tabaczka P, Kotcher G, Frank J, Adsay NV. Fas and Fas ligand expression in pancreatic adenocarcinoma. Pancreas. 2002;25:e36-e41. [PubMed] [DOI] |
| 5. | Lee TB, Min YD, Lim SC, Kim KJ, Jeon HJ, Choi SM, Choi CH. Fas (Apo-1/CD95) and Fas ligand interaction between gastric cancer cells and immune cells. J Gastroenterol Hepatol. 2002;17:32-38. [PubMed] [DOI] |
| 6. | Zietz C, Rumpler U, Stürzl M, Löhrs U. Inverse relation of Fas-ligand and tumor-infiltrating lymphocytes in angiosarcoma: indications of apoptotic tumor counterattack. Am J Pathol. 2001;159:963-970. [PubMed] [DOI] |
| 7. | Ito Y, Noguchi S, Takeda T, Matsuura N. Fas ligand expression in BRCA1-associated hereditary breast carcinoma clearly differs from that in sporadic breast carcinoma. Breast Cancer Res Treat. 2001;66:95-100. [PubMed] [DOI] |
| 8. | Radfar S, Martin H, Tilkin-Mariame AF. [Tumor escape mechanism involving Fas and Fas-L molecules in human colorectal tumors]. Gastroenterol Clin Biol. 2000;24:1191-1196. [PubMed] |
| 9. | O'Connell J, Bennett MW, Nally K, Houston A, O'Sullivan GC, Shanahan F. Altered mechanisms of apoptosis in colon cancer: Fas resistance and counterattack in the tumor-immune conflict. Ann N Y Acad Sci. 2000;910:178-192; discussion 193-195. [PubMed] [DOI] |
| 11. | 邓 清, 袁 宏银, 熊 斌, 张 明生. 细胞凋亡、增生和Fas/FasL系统在大肠癌中的作用. 武汉大学学报(医学版). 2003;24:149-151. |
| 17. | Webb SD, Sherratt JA, Fish RG. Cells behaving badly: a theoretical model for the Fas/FasL system in tumour immunology. Math Biosci. 2002;179:113-129. [PubMed] [DOI] |
| 18. | Andreola G, Rivoltini L, Castelli C, Huber V, Perego P, Deho P, Squarcina P, Accornero P, Lozupone F, Lugini L. Induction of lymphocyte apoptosis by tumor cell secretion of FasL-bearing microvesicles. J Exp Med. 2002;195:1303-1316. [PubMed] [DOI] |
| 19. | Li JH, Rosen D, Sondel P, Berke G. Immune privilege and FasL: two ways to inactivate effector cytotoxic T lymphocytes by FasL-expressing cells. Immunology. 2002;105:267-277. [PubMed] [DOI] |
| 20. | Koyama S, Koike N, Adachi S. Fas receptor counterattack against tumor-infiltrating lymphocytes in vivo as a mechanism of immune escape in gastric carcinoma. J Cancer Res Clin Oncol. 2001;127:20-26. [PubMed] [DOI] |
| 21. | Michael-Robinson JM, Pandeya N, Cummings MC, Walsh MD, Young JP, Leggett BA, Purdie DM, Jass JR, Radford-Smith GL. Fas ligand and tumour counter-attack in colorectal cancer stratified according to microsatellite instability status. J Pathol. 2003;201:46-54. [PubMed] [DOI] |
| 22. | Houston A, Waldron-Lynch FD, Bennett MW, Roche D, O'Sullivan GC, Shanahan F, O'Connell J. Fas ligand expressed in colon cancer is not associated with increased apoptosis of tumor cells in vivo. Int J Cancer. 2003;107:209-214. [PubMed] [DOI] |
| 23. | Song E, Chen J, Ouyang N, Su F, Wang M, Heemann U. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer. 2001;85:1047-1054. [PubMed] [DOI] |
| 24. | Oka S, Tanaka S, Hiyama T, Kitadai Y, Yoshihara M, Shimamoto F, Haruma K, Chayama K. Human telomerase reverse transcriptase, p53 and Ki-67 expression and apoptosis in colorectal serrated adenoma. Scand J Gastroenterol. 2002;37:1194-1200. [PubMed] [DOI] |