修回日期: 2004-03-01
接受日期: 2004-03-04
在线出版日期: 2004-07-15
目的: 研究携带mIL-12基因的增生型腺病毒对小鼠移植胃癌的治疗效果及其作用机制, 探讨胃癌治疗的新思路及途径.
方法: 以E1B55KDa缺失的增生型腺病毒ONYX-015、带有小鼠IL-12(mIL-12)基因的增生型腺病毒CNHK200-mIL-12以及非增生型腺病毒Ad-mIL-12治疗SGC-7901 BALB/C裸鼠及MFC 615小鼠胃癌移植模型, 从肿瘤大小及动物生存期等方面评价治疗效果; 并检测各治疗组的CD4+和CD8+淋巴细胞水平及NK和CTL细胞的杀伤活性, 探讨其免疫学作用机制.
结果: 实验证实增生型腺病毒(ONYX-015, CNHK200-mIL-12)可以有效地杀伤裸鼠体内的SGC-7901胃癌细胞, 但是不足以使其完全消退, 仅起到延缓生长的作用. 荷瘤615小鼠在注射Ad-mIL-12后部分(3/10)肿瘤消退, 余者(7/10)生长明显减慢, 肿瘤无破溃, 生存期明显延长; CNHK200-mIL-12治疗组部分肿瘤(4/10)停止生长, 1 wk后开始缩小, 10-14 d完全消退, 其余小鼠肿瘤(6/10)生长明显减慢, 肿瘤无破溃, 生存期明显延长. 免疫学检测发现各腺病毒治疗组的CD4+和CD8+细胞水平以及NK、CTL细胞的杀伤活性均高于对照组, 尤以携带IL-12基因者明显(P<0.001).
结论: 以增生型腺病毒为载体携带IL-12基因可大量杀伤肿瘤细胞并与增生型腺病毒协同发挥抗肿瘤活性.
引文著录: 薛绪潮, 方国恩, 王星华, 刘芳, 毕建威, 曹贵松, 钱其军. 携带mIL-12基因的增生型腺病毒对小鼠移植胃癌的治疗作用. 世界华人消化杂志 2004; 12(7): 1522-1526
Revised: March 1, 2004
Accepted: March 4, 2004
Published online: July 15, 2004
AIM: To study the treatment of the replication-competent adenovirus-mediated transfer of interleukin-12 gene in the mouse transplanted with gastric cancer in vivo, and to explore the new therapeutic approach of gastric cancer.
METHODS: The efficacy of CNHK200-mIL-12, ONYX-015, Ad-mIL-12 was tested in nude mice and 615 mice with subcutaneous human SGC-7901 and MFC 615 mice carcinomas. The immunological mechanism in xenografts gastric tumor of mice was further analyzed.
RESULTS: The replication-competent adenovirus-mediated transfer of interleukin-12 gene slowed down the tumor progress. This might be caused by the proliferation of the replication-competent adenovirus. Ad-mIL-12 had little effect on the transplanted tumor in nude mice. While 615 mice bearing MFC gastric cancer presented the best anticancer effects. Administration of Ad-mIL-12 was showed to delay the growth of transplanted MFC tumor markedly(P < 0.001), and mediate complete regression of 3/10 established tumor. In the setting of CNHK200-mIL-12, the regression ratio was 4/10. Significantly prolonged survival was observed in both Ad-mIL-12 and CNHK200-mIL-12 experimental groups of MFC gastric tumor bearing mice. The analysis of the immunological mechanism in xenografts gastric tumor of mice 615 showed that the expression of IL-12 by CNHK200-mIL-12 and Ad-mIL-12 could greatly stimulate the immune response against the transplanted gastric tumor. It was found that these therapies could enhance the natural killer cell activity and specific cytotoxic T cell activity. The CD4+ and CD8+ T cell infiltrated into the tumor more significantly than that in the group of ONYX-015 and control (P < 0.001).
CONCLUSION: The replication-competent adenovirus can specifically replicate in tumor cells and kill the tumor cells. IL-12 is an effective cytokine stimulating anticancer immune responses. When combined, it is complementary resulting in a significantly improved treatment outcome.
- Citation: Xue XC, Fang GE, Wang XH, Liu F, Bi JW, Cao GS, Qian QJ. Therapeutic effect of replication-competent adenovirus-mediated transfer of interleukin-12 gene on the mouse transplanted gastric cancer in vivo. Shijie Huaren Xiaohua Zazhi 2004; 12(7): 1522-1526
- URL: https://www.wjgnet.com/1009-3079/full/v12/i7/1522.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i7.1522
胃癌是一种高发、高恶性度、预后不良的恶性肿瘤, 目前主要以手术切除, 辅以放疗、化疗及免疫治疗等进行综合治疗. 由于胃癌起病隐匿, 早期诊断困难, 常延误治疗, 发现时往往已是中晚期, 而且手术完全切除率低, 复发率高, 术后放化疗的效果也不理想, 尤其对于晚期或有远处转移的患者, 尚缺乏有效的治疗手段, 亟需探索治疗胃癌的新方法[1-7]. 随着人们对于肿瘤组织认识的不断深入以及细胞生物学、分子生物学的迅猛发展, 肿瘤的基因治疗成为当前研究的热点. IL-12具有强大的抗肿瘤活性, 研究表明经IL-12可使肿瘤明显消退, 转移率和复发率降低, 还可拮抗肿瘤新生血管[8-15]. 我们利用携带小鼠IL-12(mIL-12)基因的增生型腺病毒对小鼠移植胃癌进行治疗观察, 并进一步探讨其作用机制.
ONYX-015(美国加州大学Berk AJ教授惠赠)及CNHK200-mIL-12, Ad-mIL-12(本实验室构建[16]), 经扩增、纯化, 用Ad-buffer调整病毒滴度为1×1013pfu/L. 其中ONYX-015, CNHK200-mIL-12为增生型腺病毒, Ad-mIL-12为复制缺陷型腺病毒. 人胃癌细胞株SGC-7901及615小鼠胃癌细胞株MFC购自中国科学院上海生命科学研究院细胞资源中心. 大鼠抗小鼠CD4, CD8单抗、FITC标记的兔抗大鼠二抗购自美国Pharmingen公司. 10 g/L 锇酸溶液、25 g/L戊二醛溶液、40 g/L甲醛、醋酸铀等购自上海化学试剂有限公司. 615小鼠, 4-6周龄, 50只, ♀, 体质量18-23 g; Balb/c裸鼠, 4-6周龄, 50只, ♂, 体质量18-20 g, 均购自上海毕凯公司. 透射电镜、游标卡尺、二氧化碳孵箱、离心机、恒温水浴箱等.
SGC-7901用100 mL/L小牛血清的DMEM培养, MFC用100 mL/L小牛血清的RPMI 1640培养. 体外培养并收集胃癌细胞株SGC-7901细胞, 调整细胞悬液浓度5×1010/L, 于Balb/c裸鼠右季肋部皮下接种(0.1 mL/只), 7 d后成瘤, 观察计算肿瘤体积, 计算公式a×b2×0.5(a为肿瘤最大径, b为前者垂直径). 收集体外培育小鼠细胞株MFC细胞, 调整细胞悬液浓度至1×1011/L, 于615鼠右季肋部皮下接种(0.1 mL/只), 5-7 d后成瘤, 同样方法计算肿瘤体积. 615小鼠共60只, 右季肋部皮下注射1×107 MFC细胞, 5 d后肿瘤可见形成, 最大径约5 mm, 随机分为Ad-buffer(空白对照)、ONYX-015及Ad-mIL-12、CNHK200-mIL-12四组, 每组15只; 分别于瘤内注射Ad-buffer, ONYX-015, CNHK200-mIL-12及Ad-mIL-12病毒悬液100 L, 每天注射1次, 共5次(病毒总量为5×109pfu); 每周测量肿瘤大小1次, 取最大径(a)及其垂直径(b), 计算肿瘤体积; 治疗1 wk后, 每组取5只动物, 折颈处死, 切取肿瘤组织及脾脏进行免疫功能指标检测. 每组余10只荷瘤鼠观察生存情况. Balb/c裸鼠治疗组40只, 皮下接种SGC-7901, 肿瘤生长至5-6 mm后, 随机分为4组. 治疗方法同上. 观察荷瘤鼠治疗后生存情况.
1.2.1 NK和CTL细胞活性检测: 采用4 h 51Cr释放法. 经不同治疗的荷瘤小鼠5只, 杀死后取脾脏, 制备脾细胞悬液. 将3×106的脾细胞与3×105的YAC-1细胞置于37 ℃培养2 h, 检测NK细胞活性. 将肿瘤细胞与脾细胞按1: 20的比例混合, 加入重组的人IL-2 200 ku/L, 置50 mL/L CO2, 37 ℃中孵育6 d, 分离淋巴细胞, Na51CrO4标记的MFC细胞作为靶细胞, 效靶比1: 50及1: 25, 检测CTL活性. 杀伤率=(实验组cpm-自然释放组cpm)/(最大释放组cpm-自然释放组cpm)×100%.
1.2.2 CD4+和CD8+T淋巴细胞流式细胞仪检测: 取615小鼠肿瘤组织, 剪成小块, 过不锈钢网, 制备成单细胞悬液. PBS洗2遍, 调整细胞浓度至108/L, 加入大鼠抗小鼠CD4和CD8单抗, 4 ℃孵育30 min, PBS洗2遍, 再加入FITC标记的兔抗大鼠二抗, 4 ℃孵育30 min, PBS洗2遍, 最后将细胞悬浮于0.2 mL PBS中, 进行流式细胞学分析, 检测CD4+和CD8+淋巴细胞.
统计学处理 以一个重复测量的双因素方差分析及Kaplam-Meier生存分析.
胃癌细胞株SGC-7901接种Balb/c裸鼠, 7-9 d成瘤. MFC细胞株接种615小鼠5 d即成瘤, 且肿瘤生长较快.
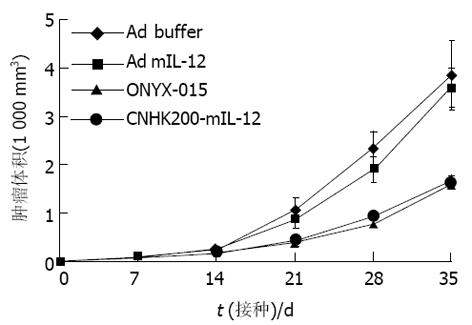
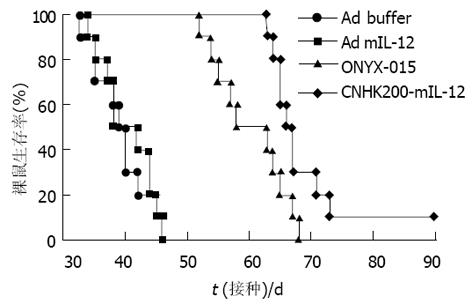
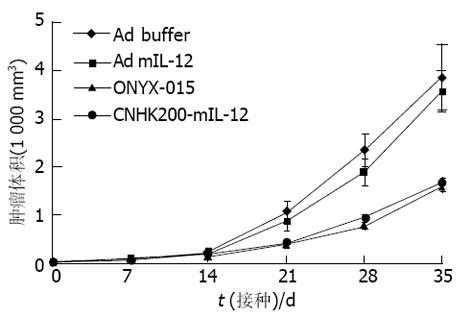
对照组肿瘤生长迅速, ONYX-015, CNHK200-mIL-12治疗组, 在治疗期内肿瘤生长明显减慢, 有些肿瘤停止生长, 少数肿瘤缩小, 未见肿瘤消退现象, 与空白对照组及Ad- mIL-12相比, 差异显著(P<0.05, 图1). 对照组与Ad- mIL-12相比肿瘤生长速度相近, 无显著差异. 各试验组的生存期相比较, Ad- mIL-12治疗组生存期未见延长, 二者相比差异不显著(t = 0.009, P>0.05); ONYX-015, CNHK200-mIL-12组间差异不显著(t = 5.73, P>0.05, 图2). ONYX-015, CNHK200-mIL-12组与对照组及Ad- mIL-12治疗组生存期相比差异显著.
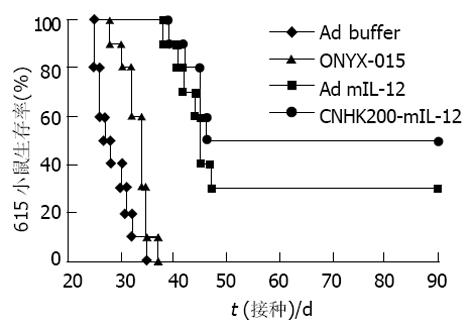
Ad buffer对照组, 肿瘤快速生长, 生存期短, 分别于接种肿瘤后25-34 d内死亡. 而ONYX-015治疗组小鼠肿瘤生长减慢, 肿瘤破溃延迟, 生存期比Ad-buffer组稍延长. CNHK200-mIL-12治疗组实行治疗后4例肿瘤停止生长, 1 wk后开始缩小, 10-14 d完全消退. 6例肿瘤生长明显减慢, 肿瘤无破溃, 生存期明显延长(图3, 4). 但CNHK200-mIL-12治疗组615小鼠在治疗后及肿瘤消退后一段时间内均表现一些毒副作用. 如精神萎靡不振, 毛发失去光泽, 凌乱, 进食减少, 腹泻, 体重下降. 停止治疗2 wk左右后开始逐渐恢复正常, 肿瘤消退后, 小鼠可长期存活, 最长观察6 mo仍健康生存. 治疗后6 wk, 对经治疗肿瘤消失的615小鼠再次于对侧皮下注射1×107MFC细胞悬液, 观察2 wk未见成瘤. 证明经CNHK200-mIL-12治疗后615小鼠获得对MFC移植瘤免疫力. Ad-mIL-12治疗组7/10肿瘤生长明显减慢, 肿瘤无破溃, 生存期明显延长, 3例治疗后肿瘤消退, 与对照组相比具有显著差异.
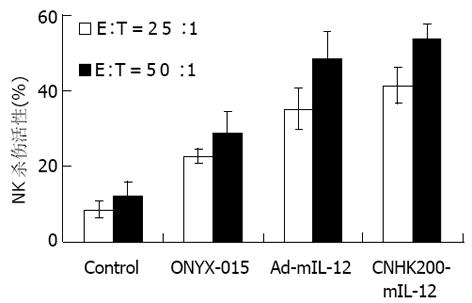
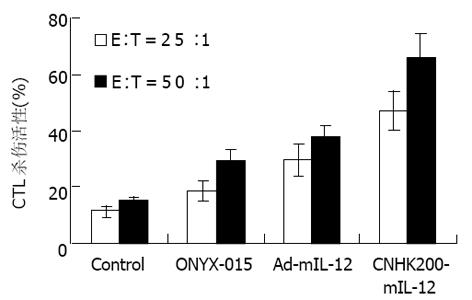
单用ONYX-015可使荷瘤615小鼠NK和CTL活性比未治疗组略升高, 与对照组相比无显著差异; Ad-mIL-12、CNHK200-mIL-12治疗后对荷瘤615小鼠NK和CTL活性较对照及ONYX-015升高更明显, 相比差异显著(P<0.01, 图5, 6).
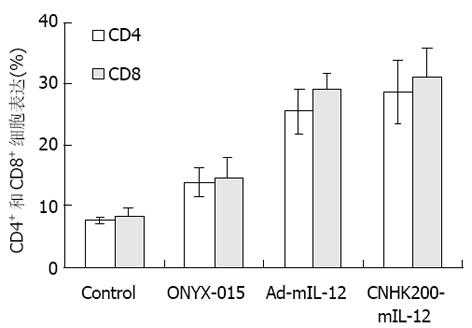
MFC细胞株为615小鼠自身胃癌肿瘤体外建株得来, 肿瘤恶性程度高, 生长迅速. 对裸鼠成瘤进行ONYX-015, CNHK200-mIL-12, Ad-mIL-12治疗后, 流式细胞仪检测显示, CNHK200-mIL-12, Ad-mIL-12治疗组CD4+, CD8+细胞阳性率增高, ONYX-015次之, 对照组阳性率低(图7).
传统基因治疗的非增生型病毒载体存在着转载效率低下、靶向性差等一些缺点[17]. 增生型病毒利用肿瘤细胞与正常细胞之间一些结构或代谢途径上的差异, 使其可以特异性的靶向肿瘤细胞内复制. E1B-55KDa蛋白缺失的腺病毒感染p53功能正常的细胞后, 其E1A激活宿主细胞的p53途径, 该细胞将发生凋亡, 病毒不能有效复制, 而在p53功能异常的细胞(50%左右肿瘤细胞的p53功能异常)内则可以复制增生并最终将其裂解[18]. ONYX-015联合化疗治疗头颈部肿瘤已进入临床试验阶段, 并显示出良好的抗肿瘤能力和安全性[19-23]. 但是由于肿瘤细胞的高度异质性、人体内普遍存在着腺病毒中和抗体以及肿瘤组织内部常有出血、坏死、纤维化等一些原因, ONYX-015单剂应用的临床有效率仅为15%[24-25]. 因此, 联合其他一些治疗手段对于病毒治疗来说是非常重要和必要的. 除联合化疗外, 以增生型病毒作为转载抗癌基因的平台进行抗肿瘤治疗(我们称之为病毒-基因治疗), 也是一种非常有前景的治疗方案[26-31]. 我们利用抗癌机制与ONYX-015相同的增生型腺病毒载体CNHK200携带小鼠IL-12基因治疗胃癌, 充分利用增生型病毒体积小、弥散能力强以及可以特异地在肿瘤细胞内复制等一些特点, 将抗癌基因携带至肿瘤病灶局部大量表达, 协同发挥抗肿瘤功能.
人腺病毒感染动物细胞(正常或肿瘤)后缺乏复制增生能力, 因此目前尚无理想的动物模型用于增生型腺病毒的体内实验[32-33]. 为解决这一问题, 我们在本研究中选用两种不同的动物模型, 其中615小鼠具有正常的免疫功能, 可观察IL-12在体内的作用情况, 但不能用于观察增生型腺病毒对肿瘤细胞杀伤情况; Balb/c裸鼠T细胞免疫缺陷, 可以接种人肿瘤细胞, 因此可以观察病毒的复制增生对肿瘤的治疗作用, 但不适于观察目的基因的免疫效应对肿瘤的作用. 将二者结合起来即可较为全面地评估携带目的基因的增生型腺病毒对肿瘤的治疗作用. 通过实验我们观察到, 荷瘤615小鼠在注射腺病毒后肿瘤生长减缓、生存期延长. 进一步的免疫学检测发现各治疗组的CD4+和CD8+细胞水平以及NK、CTL细胞的杀伤活性均高于对照组, 尤以携带IL-12基因者明显. 这表明有免疫功能的动物感染腺病毒后, 会产生针对腺病毒的免疫反应, 并清除被感染的细胞, IL-12则可以明显地放大这种免疫反应的效果. 在我们的实验中, 部分615小鼠移植瘤经携带mIL-12基因的腺病毒治疗后消退, 于其他部位再次注射MFC细胞不能成瘤, 这充分显示出IL-12强大的抗肿瘤活性及其抗肿瘤复发的功能. 另外, 通过我们的实验还证明了利用增生型腺病毒特异性靶向肿瘤细胞复制的特点可以有效地杀灭裸鼠体内的SGC-7901胃癌细胞. 同时, 我们发现单以增生型腺病毒不足以使其完全消退, 仅起到延缓生长的作用. 这可能是由于肿瘤组织内部存在着大量的纤维间隔以及坏死组织限制了腺病毒的感染能力造成的. 我们认为以增生型腺病毒为载体携带IL-12基因治疗肿瘤, 一方面可以利用增生型腺病毒特异地靶向肿瘤细胞复制、增生并最终将其裂解的特点, 大量杀伤肿瘤细胞; 另一方面可使IL-12基因在肿瘤病灶形成的局部高度表达发挥抗肿瘤活性, 而不增加其在系统中的水平(这一点对于IL-12这种剂量依赖型的细胞因子是非常重要的[34-36]), 减少副作用[37]. 当然, 我们在实验的过程中也注意到CNHK200-mIL-12治疗组615小鼠在治疗后及肿瘤消退后一段时间内均表现精神萎靡不振、毛发失去光泽、进食减少、腹泻、体重下降等一些毒副作用. 这说明增生型腺病毒载体的靶向性还有待于进一步的提高, 也为我们下一步的研究工作指明了方向.
编辑: N/A
| 1. | Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol. 2001;7:357-362. [PubMed] [DOI] |
| 2. | Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY, Fang J. Effect of jianpiyiwei capsule on gastric precancerous lesions in rats. World J Gastroenterol. 2002;8:608-612. [PubMed] [DOI] |
| 3. | Yu Y, Zhang YC, Zhang WZ, Shen LS, Hertzog P, Wilson TJ, Xu DK. Ets1 as a marker of malignant potential in gastric carcinoma. World J Gastroenterol. 2003;9:2154-2159. [PubMed] [DOI] |
| 4. | Zhang J, Su XQ, Wu XJ, Liu YH, Wang H, Zong XN, Wang Y, Ji JF. Effect of body mass index on adenocarcinoma of gastric cardia. World J Gastroenterol. 2003;9:2658-2661. [PubMed] [DOI] |
| 5. | Guo HQ, Guan P, Shi HL, Zhang X, Zhou BS, Yuan Y. Prospective cohort study of comprehensive prevention to gastric cancer. World J Gastroenterol. 2003;9:432-436. [DOI] |
| 6. | Gulmann C, Hegarty H, Grace A, Leader M, Patchett S, Kay E. Differences in proximal (cardia) versus distal (antral) gastric carcinogenesis via the retinoblastoma pathway. World J Gastroenterol. 2004;10:17-21. [PubMed] |
| 7. | Ding YB, Chen GY, Xia JG, Zang XW, Yang HY, Yang L, Liu YX. Correlation of tumor-positive ratio and number of perigastric lymph nodes with prognosis of patients with surgically-removed gastric carcinoma. World J Gastroenterol. 2004;10:182-185. [PubMed] |
| 8. | Su W, Ito T, Oyama T, Kitagawa T, Yamori T, Fujiwara H, Matsuda H. The direct effect of IL-12 on tumor cells: IL-12 acts directly on tumor cells to activate NF-kappaB and enhance IFN-gamma-mediated STAT1 phosphorylation. Biochem Biophys Res Commun. 2001;280:503-512. [PubMed] [DOI] |
| 9. | Zhang R, DeGroot LJ. Genetic immunotherapy of established tumours with adenoviral vectors transducing murine interleukin-12 (mIL12) subunits in a rat medullary thyroid carcinoma model. Clin Endocrinol (Oxf). 2000;52:687-694. [PubMed] [DOI] |
| 10. | Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V, Sangro B, Melero I, Qian C, Prieto J. Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12. Hepatology. 2001;33:52-61. [PubMed] [DOI] |
| 12. | 魏 道严, 戴 冰冰, 王 中和, 陈 诗书. 腺病毒载体介导mIL-12基因治疗联合放射治疗小鼠肝癌的研究. 中华放射医学与防护杂志. 2001;21:279-281. |
| 13. | Yamazaki M, Zhang R, Straus FH, Messina M, Robinson BG, Hashizume K, DeGroot LJ. Effective gene therapy for medullary thyroid carcinoma using recombinant adenovirus inducing tumor-specific expression of interleukin-12. Gene Ther. 2002;9:64-74. [PubMed] [DOI] |
| 14. | Nasu Y, Bangma CH, Hull GW, Lee HM, Hu J, Wang J, McCurdy MA, Shimura S, Yang G, Timme TL. Adenovirus-mediated interleukin-12 gene therapy for prostate cancer: suppression of orthotopic tumor growth and pre-established lung metastases in an orthotopic model. Gene Ther. 1999;6:338-349. [PubMed] [DOI] |
| 15. | Duda DG, Sunamura M, Lozonschi L, Kodama T, Egawa S, Matsumoto G, Shimamura H, Shibuya K, Takeda K, Matsuno S. Direct in vitro evidence and in vivo analysis of the antiangiogenesis effects of interleukin 12. Cancer Res. 2000;60:1111-1116. [PubMed] |
| 16. | 薛 绪潮, 方 国恩, 张 琪, 薛 惠斌, 毕 建威, 曹 贵松, 钱 其军. 增生型腺病毒载体介导mIL-12基因对胃癌细胞的杀伤作用(英文). 第二军医大学学报. 2004;25:75-79. |
| 17. | Galanis E, Vile R, Russell SJ. Delivery systems intended for in vivo gene therapy of cancer: targeting and replication competent viral vectors. Crit Rev Oncol Hematol. 2001;38:177-192. [PubMed] [DOI] |
| 18. | Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, Ng L, Nye JA, Sampson-Johannes A, Fattaey A. An adenovirus mutant that replicates selectively in p53-deficient human tumor cells. Science. 1996;274:373-376. [PubMed] [DOI] |
| 19. | Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, Robertson AG, Park O, Gulley ML, Heise C. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798-806. [PubMed] |
| 20. | Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, Kuhn J, McCarty T, Landers S, Blackburn A. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289-298. [PubMed] [DOI] |
| 21. | Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, Gore M, Ironside J, MacDougall RH, Heise C. a controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879-885. [PubMed] [DOI] |
| 22. | Kirn D. Oncolytic virotherapy for cancer with the adenovirus dl1520 (Onyx-015): results of phase I and II trials. Expert Opin Biol Ther. 2001;1:525-538. [PubMed] [DOI] |
| 23. | Makower D, Rozenblit A, Kaufman H, Edelman M, Lane ME, Zwiebel J, Haynes H, Wadler S. Phase II clinical trial of intralesional administration of the oncolytic adenovirus ONYX-015 in patients with hepatobiliary tumors with correlative p53 studies. Clin Cancer Res. 2003;9:693-702. [PubMed] |
| 24. | Heise CC, Williams A, Olesch J, Kirn DH. Efficacy of a replication-competent adenovirus (ONYX-015) following intratumoral injection: intratumoral spread and distribution effects. Cancer Gene Ther. 1999;6:499-504. [PubMed] [DOI] |
| 25. | Nemunaitis J, Ganly I, Khuri F, Arseneau J, Kuhn J, McCarty T, Landers S, Maples P, Romel L, Randlev B. Selective replication and oncolysis in p53 mutant tumors with ONYX-015, an E1B-55kD gene-deleted adenovirus, in patients with advanced head and neck cancer: a phase II trial. Cancer Res. 2000;60:6359-6366. [PubMed] |
| 26. | Steinwaerder DS, Carlson CA, Otto DL, Li ZY, Ni S, Lieber A. Tumor-specific gene expression in hepatic metastases by a replication-activated adenovirus vector. Nat Med. 2001;7:240-243. [PubMed] [DOI] |
| 27. | 钱 其军, 刘 新垣, 吴 孟超. 肿瘤的基因-病毒治疗的研究热点. 中国肿瘤生物治疗杂志. 2001;8:77-79. |
| 28. | Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106:763-771. [PubMed] [DOI] |
| 29. | Yu DC, Sakamoto GT, Henderson DR. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res. 1999;59:1498-1504. [PubMed] |
| 30. | Wildner O, Blaese RM, Morris JC. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410-413. [PubMed] |
| 31. | Wildner O, Morris JC. The role of the E1B 55 kDa gene product in oncolytic adenoviral vectors expressing herpes simplex virus-tk: assessment of antitumor efficacy and toxicity. Cancer Res. 2000;60:4167-4174. [PubMed] |
| 32. | Prince GA, Porter DD, Jenson AB, Horswood RL, Chanock RM, Ginsberg HS. Pathogenesis of adenovirus type 5 pneumonia in cotton rats (Sigmodon hispidus). J Virol. 1993;67:101-111. [PubMed] |
| 33. | Torres JM, Alonso C, Ortega A, Mittal S, Graham F, Enjuanes L. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J Virol. 1996;70:3770-3780. [PubMed] |
| 34. | Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665-2670. [PubMed] [DOI] |
| 35. | Mazzolini G, Narvaiza I, Pérez-Diez A, Rodriguez-Calvillo M, Qian C, Sangro B, Ruiz J, Prieto J, Melero I. Genetic heterogeneity in the toxicity to systemic adenoviral gene transfer of interleukin-12. Gene Ther. 2001;8:259-267. [PubMed] [DOI] |
| 36. | Asselin-Paturel C, Megherat S, Vergnon I, Echchakir H, Dorothée G, Blesson S, Gay F, Mami-Chouaib F, Chouaib S. Differential effect of high doses versus low doses of interleukin-12 on the adoptive transfer of human specific cytotoxic T lymphocyte in autologous lung tumors engrafted into severe combined immunodeficiency disease-nonobese diabetic mice: relation with interleukin-10 induction. Cancer. 2001;91:113-122. [PubMed] [DOI] |
| 37. | Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: a review. Toxicol Pathol. 1999;27:58-63. [PubMed] [DOI] |