修回日期: 2004-03-09
接受日期: 2004-03-16
在线出版日期: 2004-06-15
目的: 探讨肿瘤坏死因子(TNF-α)对肠黏膜上皮细胞间紧密连接的影响.
方法: 应用免疫荧光方法检测在肿瘤坏死因子(TNF-α) 0, 50, 100和200 mg/L作用下, 与结肠癌上皮细胞株(CaCo-2细胞株)共同培养24 h, 以及用TNF-α 100 mg/L与CaCo-2细胞共同培养0, 4, 8和24 h, 观察TNF-α对紧密连接蛋白(zonula occluden 1, ZO-1)表达的影响. 并应用半定量RT-PCR方法检测TNF-α用上述相应的时间和浓度作用后对肠黏膜上皮细胞ZO-1 mRNA表达的影响.
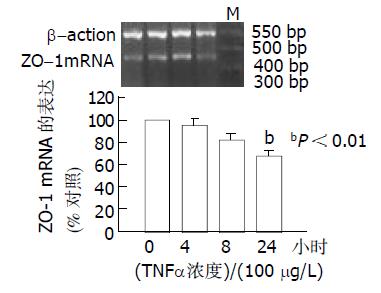
结果: 用TNF-α 100和200 mg/L作用24 h后ZO-1的免疫荧光明显减少, 并呈剂量依赖性; 用TNF-α 100 mg/L作用 24 h时ZO-1免疫荧光明显减少, 并呈时间依赖性. 应用半定量RT-PCR的方法检测结果: TNF-α作用24 h时, ZO-1 mRNA从TNF-α 0 mg/L 的1.1926降至 100 mg/L 的0.7834和200 mg/L 的0.7081; TNF-α 100 mg/L作用4 h, ZO-1mRNA为(95. 5±5.5%); 8 h为(82.0±5.4%); 24 h降至最低为(67.7±5.7%), 与对照组相比P<0.01.
结论: TNF-α是通过抑制肠上皮细胞间紧密连接蛋白ZO-1表达引起肠黏膜上皮细胞间紧密连接破坏. 并且是通过抑制ZO-1 mRNA引起肠上皮细胞ZO-1蛋白表达降低.
引文著录: 宋红丽, 吕飒, 马力, 李颖, 刘沛. TNF-α影响肠黏膜上皮细胞间紧密连接蛋白的表达. 世界华人消化杂志 2004; 12(6): 1303-1306
Revised: March 9, 2004
Accepted: March 16, 2004
Published online: June 15, 2004
AIM: To investigate the effect of TNF-α on tight junctions between the epithelial cells of the intestinal mucosal barrier.
METHODS: Colon cancer cell line (CaCo-2) was cultured. The localizations of zonula occluden-1 (ZO-1) in CaCo-2 epithelial cells were detected by immunofluorescence after exposure to TNF-α (0, 50, 100 and 200 mg/L) for 24 h and the TNF-α treatment with 100 mg/L for 0, 4, 8 and 24 h. The expression level of ZO-1 was analyzed by semi-quantitative reverse transcriptase-PCR after exposure to TNF-α with the above time and concentration.
RESULTS: Different expression levels of ZO-1 depended on different time and concentration of TNF-α treated on CaCo-2 epithelial cells. The integrity of tight junctions was disrupted and the expression level of ZO-1 decreased after treatment with TNF-α. The intensity of the immunofluorescent signal was less intensive after exposure to TNF-α 100 and 200 mg/L than that to 0 and 50 mg/L. The intensity of the immunofluorescent signal was less intensive after exposure to TNF-α 100 mg/L for 24 hours than those for 0 h, 4 h and 8 h. The RT-PCR results showed that after CaCo-2 cells were treated for 24h with the indicated concentrations (0, 50, 100 and 200 mg/L) of TNF-α and the TNF-α treatment with 100 mg/L for 0 h, 4 h, 8 h and 24 h, the expression levels of ZO-1 mRNA in CaCo-2 cells were decreased by TNF-α with 100 mg/L for 24 h, as compared with control (P < 0.01).
CONCLUSION: Tight junctions between the epithelial cells of the intestinal mucosal barrier can be destroyed by TNF-α, and decreased expression level of ZO-1 induced by TNF-α is one of the most important molecular mechanisms.
- Citation: Song HL, Lu S, Ma L, Li Y, Liu P. Effect of TNF-α on tight junctions between the epithelial cells of intestinal mucosal barrier. Shijie Huaren Xiaohua Zazhi 2004; 12(6): 1303-1306
- URL: https://www.wjgnet.com/1009-3079/full/v12/i6/1303.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i6.1303
自发性腹膜炎(spontaneous bacterial peritonitis, SBP )是重症肝炎常见的并发症, 是引起重症肝炎死亡的重要原因[1-3]. 近年来, 对重症肝炎并发SBP的发生机制有了一定的认识. 肠通透性改变和细菌易位与SBP发生密切相关[4-7], 而重症肝炎异常增高的细胞因子如肿瘤坏死因子(TNF)、γ-干扰素(IFN-γ)、白介素(IL)等与SBP发生亦有关[8-9]. 我们观察在TNF-α作用下, 体外培养的肠黏膜上皮细胞紧密连接蛋白(zonula occluden 1, ZO-1)表达状态, 探讨TNF-α在重症肝炎并发SBP的发生中所起到的作用.
主要试剂与仪器细胞培养液DMEM高糖培养基、胎牛血清、谷氨酰胺均购于 Gibco公司; 非必须氨基酸购于Hyclone公司; 胰蛋白酶, TNF-α购于Sigma公司; ZO-1兔抗鼠抗体购于SANTA CRUZ BIOTECHNOLOGY; FITC标记山羊抗兔IgG抗体购于Jackson公司; CaCo-2细胞株购自美国ATCC(American Type Culture Cellection).
CaCo-2细胞培养按照文献, 应用含有200 mL/L胎牛血清、10 g/L非必须氨基酸、10 g/L谷氨酰胺和青霉素-链霉素双抗液的DMEM培养液将CaCo-2细胞在37 ℃, 50 mL/L CO2条件下进行培养, 每7 d按1:2的比例传代, 传代后7 d左右细胞生长达到融合. 每次实验分为对照组(TNF-α非添加组)和实验组(TNF-α添加组). 实验时取3组非同代7 d左右生长达到融合的细胞, 添加0 mg/L, 50 mg/L, 100 mg/L和200 mg/L浓度的TNF-α继续培养24 h后, 进行免疫荧光检测, 并收集CaCo-2细胞提取总RNA, 置-130 ℃保存待用. 另外, 将CaCo-2细胞与TNF-α 100 mg/L共同培养0, 4, 8和24 h, 进行免疫荧光检测, 并收集CaCo-2细胞提取总RNA.
1.2.1 免疫荧光检测: 参照Heinaz Schmitz 方法取同代细胞接种于盖玻片上, 待细胞生长融合70%左右用TNF-α处理, 取出盖玻片PBS漂洗3次(每次10 min)后, 用37 g/L甲醛固定15 min, PBS漂洗, 加入0.5% Triton X-100处理10 min, PBS漂洗, 100 mL/L小牛血清白蛋白封闭1 h, 加入浓度为1:50的ZO-1抗体, 4 ℃过夜, PBS漂洗后加入用FITC标记的免疫荧光抗体37 ℃1 h, 再用PBS漂洗, 蒸馏水洗后用甘油封片.
1.2.2 半定量RT-PCR: 用TRIZOL裂解准备好的CaCo-2细胞, 用酚-氯仿抽提总RNA, 分别取2 mL总RNA加入终浓度为0.6, 0.1mmol/L的ZO-1特异引物与b-肌动蛋白内参引物进行共扩增RT-PCR反应, 反应体系总体积30 mL. ZO-1正义引物序列为[10]: 5'-CGGTCCTCTGAGCCTGTAAG-3'; 反义序列为5'-GGATCTACATGCGACGACAA-3', 扩增长度为371 bp. b-肌动蛋白正义引物序列为: 5-GTGGGGCGCCCCAGGCACCA-3'; 反义序列为5'-CTTCCTTAATGTCACGCACGATTTC-3', 扩增长度为540 bp (以上引物均由Takara Biotechnology 公司合成). 反应条件为: 50 ℃逆转录30 min, 95 ℃变性15 min, 然后进行PCR反应30次循环: 94 ℃变性45 s, 56 ℃退火45 s, 72 ℃延伸60 s, 最后行72 ℃延伸10 min. 取PCR反应产物10 mL加入2 g/L琼脂糖凝胶后电泳. 应用天能GIS凝胶图像处理软件, 对扫描获得的RT-PCR扩增产物进行量化, 然后用质控内参排除操作误差, 进行样本标准化处理. 计算方法如下: 标准化的样本数值 = 样本量化数值/同一样本内参的量化数值.
统计学处理 应用SPSS软件(10.0版本)进行统计学分析, 各组标准化的样本数值用mean±SEM表示. 对各组间数据进行方差分析, 当P<0.05时有显著性差异.
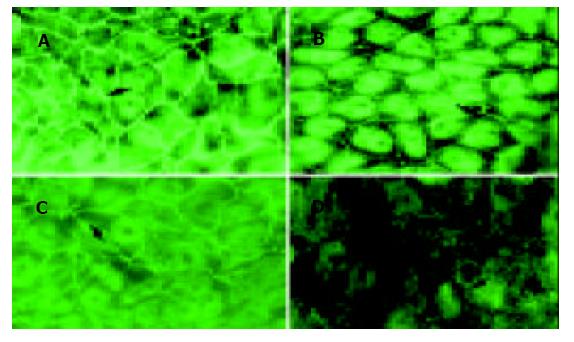
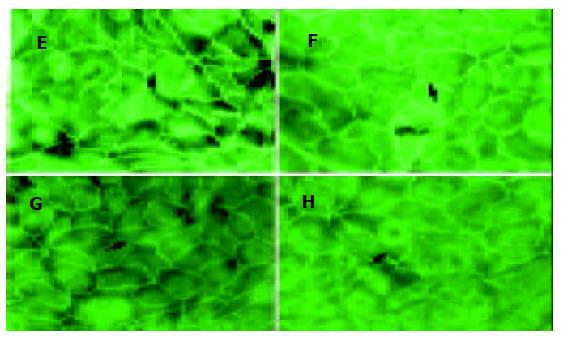
用TNF-α 0 mg/L, 50 mg/L, 100 mg/L和200 mg/L与CaCo-2细胞共同培养24 h, 应用免疫荧光检测CaCo-2细胞ZO-1蛋白表达, 发现100 mg/L和200 mg/L 组ZO-1免疫荧光明显减少或断裂(图1). 用TNF-α 100 mg/L与CaCo-2细胞共同培养, 分别在0, 4, 8和24 h时, 应用免疫荧光检测CaCo-2细胞ZO-1蛋白, 结果24 h时ZO-1免疫荧光明显减少(图2).
从浓度依赖角度, 取同代7 d左右生长达到融合的细胞, 分别添加TNF-α 0mg/L, 50 mg/L, 100 mg/L和200 mg/L与CaCo-2细胞共同培养, 结果24 h时, TNF-α 100 mg/L组, ZO-1mRNA明显降低, 从0 mg/L的1.1 926 降至 100 mg/L 的0.7 834和200 mg/L 的0.7 081. 从时间依赖角度, 取3代细胞, 加入TNF-α (100 mg/L)后, ZO-1的mRNA表达的时间曲线如图3所示. 培养4 h ZO-1的mRNA为(95.5±5.5%); 8h为(82.0±5.4%); 24 h降至最低为(67.7±5.7%), 与对照组相比P<0.01.
重症肝炎并发自发性腹膜炎的发病机制尚不十分清楚.以往学者认为: 自发性腹膜炎形成是细菌通过菌血症定植(colonization)在腹水中或肠道细菌穿过肠壁进入腹膜腔. 因肠道不仅是消化和吸收营养物质的重要脏器, 而且肠道的其他特殊功能如免疫调节、内分泌功能、黏膜屏障功能在防御细菌侵入作用受到学者们的高度重视[11]. 肠道屏障是存在于肠道内的具有高效选择性功能的屏障系统, 肠黏膜屏障在保护机体免受食物抗原、微生物及其产生的有害代谢产物的损害, 保持机体内环境的稳定方面起重要作用[12-13]. 在正常情况下, 肠黏膜具有完善的机械屏障, 以抵御较强的刺激和正常菌群的侵犯. 这种肠黏膜屏障由肠表面黏液、微绒毛、肠黏膜上皮细胞及其间的紧密连接以及黏膜的特殊结构组成[14]. 近年来, 对细胞间连接的研究受到极大重视, 肠上皮细胞间连接和大多数细胞的连接方式一样, 由紧密连接(TJ)、黏附连接(AJ)、缝隙连接(Gap junction)等构成. 构成肠上皮细胞间连接的蛋白主要有ZO-1、occludin、cadherin、claudin和b-catenin等[15]. 一旦某种组成成分发生变化, 即可导致屏障功能发生异常.
1988年Yasutoshi et al人首次发表肝衰竭与TNF-α相关, 随后相继发现重症肝炎时许多细胞因子包括IFN-γ、IL-1、IL-6等明显增加[16-18]. TNF-α是一种具有广谱生理和病理效应的细胞因子, 主要由单核和巨噬细胞分泌. TNF-α在重症肝炎、感染性休克以及炎症性肠病的发生中起着重要的作用[19-20]. 他可诱导肠上皮细胞凋亡[21-23]和细胞间紧密连接蛋白装配功能改变[24], 导致肠上皮通透性增加 [25-27]. 另有研究认为, 肠上皮通透性增加的原因大约一半是由凋亡引起 [28]. 为了研究其他引起肠上皮通透性增加原因是否与上皮细胞间紧密连接的完整性破坏有关? 有学者证实, 溃疡性结肠炎时肠黏膜屏障受损是由于紧密连接结构改变所致[29], TNF-α和IFN-γ等炎性细胞因子之间有协同作用能下调跨膜紧密连接纤维蛋白的occludin启动子的表达, 并且导致肠电生理功能紊乱[30]. 同时TNF-α有细胞毒作用, 可直接引起组织水肿和破坏.
1986年确定ZO-1为第一个紧密连接相关蛋白[31], 属于跨膜蛋白, 可以起连接跨膜蛋白occludin和肌动蛋白细胞骨架的作用, 是构成肠黏膜上皮紧密连接的重要成分之一[32]. Caco-2细胞来源于人体结肠癌细胞株, 在生长至融合后有柱状凸起, 类似于小肠微绒毛, 向培养液一侧形成刷状缘并有分化良好的紧密连接, 是目前很好的体外肠屏障模型[33], 特别是在紧密连接蛋白表达研究中得到广泛应用[34-36]. 为了探讨TNF-α对肠黏膜屏障上皮细胞间紧密连接的影响, 我们采用TNF-α与Caco-2细胞共同培养, 测定ZO-1表达. 结果发现TNF-α可直接抑制肠黏膜屏障上皮细胞间ZO-1的表达; 随着浓度增加和时间延长, ZO-1表达明显减少. 我们应用 mRNA的半定量方法测TNF-α作用后ZO-1 mRNA, 发现高浓度的TNF-α作用Caco-2细胞时间越长, 其ZO-1mRNA表达减少越明显, 并呈时间依赖关系. 故推测: TNF-α可能是通过抑制ZO-1 mRNA而降低Caco-2细胞ZO-1蛋白的表达, 从而影响肠黏膜上皮细胞间紧密连接的完整性.
本结果提示: 在重症肝炎患者并发SBP时, 高水平的TNF-α作用肠黏膜引起肠黏膜屏障改变可能是通过抑制ZO-1 mRNA而降低ZO-1蛋白表达. 由此可见: TNF-α不仅在重症肝炎肝细胞损伤的发病中起作用, 而且在其并发症-自发性腹膜炎的发生上也起一定作用. 进一步深入研究TNF-α对肠黏膜屏障上皮细胞间紧密连接的影响及其分子机制的影响有助于我们进一步了解重症肝炎并发自发性腹膜炎的发病机制、提高重症肝炎并发自发性腹膜炎、肝硬化腹水等的治疗水平、改善其预后并减少并发症的产生.
| 1. | Poddar U, Thapa BR, Prasad A, Sharma AK, Singh K. Natural history and risk factors in fulminant hepatic failure. Arch Dis Child. 2002;87:54-56. [PubMed] [DOI] |
| 2. | Thanopoulou AC, Koskinas JS, Hadziyannis SJ. Spontaneous bacterial peritonitis (SBP): clinical, laboratory, and prognostic features. A single-center experience. Eur J Intern Med. 2002;13:194-198. [PubMed] [DOI] |
| 3. | França AV, De Souza JB, Silva CM, Soares EC. Long-term prognosis of cirrhosis after spontaneous bacterial peritonitis treated with ceftriaxone. J Clin Gastroenterol. 2001;33:295-298. [PubMed] [DOI] |
| 4. | Such J, Francés R, Muñoz C, Zapater P, Casellas JA, Cifuentes A, Rodríguez-Valera F, Pascual S, Sola-Vera J, Carnicer F. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology. 2002;36:135-141. [PubMed] [DOI] |
| 5. | Chang CS, Yang SS, Kao CH, Yeh HZ, Chen GH. Small intestinal bacterial overgrowth versus antimicrobial capacity in patients with spontaneous bacterial peritonitis. Scand J Gastroenterol. 2001;36:92-96. [PubMed] [DOI] |
| 6. | Chiva M, Guarner C, Peralta C, Llovet T, Gómez G, Soriano G, Balanzó J. Intestinal mucosal oxidative damage and bacterial translocation in cirrhotic rats. Eur J Gastroenterol Hepatol. 2003;15:145-150. [PubMed] [DOI] |
| 7. | Solà R, Soriano G. Why do bacteria reach ascitic fluid? Eur J Gastroenterol Hepatol. 2002;14:351-354. [PubMed] [DOI] |
| 8. | Rodríguez-Ramos C, Galan F, Díaz F, Elvira J, Martín-Herrera L, Girón-González JA. Expression of proinflammatory cytokines and their inhibitors during the course of spontaneous bacterial peritonitis. Dig Dis Sci. 2001;46:1668-1676. [PubMed] [DOI] |
| 9. | Viallon A, Zeni F, Pouzet V, Lambert C, Quenet S, Aubert G, Guyomarch S, Tardy B, Bertrand JC. Serum and ascitic procalcitonin levels in cirrhotic patients with spontaneous bacterial peritonitis: diagnostic value and relationship to pro-inflammatory cytokines. Intensive Care Med. 2000;26:1082-1088. [PubMed] [DOI] |
| 10. | Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279-G1288. [PubMed] |
| 12. | Harari Y, Weisbrodt NW, Moody FG. Ileal mucosal response to bacterial toxin challenge. J Trauma. 2000;49:306-313. [PubMed] [DOI] |
| 13. | Kiyono H, Kweon MN, Hiroi T, Takahashi I. The mucosal immune system: from specialized immune defense to inflammation and allergy. Acta Odontol Scand. 2001;59:145-153. [PubMed] [DOI] |
| 15. | Muto Y, Nouri-Aria KT, Meager A, Alexander GJ, Eddleston AL, Williams R. Enhanced tumour necrosis factor and interleukin-1 in fulminant hepatic failure. Lancet. 1988;2:72-74. [PubMed] [DOI] |
| 16. | Souza MH, Cunha FQ, Martinelli AL. Interleukin 6 concentration in ascitic fluid of cirrhotic patients: relationship with previous episodes of spontaneous bacterial peritonitis. J Gastroenterol. 2003;38:149-152. [PubMed] [DOI] |
| 17. | Yumoto E, Higashi T, Nouso K, Nakatsukasa H, Fujiwara K, Hanafusa T, Yumoto Y, Tanimoto T, Kurimoto M, Tanaka N. Serum gamma-interferon-inducing factor (IL-18) and IL-10 levels in patients with acute hepatitis and fulminant hepatic failure. J Gastroenterol Hepatol. 2002;17:285-294. [PubMed] [DOI] |
| 18. | Nakamura T, Ushiyama C, Suzuki S, Shimada N, Ebihara I, Suzaki M, Takahashi T, Koide H. Effect of plasma exchange on serum tissue inhibitor of metalloproteinase 1 and cytokine concentrations in patients with fulminant hepatitis. Blood Purif. 2000;18:50-54. [PubMed] [DOI] |
| 19. | Corredor J, Yan F, Shen CC, Tong W, John SK, Wilson G, Whitehead R, Polk DB. Tumor necrosis factor regulates intestinal epithelial cell migration by receptor-dependent mechanisms. Am J Physiol Cell Physiol. 2003;284:C953-C961. [PubMed] [DOI] |
| 20. | Kanzler S, Galle PR. Apoptosis and the liver. Semin Cancer Biol. 2000;10:173-184. [PubMed] [DOI] |
| 21. | Pinkoski MJ, Droin NM, Green DR. Tumor necrosis factor alpha up-regulates non-lymphoid Fas-ligand following superantigen-induced peripheral lymphocyte activation. J Biol Chem. 2002;277:42380-42385. [PubMed] [DOI] |
| 22. | Bojarski C, Bendfeldt K, Gitter AH, Mankertz J, Fromm M, Wagner S, Riecken EO, Schulzke JD. Apoptosis and intestinal barrier function. Ann N Y Acad Sci. 2000;915:270-274. [PubMed] [DOI] |
| 23. | Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164-6172. [PubMed] [DOI] |
| 24. | Poritz LS, Garver KI, Tilberg AF, Koltun WA. Tumor necrosis factor alpha disrupts tight junction assembly. J Surg Res. 2004;116:14-18. [PubMed] [DOI] |
| 25. | Schmitz H, Fromm M, Bentzel CJ, Scholz P, Detjen K, Mankertz J, Bode H, Epple HJ, Riecken EO, Schulzke JD. Tumor necrosis factor-alpha (TNFalpha) regulates the epithelial barrier in the human intestinal cell line HT-29/B6. J Cell Sci. 1999;112:137-146. [PubMed] |
| 26. | Coyne CB, Vanhook MK, Gambling TM, Carson JL, Boucher RC, Johnson LG. Regulation of airway tight junctions by proinflammatory cytokines. Mol Biol Cell. 2002;13:3218-3234. [PubMed] [DOI] |
| 27. | Ma TY, Iwamoto GK, Hoa NT, Akotia V, Pedram A, Boivin MA, Said HM. TNF-αlpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367-G376. [PubMed] [DOI] |
| 28. | Gitter AH, Bendfeldt K, Schulzke JD, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-αlpha-induced single-cell apoptosis. FASEB J. 2000;14:1749-1753. [PubMed] [DOI] |
| 29. | Schmitz H, Barmeyer C, Fromm M, Runkel N, Foss HD, Bentzel CJ, Riecken EO, Schulzke JD. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301-309. [PubMed] [DOI] |
| 30. | Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085-2090. [PubMed] |
| 31. | Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA. Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol. 1986;103:755-766. [PubMed] [DOI] |
| 32. | Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745-29753. [PubMed] [DOI] |
| 33. | Cruz N, Qi L, Alvarez X, Berg RD, Deitch EA. The Caco-2 cell monolayer system as an in vitro model for studying bacterial-enterocyte interactions and bacterial translocation. J Burn Care Rehabil. 1994;15:207-212. [PubMed] [DOI] |
| 34. | Sappington PL, Han X, Yang R, Delude RL, Fink MP. Ethyl pyruvate ameliorates intestinal epithelial barrier dysfunction in endotoxemic mice and immunostimulated caco-2 enterocytic monolayers. J Pharmacol Exp Ther. 2003;304:464-476. [PubMed] [DOI] |
| 35. | Raschperger E, Engstrom U, Pettersson RF, Fuxe J. CLMP, a novel member of the CTX family and a new component of epithelial tight junctions. J Biol Chem. 2004;279:796-804. [PubMed] [DOI] |
| 36. | Neunlist M, Toumi F, Oreschkova T, Denis M, Leborgne J, Laboisse CL, Galmiche JP, Jarry A. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1028-G1036. [PubMed] [DOI] |