修回日期: 2004-01-09
接受日期: 2004-02-01
在线出版日期: 2004-05-15
目的: 为构建肝细胞永生化载体, 对猿猴病毒40大T抗原基因(SV40T)外显子进行拼接并以其为外源片段构建逆转录病毒永生化载体pLLTSN.
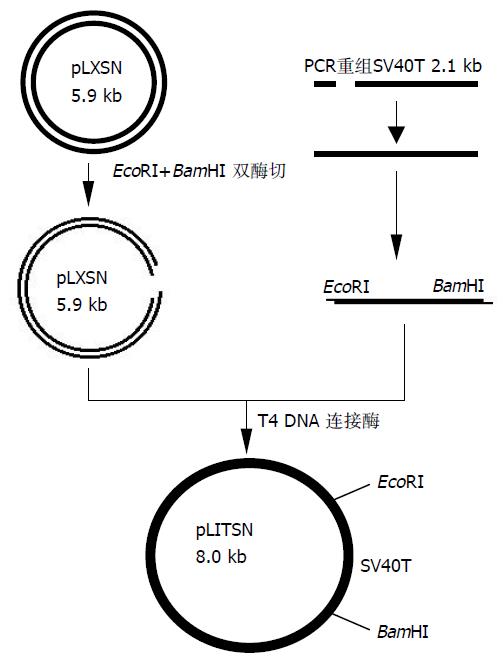
方法: 以质粒pUC19-SV40T为模板, 高保真PCR扩增SV40T的2个外显子, 重叠延伸拼接法将两个外显子拼接到一起, 然后用双粘端连接法将其克隆到空载体pLXSN的EcoRⅠ和BamHⅠ位点之间, 通过菌落PCR和酶切法筛选, 鉴定阳性克隆并经DNA测序验证.
结果: 拼接成2.1 kb的SV40T, 用菌落PCR和酶切法随机筛选的10个菌落中4个为阳性, 均含有2.1 kb SV40T插入片段. 经质粒DNA测序分析的阳性克隆, 确证无内含子.
结论: 获得重组的2.1 kb无内含子的SV40T, 成功构建了逆转录病毒载体pLLTSN.
引文著录: 李俊刚, 陈耀凯, 王宇明. SV40T外显子的拼接及逆转录病毒载体pLLTSN的构建. 世界华人消化杂志 2004; 12(5): 1104-1107
Revised: January 9, 2004
Accepted: February 1, 2004
Published online: May 15, 2004
AIM: To construct an immortalization vector for hepatocytes immortalization, and two exons of simian virus 40 large T antigen gene (SV40T) were spliced and a retroviral vector pLLTSN without intron was constructed.
METHODS: The two exons of SV40T were amplified respectively by high fidelity polymerase chain reaction (PCR) by using the plasmid pUC19-SV40T as the template. Then SV40T gene was spliced by overlapping extension (SOE), and cloned into theEcoRⅠand BamHⅠsites of the retroviral vector pLXSN. The positive recombinant clones were screened and identified by PCR by using colonies directly as templates, and by restriction endonuclease digestion analysis, and DNA sequence analysis.
RESULTS: The 2.1 kb SV40T gene was spliced. Among the ten colonies randomly screened, four were proved positive, and one of them was verified by plasmid DNA sequencing.
CONCLUSION: The retroviral vector pLLTSN containing SV40T without intron is successfully constructed.
- Citation: Li JG, Chen YK, Wang YM. Splicing of SV40T gene exons and construction of a retroviral vector pLLTSN. Shijie Huaren Xiaohua Zazhi 2004; 12(5): 1104-1107
- URL: https://www.wjgnet.com/1009-3079/full/v12/i5/1104.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i5.1104
肝细胞是生物人工肝支持系统中核心生物成分[1-7]. 由正常肝细胞转化而来的肝细胞株具有正常肝细胞的某些主要功能[8], 且在体外培养时增生能力强, 可迅速达到人工肝支持所需的细胞数量[9]. 构建逆转录病毒永生化载体是肝细胞永生化的重要步骤. 猿猴病毒40 T抗原基因(simian virus 40 large T antigen, SV40T)是常用的细胞永生基因. 野生型SV40T由2个外显子和1个内含子组成, 在转录表达过程中, 可因其mRNA的剪接与否而表达T抗原或t抗原. 由于仅T抗原即可引起细胞的永生化[10-12], 国外学者采用不表达t抗原的SV40T逆转录病毒载体成功永生化了包括肝细胞在内的多种细胞[13]. 我们在构建肝细胞永生化载体过程中, 为了避免t抗原的表达, 同时缩短DNA长度, 采用重叠延伸拼接法(splicing by overlapping extension, SOE)法[14-15]将SV40T的2个外显子拼接起来, 去除了内含子, 并将重组的SV40T克隆到逆转录病毒载体pLXSN的多克隆位点, 构建成含2.1 kb SV40T的逆转录病毒载体pLLTSN, 旨在为肝细胞永生化奠定基础.
逆转录病毒载体pLXSN及其位于多克隆位点两侧测序引物P5'和P3'购于美国Clontech公司; 质粒pUC19-SV40 T由第四军医大学段小红博士惠赠[16], 该质粒含有SV40T的全部基因序列; 限制性内切酶EcoRⅠ, BamHⅠ等购自英国NEB公司; T4 DNA连接酶为美国Promega公司产品; 大肠杆菌DH5a株为本研究所保存菌种; pfu DNA聚合酶购自北京鼎国公司; 2×PCR混合液和玻璃珠法DNA胶回收纯化试剂盒购自上海生工生物工程技术有限公司; 重组SV40T (r SV40T)PCR引物由上海生工生物工程技术有限公司合成, 序列如下: P1: 5'-GCGAATTC(EcoRI)ACCATGGATAAAGTTTTAAAC-3'; P2: 5'-CATCAGTTCCATAGGTTGGAATCTCAGTTGCATCCCAGAAGCC-3'; P3: 5'-CCAACCTATGGAACTGATGAATGGGAG-3'; P4: 5'-CCGGATCC(BamHⅠ)TTATGTTTCAGGTTCAGGG-3'. 其中P2和P3为内引物, 二者之间有19个碱基重叠互补. PCR产物纯化试剂盒为德国Roche公司产品.
SV40T外显子1的扩增: 在100 mL反应体系中分别加入无菌水91 mL, 10×PCR Buffer 10 mL, dNTP 2mL, P1, P2各2mL, 2.5 MU/L 的pfu DNA聚合酶2 mL, 模板pUC19-SV40 T质粒1 mL. 反应条件: 94 ℃预变性2 min; 然后94 ℃ 1 min, 58 ℃ 1 min, 72 ℃ 1 min共30个循环; 最后72 ℃延伸10 min. 反应产物经8 g/L琼脂糖凝胶电泳分析, 目的片段应为279 bp. SV40T外显子2的扩增: 在上述PCR体系中将P1和P2换为P3和P4, 其余成分不变. 反应条件: 94 ℃预变性2 min; 然后94 ℃ 1 min, 60 ℃ 1 min, 72 ℃ 3 min共30个循环; 最后72 ℃延伸10 min. 目的片段约为1.9 kb. PCR产物的纯化按试剂盒说明书进行操作. 每100 mL PCR产物纯化后浓缩为10 mL. SV40T基因2个外显子的拼接: 在上述PCR体系中, 引物换为P1和P4, 模板换为纯化的外显子1和2各1 mL, 反应条件: 94 ℃预变性2 min; 然后94 ℃ 1 min, 58 ℃ 1 min, 72 ℃ 3 min共30个循环; 最后72 ℃延伸10 min. 产物经8 g/L琼脂糖凝胶电泳分析后用PCR产物纯化试剂盒纯化. pLLTSN载体的构建: 按图1所示流程进行. 同时用EcoRⅠ和BamHⅠ分别双酶切载体pLXSN和纯化的2.1 kb rSV40T, 8 g/L琼脂糖凝胶电泳分离5.9 kb线性pLXSN和2.1 kb rSV40 T, 玻璃珠法DNA胶回收纯化试剂盒从凝胶中回收纯化目的片段. 将回收的pLXSN和rSV40T片段按约1:3的摩尔比混合(共约100 ng), 45 ℃水浴5 min, 再冷却到0 ℃, 在10 mL体系中用T4 DNA连接酶16 ℃过夜连接. 次日取5 mL连接产物转化氯化钙法制备的感受态DH5α细胞. pLLTSN阳性克隆的筛选和鉴定: (1)菌落PCR法[17-18]: 用分别与载体序列和SV40T序列互补的引物P5'和P4配对进行PCR, 可同时鉴定重组体是否确为pLXSN与rSV40T嵌合体, 以及SV40T在载体中插入的方向. 在20 mL反应体系中加入无菌水19.2 mL, 2×PCR混合液10 mL, P5'和P4引物各0.8 mL, 以高压灭菌牙签沾取单个菌落少许, 点入反应体系中作为模板. 反应条件: 94 ℃预变性5 min; 然后94 ℃ 30 s, 58 ℃ 30 s, 72 ℃ 50 s共30个循环; 最后72 ℃延伸10 min. 将PCR产物进行8 g/L琼脂糖凝胶电泳, 出现2.1 kb条带者为阳性. (2)酶切法: 取PCR阳性克隆接种于5 mL含100 kU/L氨苄西林的LB培养基, 37 ℃摇床中250 r/min振荡培养过夜. 碱裂解法提取质粒DNA, 以EcoRⅠ和BamHⅠ双酶切和8 g/L琼脂糖凝胶电泳分析, 电泳分离出2.1 kb条带者为阳性. 同时以空载体pLXSN行相同双酶切作为对照. 随机取一株鉴定为阳性的pLLTSN克隆进行rSV40T的DNA序列测定, 由上海生工生物工程技术有限公司完成, 测序引物为P5'和P3'.
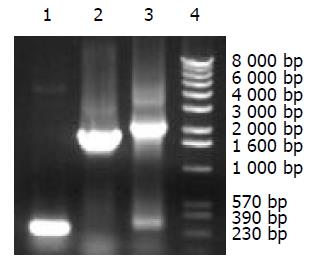
通过PCR成功地扩增出了SV40T基因279 bp和1.9 kb两个外显子DNA片段; 再以两个DNA片段为模板, 使用SOE法成功地将两个片段顺序连接成连续的, 无内含子的2.1 kb rSV40T基因(图2).
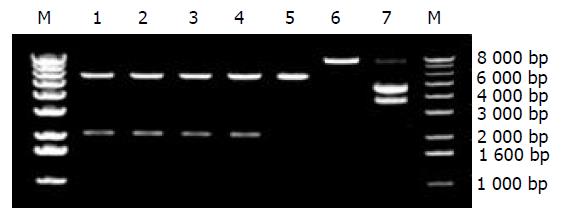
转化平板长出几百个菌落, 随机挑取10个克隆进行筛选和鉴定. 应用P5'和P4为引物, 以单个菌落的少许菌体为模板, 同时以载体pLXSN质粒作为阴性对照模板进行PCR扩增. 用8 g/L琼脂糖凝胶电泳分析PCR产物, 结果随机筛选的10个菌落中有4个可见2.1 kb的阳性条带, 阴性对照并未见任何条带.
挑取4个PCR阳性克隆进行碱裂解法提取质粒DNA, 以EcoRⅠ和BamHⅠ双酶切和8g/L琼脂糖凝胶电泳分析. 结果4个克隆均被切为5.9kb和2.1kb两个片段(图3).
经过测序, pLLTSN中的rSV40T全长2 127 bp, 共有4处点突变, 即G888A, T1063C, A1701G和T1868C突变, 但皆为同义突变, 其编码蛋白的氨基酸序列并无改变. 突变处(下划线)部分序列如下:
851 ATGTGTTGTT ATTGCTTGGG ATGTACTTGG AATTTCAATA CAGTTTTGAA...
1051 GATAGCCTAC AACTAACTAG AGAACAAATG TTAACAAACA GATTTAATGA...
1701 GATAATTCAA AGTGGCATTG CTTTGCTTCT TATGTTAATT TGGTACAGAC...
1851 TAATGTGGCT ATGGGAACTG GAGTTTTAGA TTGGCTAAGA AACAGTGATA...
基因的重叠延伸拼接(SOE)[14]又称重组PCR, 是将不相邻的两个基因或DNA片段拼接起来构成融合基因/DNA的PCR方法. 其原理是: 使用两对引物P1, P2和P3, P4经过第一轮PCR分别扩增出两个DNA片段A和B, 其中P2和P3为内引物, 具有部分序列重叠互补, 使A的3'端和B的5'端具有同源互补序列; 第二轮PCR再以A和B混合作为模板, 经变性, 退火, 复性时, A的正链和B的负链的3'端同源互补序列将结合在一起, 使两条链互为引物, 通过PCR得到延伸和扩增, 从而连接A和B. 其特点是不需要使用限制性内切酶和连接酶, 可以避免引入限制性酶切位点的核苷酸序列, 可将两个DNA片段精确地连接在一起. 使用这种方法时, 为了尽可能避免或减少碱基错配, 往往需加大模板, 引物和dNTP的用量, 同时减少PCR的循环数, 因此有时在其PCR产物的凝胶电泳图像中仍可见模板DNA的相应条带. 在图2中第三泳道出现与外显子1相同大小的较弱条带即属此种情况. 一方面是由于模板过多, 另一方面是因为内引物P2较长, 在外显子1的PCR产物纯化过程中有所残留, 导致第二轮PCR过程中产生一定扩增. 因此应用SOE时, 第一轮PCR产物最好经电泳分离后切胶回收纯化以除去多余的引物和其他非特异序列.
野生型SV40T基因全长2 473 bp, 其中前246 bp为第一外显子, 247-592 bp为内含子, 593-2 476 bp为第2外显子, 两个外显子连接后编码序列长2 127 bp.所使用的外引物引入了额外的酶切位点序列, 所以第1外显子PCR产物长279 bp. SV40T第1外显子和内含子部分序列(前561 bp)又可编码t 抗原. 在SV40转录表达过程中, 其mRNA发生剪接时则转译为T抗原, 而不剪除内含子序列时则表达t抗原. t 抗原在细胞中的作用复杂, 可以促进T抗原对细胞的转化[19], 也可增加细胞凋亡的敏感性[20], 而T抗原却可通过PI3激酶路径激活Akt抑制细胞凋亡[21]. 为了避免t抗原表达引起的其他不确定因素[10], 我们用SOE法成功地连接了SV40T的两个外显子, 删除其内含子序列, 同时减少SV40T在载体中所占容量以利于载体后续加入其他基因, 并将拼接的2.1 kb SV40T克隆到pLTSN的EcoRⅠ和BamHⅠ酶切位点之间, 构建了只有T抗原可得到表达的逆转录病毒载体pLLTSN.
SV40T是使用最广泛的永生基因, 他不但能刺激细胞增生, 延长培养细胞的生命期, 而且诱导细胞分化却不完全转化细胞.如通过分子克隆的SV40 DNA转染而永生化的猫成纤维细胞, 培养传代长达6 mo之久而不显示与其原代细胞不同的表型[22]. 应用SV40T基因永生化多种哺乳动物细胞, 这已为国内外许多研究报道所证实[8,23-28]. 体外培养的二倍体细胞是不能无限分裂增生的, 而用基因转移技术将SV40T转到二倍体细胞中并稳定表达[29], 可获得永生化细胞[30-34]. p53与pRB是调控细胞增生和细胞凋亡的抑癌基因, p53的突变和pRB的失活都可导致细胞增生周期的失控, 引起细胞的无限生长. SV40T抗原与p53[35]及pRB[36-37]结合并使后者失活, 从而延长细胞生命. 为探索用SV40T基因永生化人肝细胞的可行性, 我们构建了含SV40T的逆转录病毒表达载体. 该载体的真核表达元件为莫洛尼小鼠白血病病毒(moloney murine leukemia virus, MoMuLV)长末端重复序列(long terminal repeat, LTR), SV40T, SV40早期启动子以及新霉素耐药基因(Neor), 因此命名为pLLTSN.
逆转录病毒载体是目前哺乳动物细胞基因转移最有效的工具, 可高效转导人骨髓细胞等[38], 已被应用于遗传性疾病及癌症基因治疗的临床试验[39-40]. pLXSN是基于MoMLV构建的经典逆转录病毒载体之一. 当含目的基因的pLXSN转染包装细胞后, 可产生无复制活性的感染性病毒. 这些病毒颗粒可感染靶细胞并转移目的基因, 但不能在靶细胞中复制. 将pLLTSN包装为多嗜性复制缺陷病毒, 可感染包括人在内的多种哺乳动物细胞. 病毒颗粒感染细胞后, 其基因可整合到宿主细胞基因组, 稳定表达SV40T和Neor. 用其感染人肝细胞可望建立永生化人肝细胞系.
| 1. | Ding YT, Qiu YD, Chen Z, Xu QX, Zhang HY, Tang Q, Yu DC. The development of a new bioartificial liver and its application in 12 acute liver failure patients. World J Gastroenterol. 2003;9:829-832. [PubMed] [DOI] |
| 2. | Chen XP, Xue YL, Li XJ, Zhang ZY, Li YL, Huang ZQ. Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure. World J Gastroenterol. 2001;7:706-709. [PubMed] |
| 3. | Wang YJ, Li MD, Wang YM, Chen GZ, Lu GD, Tan ZX. Effect of extracorporeal bioartificial liver support system on fulminant hepatic failure rabbits. World J Gastroenterol. 2000;6:252-254. [PubMed] |
| 4. | Xue YL, Zhao SF, Zhang ZY, Wang YF, Li XJ, Huang XQ, Luo Y, Huang YC, Liu CG. Effects of a bioartificial liver support system on acetaminophen induced acute liver failure canines. World J Gastroenterol. 1999;5:308-311. [PubMed] [DOI] |
| 5. | Gao Y, Xu XP, Hu HZ, Yang JZ. Cultivation of human liver cell lines with microcarriers acting as biological materials of bioartificial liver. World J Gastroenterol. 1999;5:221-224. [PubMed] [DOI] |
| 6. | Wang YJ, Li MD, Wang YM, Nie QH, Chen GZ. Experimental study of bioartificial liver with cultured human liver cells. World J Gastroenterol. 1999;5:135-137. [PubMed] [DOI] |
| 8. | Maruyama M, Kobayashi N, Westerman KA, Sakaguchi M, Allain JE, Totsugawa T, Okitsu T, Fukazawa T, Weber A, Stolz DB. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446-451. [PubMed] [DOI] |
| 10. | Kobayashi N, Fujiwara T, Westerman KA, Inoue Y, Sakaguchi M, Noguchi H, Miyazaki M, Cai J, Tanaka N, Fox IJ. Prevention of acute liver failure in rats with reversibly immortalized human hepatocytes. Science. 2000;287:1258-1262. [PubMed] [DOI] |
| 11. | Allain JE, Dagher I, Mahieu-Caputo D, Loux N, Andreoletti M, Westerman K, Briand P, Franco D, Leboulch P, Weber A. Immortalization of a primate bipotent epithelial liver stem cell. Proc Natl Acad Sci USA. 2002;99:3639-3644. [PubMed] [DOI] |
| 12. | Beachy TM, Cole SL, Cavender JF, Tevethia MJ. Regions and activities of simian virus 40 T antigen that cooperate with an activated ras oncogene in transforming primary rat embryo fibroblasts. J Virol. 2002;76:3145-3157. [PubMed] [DOI] |
| 13. | Westerman KA, Leboulch P. Reversible immortalization of mammalian cells mediated by retroviral transfer and site-specific recombination. Proc Natl Acad Sci USA. 1996;93:8971-8976. [PubMed] [DOI] |
| 14. | Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61-68. [PubMed] [DOI] |
| 15. | Warrens AN, Jones MD, Lechler RI. Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene. 1997;186:29-35. [PubMed] [DOI] |
| 17. | Takumi T. Rapid cDNA cloning by PCR screening (RC-PCR). Methods Mol Biol. 2002;192:385-389. [PubMed] [DOI] |
| 18. | Liao CH, Shollenberger LM. Detection of Salmonella by indicator agar media and PCR as affected by alfalfa seed homogenates and native bacteria. Lett Appl Microbiol. 2003;36:152-156. [PubMed] [DOI] |
| 19. | Bocchetta M, Miele L, Pass HI, Carbone M. Notch-1 induction, a novel activity of SV40 required for growth of SV40-transformed human mesothelial cells. Oncogene. 2003;22:81-89. [PubMed] [DOI] |
| 20. | Cole SL, Tevethia MJ. Simian virus 40 large T antigen and two independent T-antigen segments sensitize cells to apoptosis following genotoxic damage. J Virol. 2002;76:8420-8432. [PubMed] [DOI] |
| 21. | Yu Y, Alwine JC. Human cytomegalovirus major immediate-early proteins and simian virus 40 large T antigen can inhibit apoptosis through activation of the phosphatidylinositide 3'-OH kinase pathway and the cellular kinase Akt. J Virol. 2002;76:3731-3738. [PubMed] [DOI] |
| 22. | Köksoy S, Phipps AJ, Hayes KA, Mathes LE. SV40 Immortalization of feline fibroblasts as targets for MHC-restricted cytotoxic T-cell assays. Vet Immunol Immunopathol. 2001;79:285-295. [PubMed] [DOI] |
| 23. | Yuan L, Yu WM, Qu CK. DNA damage-induced G2/M checkpoint in SV40 large T antigen-immortalized embryonic fibroblast cells requires SHP-2 tyrosine phosphatase. J Biol Chem. 2003;278:42812-42820. [PubMed] [DOI] |
| 24. | Frisa PS, Jacobberger JW. Cell density related gene expression: SV40 large T antigen levels in immortalized astrocyte lines. BMC Cell Biol. 2002;3:10. [PubMed] [DOI] |
| 25. | Ali SH, DeCaprio JA. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin Cancer Biol. 2001;11:15-23. [PubMed] [DOI] |
| 26. | van Leeuwen EB, Wisman GB, Tervaert JW, Palmans LL, van Wijk RT, Veenstra R, Molema G, van der Zee AG, van der Meer J, Ruiters MH. An SV40 large T-antigen immortalized human umbilical vein endothelial cell line for anti-endothelial cell antibody detection. Clin Exp Rheumatol. 2001;19:283-290. [PubMed] |
| 27. | Darimont C, Avanti O, Tromvoukis Y, Vautravers-Leone P, Kurihara N, Roodman GD, Colgin LM, Tullberg-Reinert H, Pfeifer AM, Offord EA. SV40 T antigen and telomerase are required to obtain immortalized human adult bone cells without loss of the differentiated phenotype. Cell Growth Differ. 2002;13:59-67. [PubMed] |
| 28. | Tsao SW, Wang X, Liu Y, Cheung YC, Feng H, Zheng Z, Wong N, Yuen PW, Lo AK, Wong YC. Establishment of two immortalized nasopharyngeal epithelial cell lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim Biophys Acta. 2002;1590:150-158. [PubMed] [DOI] |
| 29. | Berghella L, De Angelis L, Coletta M, Berarducci B, Sonnino C, Salvatori G, Anthonissen C, Cooper R, Butler-Browne GS, Mouly V. Reversible immortalization of human myogenic cells by site-specific excision of a retrovirally transferred oncogene. Hum Gene Ther. 1999;10:1607-1617. [PubMed] [DOI] |
| 30. | Behrens A, Gordon EM, Li L, Liu PX, Chen Z, Peng H, La Bree L, Anderson WF, Hall FL, McDonnell PJ. Retroviral gene therapy vectors for prevention of excimer laser-induced corneal haze. Invest Ophthalmol Vis Sci. 2002;43:968-977. [PubMed] |
| 31. | Jha KK, Banga S, Palejwala V, Ozer HL. SV40-Mediated immortalization. Exp Cell Res. 1998;245:1-7. [PubMed] [DOI] |
| 32. | Nelson K, Melville EL, Meikle PJ, Anson DS. Immortalisation of a mucopolysaccharidosis type IIIC fibroblast cell line via expression of SV40 T antigen. Cell Biol Int. 2003;27:567-570. [PubMed] [DOI] |
| 33. | Chen W, Hahn WC. SV40 early region oncoproteins and human cell transformation. Histol Histopathol. 2003;18:541-550. [PubMed] |
| 34. | Hattori K, Muta M, Toi M, Iizasa H, Shinsei M, Terasaki T, Obinata M, Ueda M, Nakashima E. Establishment of bone marrow-derived endothelial cell lines from ts-SV40 T-antigen gene transgenic rats. Pharm Res. 2001;18:9-15. [PubMed] [DOI] |
| 35. | Khandjian EW, Rose TM. Disruption of LT-antigen/p53 complex by heat treatment correlates with inhibition of DNA synthesis during transforming infection with SV40. Biochem Cell Biol. 2000;78:437-445. [PubMed] [DOI] |
| 36. | Modi S, Kubo A, Oie H, Coxon AB, Rehmatulla A, Kaye FJ. Protein expression of the RB-related gene family and SV40 large T antigen in mesothelioma and lung cancer. Oncogene. 2000;19:4632-4639. [PubMed] [DOI] |
| 37. | Lee C, Cho Y. Interactions of SV40 large T antigen and other viral proteins with retinoblastoma tumour suppressor. Rev Med Virol. 2002;12:81-92. [PubMed] [DOI] |
| 38. | Hong Y, Lee K, Choi JY, Yu SS, Kim E, Sugimoto Y, Ueno M, Kato I, Kim S. High efficiency gene transfer to human CD34+ cells. Int J Hematol. 2002;76 Suppl 1:264-265. [PubMed] [DOI] |
| 39. | McTaggart S, Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol Adv. 2002;20:1-31. [PubMed] [DOI] |
| 40. | Hong Y, Yu SS, Kim JM, Lee K, Na YS, Whitley CB, Sugimoto Y, Kim S. Construction of a high efficiency retroviral vector for gene therapy of Hunter's syndrome. J Gene Med. 2003;5:18-29. [PubMed] [DOI] |