修回日期: 2003-10-25
接受日期: 2003-11-13
在线出版日期: 2004-03-15
目的: 研究鉴定激活 mfgl2凝血酶原酶基因之冠状病毒3型或A59型鼠肝炎病毒 (MHV-3, MHV-A59) 核心(N)蛋白的功能区域.
方法: 应用定点突变技术、与mfgl2启动子共转染实验明确mfgl2凝血酶原酶基因之MHV-3或MHV-A59 N蛋白的功能区域. N蛋白内含I基因突变病毒株A1b 110和其野生株A1b 111体外感染Balb/cJ小鼠巨噬细胞、I基因表达载体与mfgl2启动子共转染实验阐明I蛋白在mfgl2基因激活中的作用.
结果: N蛋白包含由两个可变间隔区(A, B)隔开的三个结构区(I, II, III), MHV-A59 N蛋白I区可增强mfgl2转录活性, 当其基因序列突变为非嗜肝性MHV-JHM或MHV-2 I区序列时, 则丧失激活mfgl2启动子转录活性的功能. I基因突变病毒株A1b 110和其野生株A1b 111体外感染Balb/cJ小鼠巨噬细胞后对mfgl2的激活无显著差异, 共转染实验阐明I蛋白并非mfgl2基因激活中的必备因素, 该组mfgl2启动子转录活性与对照组无显著差异, 而N蛋白可激活mfgl2启动子, 使其转录活性提高62倍.
结论: 鼠肝炎病毒N蛋白I区为激活 mfgl2凝血酶原酶基因的病毒蛋白功能区域.
引文著录: 宁琴, 严伟明, 汪之沫, 习东, 刘铭锋, GaryLevy, 罗小平. 鼠肝炎病毒3型N蛋白I区激活 mfgl2凝血酶原酶基因. 世界华人消化杂志 2004; 12(3): 594-599
Revised: October 25, 2003
Accepted: November 13, 2003
Published online: March 15, 2004
AIM: To investigate the responsible domain(s) of N protein and the I gene within the N gene of MHV-3 or MHV-A59 in the activation of mfgl2.
METHODS: To investigate the responsible domain(s) of N protein of MHV-3 or MHV-A59 in the activation of fgl2 gene, four ways comparison of the N protein was carried out and the site directed mutated N gene expression constructs within domain I and domain III were cotransfected respectively with mfgl2 promoter/luciferase reporter gene in CHO cells. Macrophages from Balb/cJ mice were infected with I gene mutated MHV virus Alb110 and its isogenic Alb111 for 8-10 hours, procoagulant activity (PCA) were measured. MHV-A59 I gene expression construct was cotransfected with mfgl2 promoter-reporter gene in Chinese hamster ovary (CHO) cells, and luciferase activity was detected for the assessment of promoter function.
RESULTS: Mutations of residues Gly-12, Pro-38, Asn-40, Gln-41 and Asn42 within domain I of the N protein of MHV-A59 to their corresponding residues were found in MHV-2 abrogated mfgl2 transcription, whereas mutation of other N protein domain III did not affect mfgl2 gene transcription. Alb 110 and Alb 111 infected macrophages showed a remarkable increasing in PCA activity compared with no virus or MHV-2 or MHV-JHM infected macrophages. There was no significant difference in PCA activity between Alb 110, Alb 111 infected group and MHV-A59 group. Cotransfection I gene expression construct with a reporter construct containing mfgl2 promoter in CHO cells displayed no significant difference in luciferase activity compared with nontransfected CHO cells.
CONCLUSION: Domain I of nucleocapsid protein of murine hepatitis virus strain 3 upregulates the transcription of mfgl2 prothrimbinase/fibroleukin gene. The MHV-A59 I gene is not essential for activation of mfgl2 gene. Our study may shed lights on the investigation of current worldwide-distributed disease, severe acute respiratory syndrome (SARS).
- Citation: Ning Q, Yan WM, Wang ZM, Xi D, Liu MF, Levy G, Luo XP. Domain I of nucleocapsid protein of murine hepatitis virus strain 3 upregulates transcription of mfgl2 prothrimbinase/fibroleukin gene. Shijie Huaren Xiaohua Zazhi 2004; 12(3): 594-599
- URL: https://www.wjgnet.com/1009-3079/full/v12/i3/594.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i3.594
肝脏枯否细胞和血窦内皮mfgl2和hfgl2凝血酶原酶的高表达在冠状病毒-鼠肝炎病毒所诱导的小鼠暴发型病毒性肝炎和重症乙型肝炎的病情进展中起重要作用[1-2]. 我们的前期研究显示并非所有鼠肝炎病毒株都可诱导mfgl2基因表达, 仅嗜肝性鼠肝炎病毒3型(MHV-3)和A59型鼠肝炎病毒(MHV-A59)感染可在体内外诱导Balb/cJ小鼠mfgl2基因高表达, 而非嗜肝性2型和JHM型鼠肝炎病毒(MHV-2和MHV-JHM)在体内外不能激活mfgl2基因[3]. 已知MHV-3或MHV-A59 核心(N)蛋白和宿主肝细胞核因子4(HNF4)为mfgl2基因激活的必要因素[4]. N蛋白由3个保守结构区I, II和III组成, II区为与基因组RNA结合的部位, 而I区和III区的生物学功能尚未明了[5-8]. 近来Masters研究组又在大多数MHV N基因发现一内在开放阅读框架(internal ORF) I基因[9]. 我们阐明激活mfgl2凝血酶原酶基因的病毒蛋白功能区域.
Balb/cJ♀小鼠6-8周龄购自Jackson实验室(Bar Harbor, ME), 在本院动物中心饲养. MHV-3, MHV-A59, MHV-JHM和MHV-2购自美国典型生物保藏中心(ATCC). MHV-A59 I基因突变病毒株Alb 110和其野生型Alb 111, 特异性I蛋白mAb由美国纽约州立研究院Paul Marsters教授提供[7]. Alb110 病毒株I基因读码框起始密码和终止密码均被突变, 此株病毒不产生I蛋白. 所有的病毒株均采用鼠17CL1细胞系进行扩增培养, 鼠L2细胞系蚀斑纯化和滴度测定. 小鼠原代巨噬细胞: 将30 g/L硫羟乙酸盐1.5 mL注射到Balb/cJ小鼠腹腔内, 4 d后从该小鼠腹腔抽取巨噬细胞悬浮于RPMI 1640培养液中(含2 mmol/L谷胺酰胺和20 mL/L胎牛血清). 形态学和非特异酯酶染色检测巨噬细胞纯度超过95%. 锥虫蓝摄排实验检测巨噬细胞存活率超过95%. 中国仓鼠卵巢(CHO)细胞系来自ATCC.
N基因、I基因表达载体和mfgl2启动子虫荧光素酶(LUC)报告基因载体的构建[4]: 通过RT-PCR对MHV全长N基因、I基因编码区和3 非编码(UTRS)序列进行扩增, 并将其亚克隆到表达载体pCR 3.1. 所用引物序列为: N基因上游引物5'ACG ATG TCT TTT GTT CCT GGG 3'. N基因下游引物5'TTT TTT TTT GTG ATT CTT CCA 3', I基因上游引物5'CGC GCT GGT AAT GGA AT 3', I 基因下游引物5'CTT CGG CCA TAT CAG GTT 3'. 此载体含CMV启动子和小牛生长激素3'端信号控制系统. 所克隆之MHV-2 N基因序列已提交至NIH基因文库(序列号AF061853)[3]. 从鼠基因组P1质粒的亚克隆PM166 (pBluescript-m166)中酶解出mfgl2基因5'端1.3 kb的启动子DNA片段, 并将此1.3 kb DNA片段克隆至pGL2虫荧光素酶报告质粒(pGL2-basic, Promega)Sma I和Xho I位点, 从而形成了mfg12启动子/虫荧光素酶报告质粒Pfg12-LUC. 5'端缺失mfg12启动子/虫荧光素酶报告质粒的构建方法为以PM166为模板, 扩增5'端缺失mfg12启动子DNA片段, 然后将其克隆到pCR 2.1载体, 再一次亚克隆到pGL2虫荧光素酶报告质粒Xho I和HindIII位点. 呼吸道合胞病毒(RSV)-半乳糖甘酶(-Gal)表达质粒购自Promega公司. 突变体: 使用Dnasis软件对嗜肝性MHV (MHV-3, 序列号M35254和MHV-A59) 和非嗜肝性MHV (MHV-2和MHV-JHM, 序列号M25875) N蛋白氨基酸序列进行了比较(表1). 根据QuikChangTM (stratagene) 定点诱变试剂盒操作指导设计上下游引物, 以MHV-A59 N基因表达载体为模板进行扩增制备相应N基因突变体, 其氨基酸突变位点和引物序列见表2. 以上质粒均经测序鉴定无误. 分别将N蛋白1 L或I蛋白表达质粒或N 蛋白突变体表达质粒pGL2-basic或pfgl2-LUC DNA野生型启动子虫荧光酶报告质粒0.5 g以及-gal基因DNA 0.25 g溶于 OPTI-DMEM培养液100 L, 后与OPTI-DMEM培养液100 L混合(含lipofectAMINETM 3.5 L), 将混合物置于室温中孵育30 min. 向混合物中加入OPTI-DMEM培养液1.8 mL使总量达2 mL. 将上述混合物按每孔1 mL的体积加入六孔平板上汇合率达50%-80% 的CHO细胞内, 37 ℃ 50mL/L CO2条件培养5 h, 换20 g/L FBS DMEM 培养液, 44-48 h后, 收集细胞并经液氮冻融3次, 取上清进行-gal和LUC活性测定.
| * G12S | * P38 del | P38L * | * NQN 40-42 del | ||||
| MHV-A59 | 1 | MSFVPGQENA | GGRSSSVNRA | GNGILKKTTW | ADQTERGPNN | QNRGRRNQPK | 50 |
| MHV-3 | G | G | |||||
| MHV-2 | S | G | - - - - | H | |||
| MHV-JHM | S | G | L | K | |||
| *E85Q | |||||||
| MHV-A59 | 51 | QTATTQPNSG | SVVPHYSWFS | GITQFQKGKE | FQFAEGQGVP | IANGIPASEQ | 100 |
| MHV-3 | E | ||||||
| MHV-2 | A | Q | S | ||||
| MHV-JHM | Q | Q | |||||
| MHV-A59 | 101 | KGYWYRHNRR | SFKTPDGQQK | QLLPRWYFYY | LGTGPHAGAS | YGDSIEGVFW | 150 |
| MHV-3 | S | ||||||
| MHV-2 | E | ||||||
| MHV- JHM | H | E | |||||
| MHV-A59 | 151 | VANSQADTNT | RSDIVERDPS | SHEAIPTRFA | PGTVLPQGFY | VEGSGRSAPA | 200 |
| MHV-3 | NS | S | |||||
| MHV-2 | SQ K | TA V | K | K | |||
| MHV-JHM | SQ E R | SA | |||||
| MHV-A59 | 201 | SRSGSRSQSR | GPNNRARSSS | NQRQPASTVK | PDMAEEIAAL | VLAKLGKDAG | 250 |
| MHV-3 | A | ||||||
| MHV-2 | |||||||
| MHV-JHM | P | ||||||
| MHV-A59 | 251 | QPKQVTKQSA | KEVRQKILNK | PRQKRTPNKQ | CPVQQCFGKR | GPNQNFGGSE | 300 |
| MHV-3 | |||||||
| MHV-2 | T | ||||||
| MHV-JHM | P | ||||||
| *V321A | |||||||
| MHV-A59 | 301 | MLKLGTSDPQ | FPILAELAPT | VGAFFFGSKL | ELVKKNSGGA | DEPTKDVYE | 350 |
| MHV-3 | |||||||
| MHV-2 | PS | ||||||
| MHV-JHM | A | G | |||||
| MHV-A59 | 351 | QYSGAVRFDS | TLPGFETIMK | VLNENLNAYQ | K-DGGADVVS | PKPQRKGRRQ | 400 |
| MHV-3 | _ | ||||||
| MHV-2 | I | T | DQA SV L | P R | |||
| MHV-JHM | NQ | RGTK | |||||
| MHV-A59 | 401 | AQEKKDEVDN | VSVAKPKSSV | QRNVSRELTP | EDRSLLAQIL | DDGVVPDGLE | 450 |
| MHV-3 | |||||||
| MHV-2 | L | ||||||
| MHV-JHM | QKAQ | ||||||
| MHV-A59 | 451 | DDSNV* .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. .. | |||||
| 500 MHV-3 | |||||||
| MHV-2 | |||||||
| MHV-JHM | |||||||
| Mutant Name | Mutation | Location of Mutation | Sense Primer (5'-3')/Antisense Primer (5'-3') |
| A59G12S | Gly12 to Ser12 | Domain I | CCT GGG CAA GAA AAT GCC GGT AGC AGA AGC TCC TCT G/ |
| C AGA GGA GCT TCT GCT ACC GGC ATT TTC TTG CCC AGG | |||
| A59P38L | Pro38 to Leu38 | Domain I | GAC CAA ACC GAG CGT GGA CTA AAT AAT CAA AAT AGA GGC/ |
| GCC TCT ATT TTG ATT ATT TAG TCC ACG CTC GGT TTG GTC | |||
| A59P38del | Pro38 deletion | Domain I | GCT GAC CAA CAA ACC GAG CGT GGA CCA AAT AAT CAA AAT AGA GGC AGA AGG/ |
| CCT TCT GCC TCT ATT TTG ATT ATT TGG TCC ACG CTC GGT TTG TTG GTC AGC | |||
| A59NQN | Asn40-Gln41- | Domain I | GG GCT GAC CAA ACC GAG CGT GGA CCA AAT (AAT CAA AAT) AGA GGC AGA AGG |
| 40-42del | Asn42 deletion | AAT CAG CCA AAG CAG ACT GC | |
| GC AGT CTG CTT TGG CTG ATT CCT TCT TCC TCT (ATT TTG ATT) ATT TGG TCC ACG | |||
| CTC GGT TTG TTC AGC CC | |||
| A59E85Q | Glu85 to Gln85 | Domain I | GGA AAG GAG TTT CAG TTT GCA CAG GGA CCA GGA GTG CCT ATT GCC |
| GGC AAT AGG CAC TCC TGG TCC CTG AGC AAA CTG AAA CTC CTT TCC | |||
| A59V321A | Val321 to Ala321 | Domain II | GCA GAG TTG GCT CCA ACA CCT GGT GCC GGT GCC TTC TTC GG |
| CC GAA GAA GGC ACC GGC ACC AGG TGT TGG AGC CAA CTC TGC |
1.2.1 前凝血质活性: 感染MHV及突变株的巨噬细胞(MOI=2.5)在含有100 mL/L胎牛血清和200 mmol/L谷胺酰胺的RPMI 1640中培养8 h,未感染的巨噬细胞和MHV-3感染的巨噬细胞分别作为阴性和阳性对照.采用单相凝集试验(one-stage clotting assay)[10] ,测定mfgl2凝血酶原酶的功能(PCA).
1.2.2 Western-blot鉴定I蛋白的表达: 转染24 h后, 向培养孔内加Triton裂解液 [pH 7.8 100 mmol/L磷酸钾, 2 mL/L Triton X-100, 1 mmol/L DTT(临用前新鲜加入)], 收集裂解液上清, 用邻硝基苯--D-半乳糖苷(ONPG)于420 nm处进行比色分析-gal活性, 以判断转染效率. 收集另设平衡实验孔转染细胞, 凝胶加样缓冲液裂解后进行SDS-PAGE (150 g/L 聚丙烯酰胺凝胶)电泳. 半干法将蛋白质从SDS聚丙烯凝胶转移至硝酸纤维素膜(NC膜)上, 丽春红S染色确认蛋白质已转移到NC膜上, 蒸馏水漂洗脱色以备免疫分析. 将NC膜置于封闭液中于平缓摇动的摇床平台上室温温育2 h. 弃去封闭液, 加入兔抗MHV I蛋白mAb(1: 200), 平缓摇动的摇床平台上室温温育1 h. PBS洗涤10 min ×5次. 加入辣根过氧化物酶标记山羊抗兔IgG (1: 1 500), 摇床平台上室温温育1 h观察结果.
统计学处理 采用ANOVA统计顾问软件(sigmastat advisary statistical software, jandel corporation) 对统计资料进行单向方差分析.
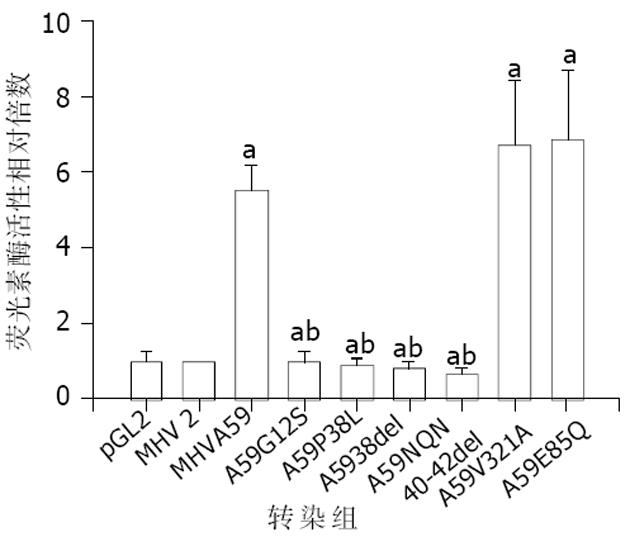
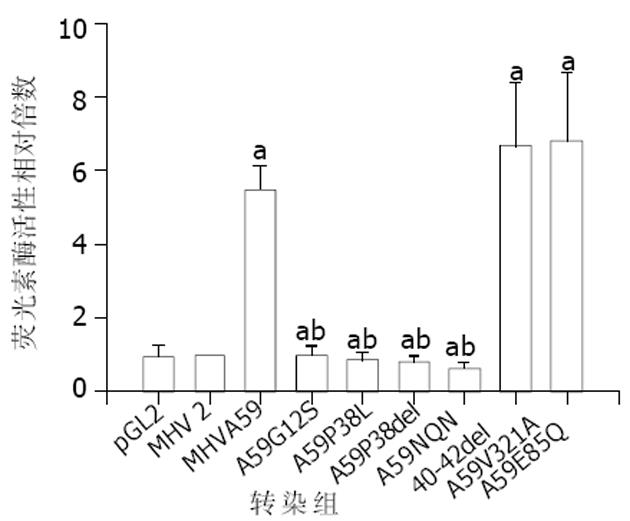
我们比较了嗜肝性MHV(MHV-A59, MHV-3)与非嗜肝MHV(MHV-2, MHV-JHM)的N蛋白氨基酸序列(表1), 并对差异部分进行了系列定点突变. 瞬时转染试验显示, 嗜肝性MHV N蛋白I区有4个位点(Gly12, Pro38, Asn40, Gln41, Asn42)经突变后(表2), 其激活mfgl2启动子的转录活性降为水平线(图1), 而N蛋白Ⅱ区突变体对mfg12的转录活性没有明显下降, 表明嗜肝性MHV N蛋白I区为激活mfg12凝血酶原酶基因的功能区域.
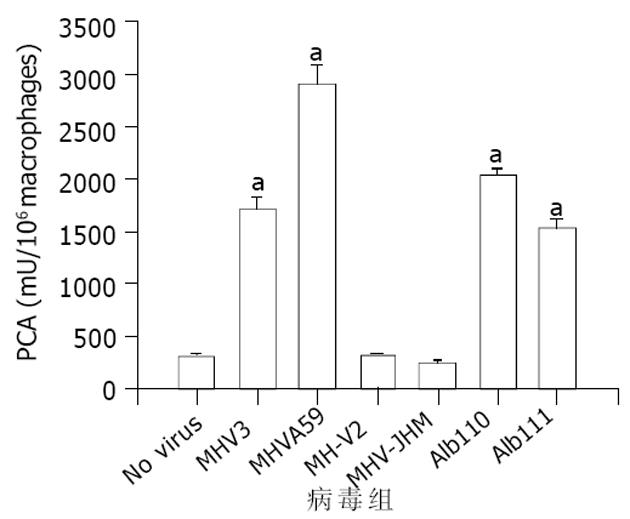
我们将MHV-A59 I 基因突变病毒株A1b110及其同基因型野生株A1B111感染Ba1b/cJ小鼠腹腔巨噬细胞并检测PCA活性. 结果显示, A1b 110 与A1B 111对小鼠巨噬细胞PCA活性的诱导程度无显著差异, 与MHV-A59、MHV-3组亦无显著差异(图2)提示I 基因可能对嗜肝性MHV N蛋白激活mfg12启动子的作用无影响.
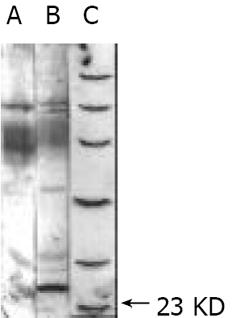
我们克隆了MHV-A59 I 基因并构建了其表达载体. 并在CHO细胞进行了I基因的真核表达. 采用特异性I蛋白单克隆抗体进行Western-blot显示一特异性的23 KD蛋白条带, 见图3箭头处.
应用SPSS统计学分析软件对3组相对荧光素酶活性倍数进行单因素方差分析, 结果I蛋白组与pCR3.1空质粒组比较P>0.05 (0.234), 与N蛋白组比较P<0.05 (0.038), N蛋白组与pCR3.1空质粒组比较P<0.01 (0.005). 说明I蛋白对mfgl2基因转录活性无显著作用(图4).
我国重症乙型肝炎发病率高, 临床治疗手段有限, 其分子致病机制不甚明了 [11-12] . MHV-3或MHV-A59感染引起的小鼠病毒性肝炎模型已被认为是研究人类重症肝炎发病机制的良好工具[13-14]. mfgl2、hfgl2凝血酶原酶的组织特异性表达已被证明在MHV-3引起的鼠暴发型肝功能衰竭和人类重症乙型肝炎的发病中起关键作用[1-2,15]. MHV-3 N 蛋白通过肝脏特异性转录因子肝核因子4 (HNF4)诱导mfgl2/fibroleukin基因的表达[4,16]. MHV-A59 N蛋白与MHV-3 N蛋白仅有1 个氨基酸的差异, 均可特异性地上调mfgl2基因表达[2-3], 因此亦是进行嗜肝性MHV相关研究的代表病毒株. 嗜肝性和非嗜肝性MHV N蛋白均可进入被感染的细胞核内, 提示MHV介导的mfgl2转录激活与嗜肝性MHV N蛋白特异性性状有关[17-19]. N蛋白I区和II区富含几种碱性氨基酸残基: lys, arg和his. 对MHV-A59, MHV-3, MHV-JHM和MHV-2的N蛋白氨基酸序列分析初步显示这些碱性氨基酸残基可形成簇状结构(至少有两个簇), 由可变长度间隔区隔开, 单独或协同作用均有可能为核定位信号. 已知MHV N蛋白II区主要作用是与病毒基因组结合, 而I区和III区的功能尚不明了[20-21].
为确定嗜肝性MHV N蛋白对激活mfgl2表达发挥关键性作用之氨基酸功能域, 将mfgl2启动子/LUC报告载体和MHV-A59 N基因野生型表达质粒或其氨基酸定点突变质粒共转染到CHO细胞, 结果显示位于N蛋白I区的氨基酸残基与mfgl2转录激活有关(图1); 而II区氨基酸残基突变则不影响. mfgl2转录活性提示N蛋白I区的Gly12, Pro38, Asn40, Gln41, Asn42氨基酸残基很可能形成一个基元 (motif), 在mfgl2转录激活过程中发挥重要作用. 关于这些残基如何相互作用尚有待MHV N蛋白晶体结构的明确以深入研究.
I蛋白是一个23 ku的疏水多肽, 由一个嵌入MHV N基因5 端的开放阅读框编码, 这个内在的基因被称为I基因, 其序列也已确证[22-24]. 目前已经发现在MHV感染的细胞上均有I蛋白的表达, 实验表明I蛋白并非MHV在组织培养或天然宿主体内复制所必须的结构蛋白, 但I基因沉寂病毒的噬斑直径要明显小于同基因的野生株病毒噬斑直径, 表明I基因的表达利于MHV病毒的生长[9]. 为阐明N基因内含I基因是否参与调节mfgl2的作用, 我们将I基因突变病毒株Alb110及其野生株Alb111体外感染Balb/cJ小鼠巨噬细胞, 检测其PCA活性, 将I基因表达载体与mfgl2荧光素酶报告质粒共转染以期明确I蛋白在mfgl2基因激活中的作用, 结果显示I蛋白非mfgl2基因激活的必备因素, 进一步论证了嗜肝性病毒株MHV-3和MHV-A59之N蛋白是促进前凝血质活性提高、诱导mfgl2凝血酶原酶基因转录激活的病毒因素. 已知丙型肝炎核心蛋白对某些宿主基因的调节作用[25-27], 我们的结果对人类乙型和丙型肝炎病毒核心蛋白调节宿主基因转录的研究具重要的指导意义.
目前累及全球28个国家和地区的Urbani SARS相关冠状病毒, 经测序核实其序列与Ⅱ类冠状病毒其中包括MHV在基因序列同源性最大[28-30], 目前尚未见到有关其发病机制、传播与防治动物模型的文献报道. 我们在MHV-3领域成功的科研经验对探讨Urbani SARS相关冠状病毒及其所致疾病的基础研究和防治有重要的借鉴意义.
感谢胡锦裳女士协助本文文字和图表处理!
编辑: N/A
| 1. | Ding JW, Ning Q, Liu MF, Lai A, Leibowitz J, Peltekian KM, Cole EH, Fung LS, Holloway C, Marsden PA. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J Virol. 1997;71:9223-9230. [PubMed] |
| 2. | 陈 悦, 宁 琴, 王 宝菊, 张 东绅, 严 福明, 孙 奕, 习 东, 严 伟明, 郝 连杰, Levy G. 重症乙型肝炎人纤维介素基因的表达 及意义. 中华医学杂志. 2003;83:446-450. |
| 3. | Ning Q, Liu M, Kongkham P, Lai MM, Marsden PA, Tseng J, Pereira B, Belyavskyi M, Leibowitz J, Phillips MJ. The nucleocapsid protein of murine hepatitis virus type 3 induces transcription of the novel fgl2 prothrombinase gene. J Biol Chem. 1999;274:9930-9936. [PubMed] [DOI] |
| 4. | 宁 琴, 罗 小平, 汪 之沫, 韩 梅芳, 严 伟明, 刘 铭锋, Levy G. Mfgl2凝血酶原酶/fibroleukin基因转录调控元件HNF4的研究. 中华医学杂志. 2003;83:678-683. |
| 5. | Lai MM, Cavanagh D. The molecular biology of coronaviruses. Adv Virus Res. 1997;48:1-100. [PubMed] [DOI] |
| 6. | Cologna R, Spagnolo JF, Hogue BG. Identification of nucleocapsid binding sites within coronavirus-defective genomes. Virology. 2000;277:235-249. [PubMed] [DOI] |
| 7. | Narayanan K, Kim KH, Makino S. Characterization of N protein self-association in coronavirus ribonucleoprotein complexes. Virus Res. 2003;98:131-140. [PubMed] [DOI] |
| 8. | Wu HY, Guy JS, Yoo D, Vlasak R, Urbach E, Brian DA. Common RNA replication signals exist among group 2 coronaviruses: evidence for in vivo recombination between animal and human coronavirus molecules. Virology. 2003;315:174-183. [PubMed] [DOI] |
| 9. | Fischer F, Peng D, Hingley ST, Weiss SR, Masters PS. The internal open reading frame within the nucleocapsid gene of mouse hepatitis virus encodes a structural protein that is not essential for viral replication. J Virol. 1997;71:996-1003. [PubMed] |
| 10. | Ning Q, Brown D, Parodo J, Cattral M, Gorczynski R, Cole E, Fung L, Ding JW, Liu MF, Rotstein O. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487-3493. [PubMed] |
| 11. | 冯 志华, 聂 青和, 白 宪光, 白 雪帆, 周 永兴, 贾 战生, 郝 春秋. 膜式血浆置换治疗慢性重症肝炎肝功能衰竭疗效. 世界华人消化杂志. 2002;10:638-641. [DOI] |
| 13. | Jodie H, Stanley P. Mouse hepatitis virus. Cur Opin Microbiol. 2001;4:462-466. [DOI] |
| 14. | Liu MF, Chan CWY, McGilvray ID, Ning Q, Levy GA. Fulminant viral hepatitis. Molecular and cellular basis, and clinical implications. Exp Rev Mol Med. 2001;1-19. |
| 15. | Marsden PA, Ning Q, Fung LS, Luo X, Chen Y, Mendicino M, Ghanekar A, Scott JA, Miller T, Chan CW. The Fgl2/fibroleukin prothrombinase contributes to immunologically mediated thrombosis in experimental and human viral hepatitis. J Clin Invest. 2003;112:58-66. [PubMed] [DOI] |
| 16. | Ning Q, Lakatoo S, Liu M, Yang W, Wang Z, Phillips MJ, Levy GA. Induction of prothrombinase fgl2 by the nucleocapsid protein of virulent mouse hepatitis virus is dependent on host hepatic nuclear factor-4 alpha. J Biol Chem. 2003;278:15541-15549. [PubMed] [DOI] |
| 17. | Wurm T, Chen H, Hodgson T, Britton P, Brooks G, Hiscox JA. Localization to the nucleolus is a common feature of coronavirus nucleoproteins, and the protein may disrupt host cell division. J Virol. 2001;75:9345-9356. [PubMed] [DOI] |
| 18. | Hiscox JA, Wurm T, Wilson L, Britton P, Cavanagh D, Brooks G. The coronavirus infectious bronchitis virus nucleoprotein localizes to the nucleolus. J Virol. 2001;75:506-512. [PubMed] [DOI] |
| 19. | Brockway SM, Clay CT, Lu XT, Denison MR. Characterization of the expression, intracellular localization, and replication complex association of the putative mouse hepatitis virus RNA-dependent RNA polymerase. J Virol. 2003;77:10515-10527. [PubMed] [DOI] |
| 20. | Masters PS, Parker MM, Ricard CS, Duchala C, Frana MF, Holmes KV, Sturman LS. Structure and function studies of the nucleocapsid protein of mouse hepatitis virus. Adv Exp Med Biol. 1990;276:239-246. [PubMed] [DOI] |
| 21. | Parker MM, Masters PS. Sequence comparison of the N genes of five strains of the coronavirus mouse hepatitis virus suggests a three domain structure for the nucleocapsid protein. Virology. 1990;179:463-468. [PubMed] [DOI] |
| 22. | Lapps W, Hogue BG, Brian DA. Sequence analysis of the bovine coronavirus nucleocapsid and matrix protein genes. Virology. 1987;157:47-57. [PubMed] [DOI] |
| 23. | Homberger FR. Sequence analysis of the nucleoprotein genes of three enterotropic strains of murine coronavirus. Arch Virol. 1995;140:571-579. [PubMed] [DOI] |
| 24. | Kunita S, Mori M, Terada E. Sequence analysis of the nucleocapsid protein gene of rat coronavirus SDAV-681. Virology. 1993;193:520-523. [PubMed] [DOI] |
| 25. | Lai MM, Ware CF. Hepatitis C virus core protein: possible roles in viral pathogenesis. Curr Top Microbiol Immunol. 2000;242:117-134. [PubMed] [DOI] |
| 26. | McLauchlan J. Properties of the hepatitis C virus core protein: a structural protein that modulates cellular processes. J Viral Hepat. 2000;7:2-14. [PubMed] [DOI] |
| 27. | Webster G, Barnes E, Brown D, Dusheiko G. HCV genotypes--role in pathogenesis of disease and response to therapy. Baillieres Best Pract Res Clin Gastroenterol. 2000;14:229-240. [PubMed] [DOI] |
| 28. | Poutanen SM, Low DE, Henry B, Finkelstein S, Rose D, Green K, Tellier R, Draker R, Adachi D, Ayers M. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995-2005. [PubMed] [DOI] |
| 29. | Drosten C, Günther S, Preiser W, van der Werf S, Brodt HR, Becker S, Rabenau H, Panning M, Kolesnikova L, Fouchier RA. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967-1976. [PubMed] [DOI] |
| 30. | Ksiazek TG, Erdman D, Goldsmith CS, Zaki SR, Peret T, Emery S, Tong S, Urbani C, Comer JA, Lim W. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953-1966. [PubMed] [DOI] |