修回日期: 2004-06-10
接受日期: 2004-06-17
在线出版日期: 2004-11-15
目的: 根据证据医学的原理探讨消化系肿瘤术后r-hGH联合低热量肠外营养的安全性、有效性及对预后的影响.
方法: 将消化系肿瘤患者100例双盲、随机分为受试组和对照组, 受试组术后给予低热量肠外营养6 d, 同时sc生长激素7 d, 对照组接受传统TPN治疗6 d; 对比观察术后两组患者血浆蛋白质水平、氮平衡、免疫功能状况、感染有关并发症、住院时间、术后生存率和复发率.
结果: 在进行6 d的肠外营养以后, 两组患者生命体征、血常规、肝肾功能、血脂和全身副反应均无显著性差异; 术后7 d, 受试组蛋白质水平恢复至术前水平, IgG、IgA、IgM和CD3、CD4下降不明显(P>0.05); 对照组术后10 d蛋白质水平才恢复至术前水平, IgG、IgM、CD3和CD4下降明显(IgG: P = 0.02<0.05; IgM: P = 0.04<0.05; CD3: P = 0.02<0.05; CD4: P = 0.03<0.05.); 术后7 d、10 d, 对照组前清蛋白、转铁蛋白、纤维连接蛋白浓度、IgG、IgM 、CD3和CD4均显著低于受试组; 受试组氮平衡明显优于对照组, 术后住院时间显著低于对照组(P = 0.03<0.05); 术后1, 2和3年生存率、复发率两组患者差异无显著性(P>0.05).
结论: 对于能手术切除的消化系肿瘤, 术后短期合理剂量使用生长激素联合低热量肠外营养, 是安全、有效的, 有利于患者术后的恢复.
引文著录: 刘权焰, 薛平惠, 刘志苏. 消化系肿瘤术后应用生长激素联合低热量肠外营养的随机分组研究. 世界华人消化杂志 2004; 12(11): 2651-2654
Revised: June 10, 2004
Accepted: June 17, 2004
Published online: November 15, 2004
AIM: To investigate the role of recombinant human growth hormone (r-hGH) and hypocaloric parenteral nutrition (HPN) for postoperative patients with gastrointestinal cancer.
METHODS: A prospective, randomized and double-blinded clinical trial was performed. One hundred postoperative patients with gastrointestinal cancer were randomly divided into two groups: research group (n = 50) and control group (n = 50). Patients in research group were given HPN (6 d) plus r-hGH (7 d, sc) and patients in control group were treated with short-time standard TPN. The level of serum protein, immune function, nitrogen balance, infection related complications, hospital stay, survival rate and relapse rate were compared between the two groups.
RESULTS: The serum protein returned to pre-operative level in research group after 7 days, and the levels of IgG, IgA, IgM, CD3 and CD4 were not significantly different from those before operation. The serum protein returned to pre-operative level in control group after 10 days, and the levels of IgG, IgM, CD3 and CD4 significantly decreased (IgG: P = 0.02 < 0.05; IgM: P = 0.04 < 0.05; CD3: P = 0.02 < 0.05; CD4: P = 0.03 < 0.05). Concentrations of pre-albumin (Pr), transferring (T) and fibronectin (F) after 10 days were significantly higher in research group than those in control group (Pr: 0.25 ± 0.07g/L vs 0.18 ± 0.06 g/L, P < 0.01; T: 2.4 ± 0.54 g/L vs 2.05 ± 0.61 g/L, P < 0.05; F: 2.72 ± 0.44 g/L vs 2.38 ± 0.89 g/L, P < 0.05). Nitrogen balance in research group was better than that in controls. Hospital stay was significantly shorter in research group than that in controls (P = 0.03 < 0.05). The 1-, 2- and 3-year survival rates and relapse rate were not significantly different between the two groups.
CONCLUSION: For patients with resectable gastrointestinal tumor, it is safe and beneficial to use short-term of r-hGH plus HPN after operation in order to accelerate recovery.
- Citation: Liu QY, Xue PH, Liu ZS. Impact of postoperative treatment with recombinant human growth hormone and hypocaloric parenteralnutrition on gastrointestinal cancer. Shijie Huaren Xiaohua Zazhi 2004; 12(11): 2651-2654
- URL: https://www.wjgnet.com/1009-3079/full/v12/i11/2651.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i11.2651
消化道肿瘤术后早期, 患者处于应激状态, 其体内代谢紊乱, 胰岛素抵抗导致营养素利用障碍, 传统的营养支持不能有效地逆转其高分解代谢状态[1-3]. 因此, 许多学者试图通过代谢支持和调理来降低分解代谢, 促进合成代谢, 从而减轻损伤, 提高组织修复能力, 有利于机体康复. 近年来, 生长激素(r-hGH)在这一领域的作用受到越来越多的重视. 研究表明, 生长激素联合低热量肠外营养通过调控细胞因子作用, 调整激素水平及代谢调理来截止肌肉和内脏蛋白的大量丢失, 从而保护脏器功能[4-8]. 但是, 应用r-hGH特别是短期大剂量应用r-hGH是否会刺激、促进实体肿瘤的生长和复发, 是目前国内外学者最为关注的问题[9-11]. 据此, 我们根据循证医学的原理探讨消化道肿瘤术后r-hGH联合低热量肠外营养的安全性、有效性及对预后的影响.
我院2000-01/2000-12消化系肿瘤患者100例, 其中肝癌21例, 胆管癌4例, 胰腺癌6例, 胃癌35例, 结肠癌20例, 直肠癌14例. 术前均有明确的诊断和手术指征, 术后经病理学证实. 且满足下列条件: 无明显的心、肺、肝功能衰竭, 无甲亢、糖尿病等代谢性疾病, 无全身感染, 免疫缺陷病, 且无肿瘤远处转移. 所有病例分为受试组和对照组, 每组各50例, 每例患者均知情同意.
1.2.1 研究设计: 本研究为随机双盲、有对照的临床研究. 研究时间从2000-01/2003-12, 采用由武汉大学医学院公共卫生学院提供的随机表, 总表内容不让临床医师和患者知道. 每一个入选患者准备营养液, 该序号也出现在患者的处方和输液袋上.
1.2.2 营养配方: 营养液均在超净TPN室配制. 于术后1 d开始给药, 连续6 d. 受试组给予以下营养支持: 每日热量62.9-83.9 kJ/kg, 氮量为0.12-0.15 g/kg, 热氮比为100-120:1, PN能源由25%葡萄糖和20%脂肪乳剂提供, 糖脂能量比为6:4, 氮源选择85 g/L的乐凡命(SSPC产品)和支链氨基酸溶液, 适量补充维生素及电解质; 在提供非蛋白热卡时, 降低葡萄糖用量, 增加脂肪乳剂供能, 但以脂肪供能不超过总量的40%为宜. 术后1 d开始应用r-hGH(Saizen)8 IU/d, 于08:00和20:00分2次皮下注射使用, 连续用药7 d. 对照组接受传统TPN治疗, 每日热量为105 kJ/kg, 氮量为0.2-0.25 g/kg.
1.2.3 患者一般情况: 两组患者的年龄、性别、手术前的体重及病因差异无显著性意义(表1).
| 分组 | n | 年龄/a | 男 | 女 | 体重/Kg | 肝癌 | 胆管癌 | 胰腺癌 | 结肠癌 | 直肠癌 | 胃癌 |
| 受试组 | 50 | 50.4±10.2 | 28 | 22 | 54.7±10.8 | 12 | 1 | 4 | 11 | 6 | 16 |
| 对照组 | 50 | 51.5±11.8 | 30 | 20 | 53.5±9.7 | 9 | 3 | 2 | 9 | 8 | 19 |
1.2.4 安全性评价: 两组患者均进行血常规, 肝肾功能, 血脂和血糖检查, 并监测患者的生命体征和全身副作用.
1.2.5 替代有效性终点指标: (1)血浆蛋白质水平变化: 两组患者分别于术前、术后7 d、10 d采静脉血测定血浆清蛋白、前清蛋白、转铁蛋白、纤维连接蛋白浓度; (2)氮平衡测定: 手术后2 d至手术后10 d计算氮平衡. 每天收集24 h尿、粪、引流液, 应用微量凯氏定氮法测定氮, 按氮平衡 = 入氮量-(尿氮量+粪氮量+引流物氮量)公式计算; (3)免疫功能状况: 于术后7 d、10 d, 采用静脉血用贝克曼array特定蛋白分析仪测定IgG, IgA, IgM, 用免疫荧光法测定T细胞亚群.
1.2.6 临床预后终点指标: (1)感染有关并发症: 感染性并发症是按照美国胸科医师协会/危重医学协调会(ACCP/SCCM)的定义和标准进行测定; (2)住院时间: 按手术日到出院时间的天数计算; (3)术后生存率和复发率: 从出院日开始计算, 术后1年为3 mo随访一次, 2-3年为6 mo随访1次.
统计学处理 数据以mean±SD表示. 采用SPSS10.0软件, 用方差分析比较两组间计量资料, 计数资料采用χ2检验.
在进行6 d的肠外营养以后, 两组患者生命体征、血常规、肝肾功能、血脂和全身副反应均无显著性差异.
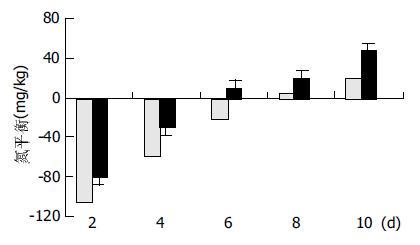
两组患者术前蛋白质水平差别无显著意义(P>0.05). 术后7 d, 受试组蛋白质水平恢复至术前水平, 而对照组术后10 d才恢复至术前水平. 两组间比较, 术后7 d、10 d, 对照组前清蛋白、转铁蛋白、纤维连接蛋白浓度均显著低于受试组(P<0.05, 表2). 受试组氮平衡明显优于对照组, 两组间存在显著差异(P<0.05, 图1).
与术前相比较, 对照组血清IgG, IgM, CD3和CD4水平在术后7 d显著下降, 在术后10 d仍无明显恢复; 受试组在术后7 d, IgG、IgA、IgM和CD3、CD4下降不明显(P>0.05). 两组间比较, 术后7 d、10 d, 对照组IgG、IgM、CD3和CD4均显著低于受试组, 而CD8则高于受试组(表3). 所有患者均未发生切口感染, 但对照组1例直肠癌发生吻合漏, 1例胰头癌发生肺部感染.
| 分组 | IgG(g/L) | IgM(g/L) | IgA(g/L) | CD3% | CD4% | CD8% | |
| 术前 | 受试组 | 14.24±4.05 | 3.72±1.42 | 2.18±1.01 | 56.45±5.71 | 39.8±3.75 | 26.14±2.48 |
| 对照组 | 13.96±5.18 | 3.58±1.76 | 2.28±1.24 | 54.83±6.41 | 40.75±4.2 | 25.03±3.17 | |
| 术后7d | 受试组 | 13.48±3.01d | 3.64±1.85d | 2.07±2.14 | 53.15±4.32c | 37.29±4.15d | 23.37±4.11 |
| 对照组 | 9.74±4.92b | 2.89±3.16b | 1.95±3.22 | 42.29±4.78a | 30.28±5.17b | 27.58±3.52 | |
| 术后10d | 受试组 | 18.66±5.21d | 4.21±2.31d | 2.26±3.45 | 58.42±5.94d | 42.12±6.29d | 20.15±4.93d |
| 对照组 | 10.19±3.74a | 2.92±2.01a | 2.14±2.73 | 45.25±7.12a | 33.92±5.24b | 29.74±6.12 |
由于恶性肿瘤对氮的需求, 消化道肿瘤患者术前存在不同程度的营养不良. 手术创伤后应激期蛋白分解代谢必然会进一步加剧蛋白质的分解. 然而, 传统的肠外营养虽提供了充足的热量和底物, 却不能有效地逆转其高分解代谢状态, 促进蛋白质合成[12]. 为追求正氮平衡而带来的高氮量、高热量输注往往适得其反, 反而会加重患者的代谢负担, 引起更多的代谢紊乱[13]. 为了达到既能改善患者的营养状况, 又不致于加重患者的代谢负担, 许多学者希望通过生长激素的代谢调理和代谢支持来改善肿瘤患者异常代谢状态, 促进蛋白质合成, 纠正负氮平衡, 提高机体的免疫防御功能[14-17]. 生长激素主要促进葡萄糖氧化, 从而提高能量水平, 促进脂肪分解及糖异生, 在转录与翻译水平上促进蛋白质合成[18-20]. 研究表明: 单纯低热量肠外营养一般不能使机体达到正氮平衡, 但同时使用生长激素, 则能明显改善氮平衡[13,21]. 我们的研究结果显示, 生长激素有明显的储氮效应, 受试组前清蛋白、转铁蛋白、纤维连接蛋白浓度在术后7 d, 恢复至术前水平, 在术后10 d, 明显高于术前水平; 而对照组直至术后10 d才恢复至术前水平; 且在术后7 d、10 d, 上述蛋白浓度, 受试组显著高于对照组; 氮平衡测定结果亦显示, 受试组氮平衡显著优于对照组, 表明受试组外源性氮得到较好的利用.
消化系肿瘤患者普遍存在免疫功能低下, 机体处于低应答或不应答状态, T淋巴细胞亚群的数量及功能发生异常时, 将直接影响肿瘤的发生、发展及预后. 众多研究表明, 生长激素可刺激机体产生IgG、IgM、IgA, 改善体液免疫功能, 对T、B淋巴细胞, K细胞及巨噬细胞均有调节作用[14,22-23]. 我们的研究显示消化道肿瘤术后7 d, 对照组血清IgG, IgM和CD3, CD4水平显著下降, 术后10 d仍无明显恢复; 受试组IgG, IgA, IgM和CD3, CD4下降不明显, 且在术后10 d较术前有明显升高; 表明生长激素联合低热量肠外营养能改善宿主的细胞免疫和体液免疫功能, 其机制可能与宿主营养状况的改善有关. 术后感染并发症、住院时间, 受试组均显著低于对照组, 进一步表明, 生长激素联合低热量肠外营养可作为消化道肿瘤术后患者较为合理的营养支持.
近来, 应用生长激素, 特别是短期大剂量应用生长激素是否会刺激、促进实体肿瘤的生长和复发, 是目前最为关注问题. 有研究报道, 生长激素对机体代谢, 尤其是蛋白质代谢的作用主要是通过IGF-1受体介导实现的, 绝大多数消化道肿瘤细胞表面有IGF-1受体[24], 因此, 消化道肿瘤术后使用生长激素具有潜在的促肿瘤生长作用[10,24]. 但是, 生长激素对机体的作用是多方面的, 肿瘤在体内的发生、发展和转移是多步骤、复杂的. 目前, 临床上还没有证据表明, 生长激素具有刺激肿瘤生长的作用[25-26]; 相反大量临床结果显示, 围手术期使用外源性生长激素是安全的, 他可以保存宿主自体成分, 不会刺激肿瘤生长, 并能降低围手术期并发症的发生率和死亡率.
我们采用证据医学原理, 系随机、双盲、对照、前瞻性研究, 其结果证实消化道肿瘤术后, 生长激素联合低热量肠外营养明显改善了术后营养状况和免疫功能, 降低了感染并发症. 术后生存时间随访结果表明, 外源性生长激素应用与否, 对消化道肿瘤术后宿主生存率和肿瘤复发率没有明显的影响.
编辑: N/A
| 1. | Bozzetti F, Gavazzi C, Ferrari P, Dworzak F. Effect of total parenteral nutrition on the protein kinetics of patients with cancer cachexia. Tumori. 2000;86:408-411. [PubMed] |
| 2. | Nygren J, Thorell A, Brismar K, Essen P, Wernerman J, McNurlan MA, Garlick PJ, Ljungqvist O. Glucose flux is normalized by compensatory hyperinsulinaemia in growth hormone-induced insulin resistance in healthy subjects, while skeletal muscle protein synthesis remains unchanged. Clin Sci (Lond). 2002;102:457-464. [DOI] |
| 3. | Bozzetti F, Gavazzi C, Cozzaglio L, Costa A, Spinelli P, Viola G. Total parenteral nutrition and tumor growth in malnourished patients with gastric cancer. Tumori. 1999;85:163-166. [PubMed] |
| 4. | Wolf RF, Pearlstone DB, Newman E, Heslin MJ, Gonenne A, Burt ME, Brennan MF. Growth hormone and insulin reverse net whole body and skeletal muscle protein catabolism in cancer patients. Ann Surg. 1992;216:280-8; discussion 288-90. [PubMed] [DOI] |
| 5. | Wong WK, Soo KC, Nambiar R, Tan YS, Yo SL, Tan IK. The effect of recombinant growth hormone on nitrogen balance in malnourished patients after major abdominal surgery. Aust N Z J Surg. 1995;65:109-113. [DOI] |
| 6. | Vara-Thorbeck R, Ruiz-Requena E, Guerrero-Fernández JA. Effects of human growth hormone on the catabolic state after surgical trauma. Horm Res. 1996;45:55-60. [PubMed] [DOI] |
| 7. | Hammarqvist F, Sandgren A, Andersson K, Essén P, McNurlan MA, Garlick PJ, Wernerman J. Growth hormone together with glutamine-containing total parenteral nutrition maintains muscle glutamine levels and results in a less negative nitrogen balance after surgical trauma. Surgery. 2001;129:576-586. [PubMed] [DOI] |
| 8. | Carroll PV, Jackson NC, Russell-Jones DL, Treacher DF, Sönksen PH, Umpleby AM. Combined growth hormone/insulin-like growth factor I in addition to glutamine-supplemented TPN results in net protein anabolism in critical illness. Am J Physiol Endocrinol Metab. 2004;286:E151-E157. [PubMed] [DOI] |
| 9. | Ruokonen E, Takala J. Dangers of growth hormone therapy in critically ill patients. Curr Opin Clin Nutr Metab Care. 2002;5:199-209. [PubMed] [DOI] |
| 11. | Bartlett DL, Charland S, Torosian MH. Growth hormone, insulin, and somatostatin therapy of cancer cachexia. Cancer. 1994;73:1499-1504. [DOI] |
| 12. | Irgau I, Fulda GJ. Effects of growth hormone on nitrogen balance in the hypermetabolic state: a selected review of the literature. Del Med J. 1993;65:767-772. [PubMed] |
| 13. | Manson JM, Smith RJ, Wilmore DW. Growth hormone stimulates protein synthesis during hypocaloric parenteral nutrition. Role of hormonal-substrate environment. Ann Surg. 1988;208:136-142. [PubMed] [DOI] |
| 14. | Vara-Thorbeck R, Guerrero JA, Rosell J, Ruiz-Requena E, Capitán JM. Exogenous growth hormone: effects on the catabolic response to surgically produced acute stress and on postoperative immune function. World J Surg. 1993;17:530-7; discussion 537-8. [PubMed] [DOI] |
| 15. | Bjarnason R, Wickelgren R, Hermansson M, Hammarqvist F, Carlsson B, Carlsson LM. Growth hormone treatment prevents the decrease in insulin-like growth factor I gene expression in patients undergoing abdominal surgery. J Clin Endocrinol Metab. 1998;83:1566-1572. [PubMed] [DOI] |
| 16. | Bozzetti F, Gavazzi C, Miceli R, Rossi N, Mariani L, Cozzaglio L, Bonfanti G, Piacenza S. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24:7-14. [PubMed] [DOI] |
| 17. | Jackson NC, Carroll PV, Russell-Jones DL, Sönksen PH, Treacher DF, Umpleby AM. Effects of glutamine supplementation, GH, and IGF-I on glutamine metabolism in critically ill patients. Am J Physiol Endocrinol Metab. 2000;278:E226-E233. [PubMed] |
| 18. | Zhang X, Li J, Li N. Growth hormone improves graft mucosal structure and recipient protein metabolism in rat small bowel transplantation. Chin Med J (Engl). 2002;115:732-735. |
| 19. | Kolstad O, Jenssen TG, Ingebretsen OC, Vinnars E, Revhaug A. Combination of recombinant human growth hormone and glutamine-enriched total parenteral nutrition to surgical patients: effects on circulating amino acids. Clin Nutr. 2001;20:503-510. [PubMed] [DOI] |
| 20. | O'Leary MJ, Koll M, Ferguson CN, Coakley JH, Hinds CJ, Preedy VR, Garlick PJ. Liver albumin synthesis in sepsis in the rat: influence of parenteral nutrition, glutamine and growth hormone. Clin Sci (Lond). 2003;105:691-698. [PubMed] [DOI] |
| 21. | Carli F, Webster JD, Halliday D. A nitrogen-free hypocaloric diet and recombinant human growth hormone stimulate postoperative protein synthesis: fasted and fed leucine kinetics in the surgical patient. Metabolism. 1997;46:796-800. |
| 22. | Liu W, Jiang Z, Wang X, Shu H, Cui W, Wilmore DW. Impact of perioperative treatment of recombinant human growth hormone on cell immune function and intestinal barrier function: randomized, double-blind, controlled trial. World J Surg. 2003;27:412-415. [PubMed] [DOI] |
| 23. | Shim M, Cohen P. IGFs and human cancer: implications regarding the risk of growth hormone therapy. Horm Res. 1999;51 Suppl 3:42-51. [PubMed] |
| 24. | Mercadante S. Parenteral versus enteral nutrition in cancer patients: indications and practice. Support Care Cancer. 1998;6:85-93. [PubMed] [DOI] |