修回日期: 2004-08-23
接受日期: 2004-08-30
在线出版日期: 2004-10-15
目的: 研究腺病毒感染成体肝干细胞介导外源基因表达的可行性及建立稳定表达EGFP 的成体肝干细胞.
方法: 采用细菌同源重组法构建CMV驱动的EGFP报告基因腺病毒载体pAd-CMV-EGFP, 在293细胞中包装制备腺病毒, 并用重组腺病毒感染体外培养的成体肝干细胞WB-F344, 观察腺病毒携带的外源基因EGFP 在WB-F344细胞中的表达, 及分析病毒对肝干细胞的感染效率. 进一步单克隆培养纯化建立稳定表达EGFP 的细胞株(WB-EGFP), 并采用流式细胞技术、免疫细胞化学技术、诱导分化实验以观察其生物学特性.
结果: 成功构建腺病毒载体, 并包装制备高滴度的重组腺病毒Ad-EGFP. 重组腺病毒有效的感染WB-F344细胞, 感染率约40-70%.建立WB-EGFP细胞株, 持续传代8-9代均较稳定表达EGFP及肝干细胞表面标记, 仍保持干细胞样的增生、分化等基本生物学特性.
结论: 腺病毒可高水平的介导外源基因表达于成体肝干细胞, 是有效的基因转移系统. 稳定表达EGFP的肝干细胞株观察直观、可连续传代, 并保持干细胞的生物学特性, 可用于体内外肝细胞研究中的追踪、鉴定.
引文著录: 钟晓刚, 何生, 殷舞, 邓靖宇, 陈波. 腺病毒介导增强型绿色荧光蛋白基因在大鼠成体肝干细胞中的表达. 世界华人消化杂志 2004; 12(10): 2341-2344
Revised: August 23, 2004
Accepted: August 30, 2004
Published online: October 15, 2004
AIM: To investigate the feasibility of adenoviral-mediated exogenous gene expression in adult liver stem cells of rats and to establish a cell line that stably and efficiently express enhanced green fluorescence protein (EGFP).
METHODS: A pAd-CMV-EGFP vector under the control of CMV promoter was constructed by homologous recombination in E.coilBJ 5 183, and the recombinant virus was Packaged in HEK 293 cell line. Hepatic adult stem cells cultured in vitro were infected with recombinant adenovirus. Expression of EGFP was observed by fluorescent microscopy and infection efficiency was analyzed. Adult liver stem cells were further cultured to estabilish a cell line that stably and efficiently expressed EGFP through cloning culture and the biological characteristics of the cell line were observed and analyzed by fluorescence microscopy, immunocytochemistry and differentiation-inducing experiment.
RESULTS: Adenovirus vector of pAd-CMV-EGFP was constructed and high titer recombinant virus were produced successfully. EGFP, mediated by adenovirus, could be transfected into hepatic adult stem cells with a high efficiency (about 40-70%). After cloning culture, WB-EGFP cell line was established, and it could stably express EGFP in 8-9 generations. Furthermore, biological characteristics such as marker of stem cells, proliferation speed and differentiation capability had not been affected.
CONCLUSION: Target gene can be efficiently transfected into hepatic adult stem cells through adeno-vector system. EGFP can be stably and long-term expressed in transfected cells and their offspring. It can serve as a tracker in the research of stem cells.
- Citation: Zhong XG, He S, Yin W, Deng JY, Chen B. Adenoviral-mediated efficiency expression of enhanced green fluorescence protein in adult liver stem cells of rats. Shijie Huaren Xiaohua Zazhi 2004; 12(10): 2341-2344
- URL: https://www.wjgnet.com/1009-3079/full/v12/i10/2341.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v12.i10.2341
在大多数成年机体器官中有干细胞, 即成体干细胞. 成年哺乳类动物肝脏中存在的肝干细胞, 又称肝卵圆细胞, 以小卵圆形细胞的形式分布于终末小胆管(Hering管), 具有双向分化增生潜力, 可分化为肝细胞或胆管上皮细胞[1-4]. 然而, 肝干细胞在肝脏的多种生理和病理过程中的作用还远未阐明. 增强型绿色荧光蛋白(enhanced green fluorescent protein, EGFP)基因是在GFP的基础上经碱基修饰改造而来, 是一种较好的报告基因, 广泛用于多种基因转移系统[5-7]. 采用EGFP标记成体肝干细胞, 来探索其在体内分化、发育规律, 以及与肝硬化、肝癌等肝脏疾病的关系有重要的意义. 因此, 我们采用重组腺病毒转染大鼠成体肝干细胞、克隆纯化, 使其高效稳定表达EGFP, 并对其生物学特性进行了观察研究, 同时也为成体肝干细胞基因转移修饰提供可用的模式.
大鼠成体肝卵圆细胞株WB-F344(简称WB细胞), 由美国北卡罗来那大学Grisham教授建立[8-9], 购自中国医学科学院药物研究所. 质粒pAdEasy-1为缺失E1区和E3区5型野生型腺病毒(Ad5dE1/3)基因组质粒, 带有氨苄青霉素抗性基因. 穿梭质粒pAdTack-CMV在CMV启动子驱动下表达增强型绿色荧光蛋白(EGFP), 带有卡那霉素抗性基因[10]. E.Coli菌种BJ5183由生化研究室李明博士惠赠, DH5a菌种及293细胞由病理研究室提供, 引自美国典型生物物种保藏中心(ATCC). 限制性内切酶PmeⅠ、PacⅠ,购自NEB公司, Lamda DNA/HindⅢ , BamHⅠ购自 Takara公司, 小量质粒DNA抽提纯化试剂盒购自美国Sigma公司, 转染脂质体Lipofect AMINE, Opti-MEM(OMEM)购自美国Invitrogen公司. 其他试剂为国产分析纯.
WB细胞生长于含100 mL/L胎牛血清、25 mmol/L Hepes、100 ku/L青霉素与100 mg/L链霉素的DMEM/F-12(1:1)培养基, 在37 ℃、50 mL/L CO2、饱和湿度条件下培养. 参照文献[11-12]用两步法构建重组腺病毒: 超螺旋的腺病毒骨架质粒pAdEasy-1转化重组菌BJ5183, 经氨苄青霉素筛选鉴定后, 用氯化钙法制备感受态细菌BJ5183pAdEasy-1. 将含有EGFP报告基因的穿梭质粒pAdTrack-CMV用限制性内切酶PmeI酶切线性化, 转化感受态细菌BJ5183pAdEasy-1, 线性化质粒pAdTrack-CMV与pAdEasy-1在细菌内发生同源重组. 重组质粒经筛选及PacI酶切鉴定正确后, 转入大肠杆菌DH5a扩增获得高拷贝重组质粒pAd-CMV-EGFP. PacI酶切线性化重组质粒pAd-CMV-EGFP, 用脂质体转染法转染人胚肾293细胞包装腺病毒. 10-12 d收获病毒, 再感染扩增纯化提高病毒滴度. 调整病毒滴度, 以0.1 , 1, 10, 100, 200, 1 000感染复数(MOI)感染60-80%融合的WB细胞, 37℃孵育24 h后, 换新的培养基培养. 荧光显微镜下观察EGFP的表达, 分析转染效率. 采用有限稀释法选择荧光表达强的单克隆, 传代培养, 建立稳定表达GFP的细胞株WB-EGFP[13]. 按1.0×107/L接种细胞于24孔板, 接种后0-9 d, 每天终止3孔, 消化后用台盼蓝拒染法计数, 取均值, 绘成生长曲线. 收集对数生长期细胞, 制备单细胞悬液, 碘化丙啶(PI)染色, 流式细胞仪作细胞周期分析.用丁酸钠(sodium butyrate, SB)或二甲亚砜(dexamethasone, DMSO)诱导肝细胞分化[14-15]. 按1.0×108/L 接种WB, WB-EGFP细胞于6孔板, 24 h后加入2-4 mol/L浓度丁酸钠或1-4 umol/L二甲亚砜, 以等量PBS液代替丁酸钠或二甲亚砜作为对照, 观察细胞形态学改变. 制备细胞爬片, 免疫细胞化学染色检测细胞中c-kit, ck19, AFP, a1-ATT表达.
统计学处理 所有数据以(mean±SD)表示, 采用SPSS医学统计学软件分析, 两组间均数差异的比较采用t检验.
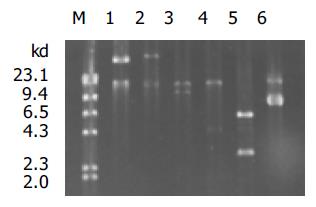
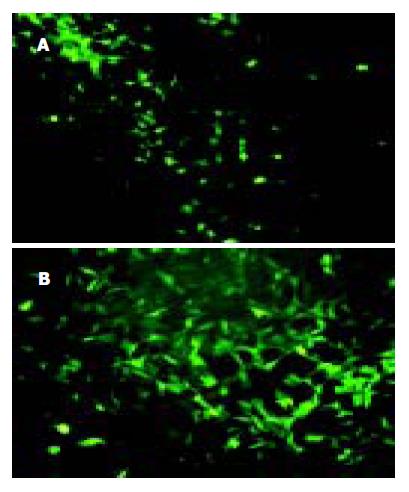
穿梭质粒pAdTrack-CMV和腺病毒质粒 pAdEasy-1经同源重组生成重组质粒pAd-CMV-EGFP, PacI酶切产生特异性酶切条带, 表明重组腺病毒载体正确(图1). Pac I酶切线性化重组质粒pAd-CMV-EGFP, 用脂质体转染法转染人胚肾293细胞包装腺病毒. 转染后4, 5 d, 293细胞内可见明显绿色荧光表达(图2). 7-10 d收集裂解细胞获得病毒, 经再感染293细胞扩增、浓缩, 病毒滴度可达到109 nfu/L原液. 该病毒命名为Ad-EGFP.
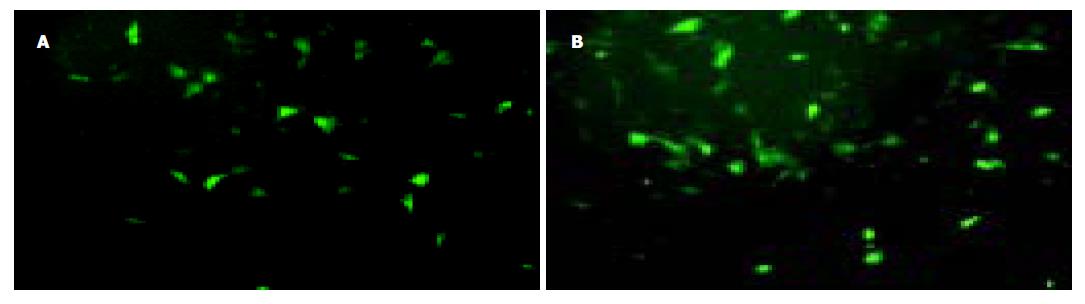
重组腺病毒Ad-EGFP均可感染WB细胞, 感染12 h后可见荧光表达, 24 h后明显(图3). 1, 10, 100感染复数(MOI)感染效率较高, 约40-70%, 荧光显微镜下细胞形态正常. 感染复数大于100时, 90%的WB细胞可表达绿色荧光蛋白, 但细胞出现明显的细胞病理现象, 细胞存活和贴壁功能严重受损. 采用有限稀释法挑取形态正常、表达EGFP强的单克隆细胞, 传代培养, 获得比较稳定表达EGFP的细胞株(WB-EGFP). 持续的传代培养, 细胞表达EGFP能够维持8-9代(42 d).
有限稀释法单克隆培养得到转染细胞株WB-EGFP, 生长状态良好, 细胞形态一致, 生长曲线与WB细胞相似. 碘化丙啶(PI)染色, 流式细胞仪作细胞周期分析, 两种细胞均主要分布在G1期, 统计学分析无显著差异(P >0.05), 表明WB-EGFP细胞生长特性与WB细胞基本一致, EGFP的表达并不影响肝干细胞的生长、增生(表1).
| 组别 | G0/G1 | S | G2/M |
| WB | 61.7±1.7 | 19.9±4.1 | 18.6±2.1 |
| WB-EGFP | 62.0±2.1 | 19.6±3.8 | 18.3±2.3 |
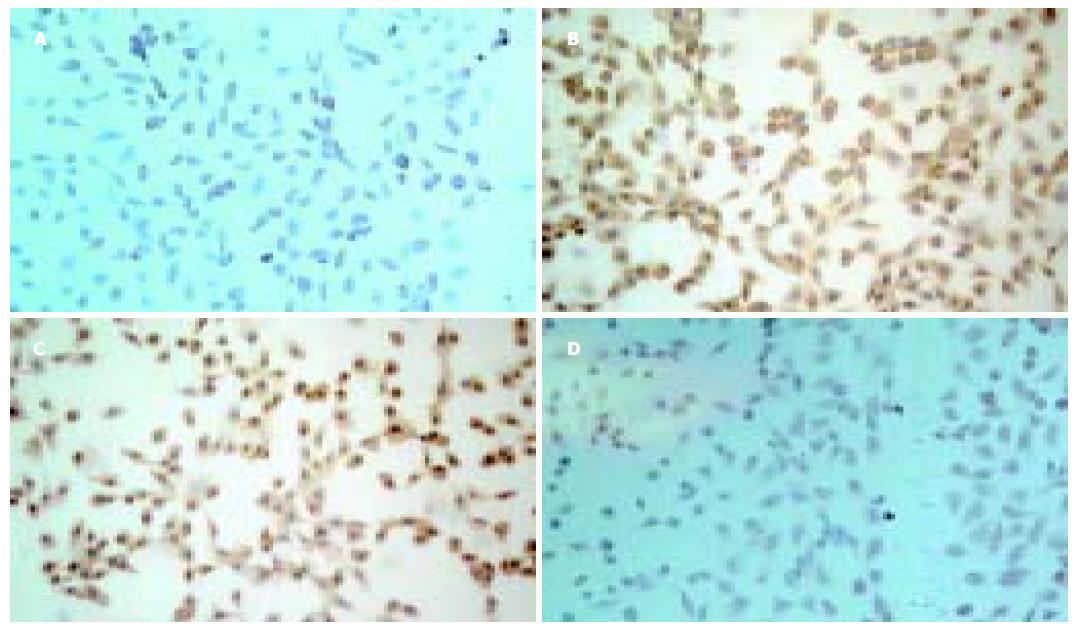
未分化的WB及WB-EGFP细胞, 均表达干细胞的生物标志, 免疫细胞化学染色c-kit(+), AFP(+), ck19(+), a1-ATT(-). 2-3 mmol/L浓度丁酸钠(SB)或1-2 mmol/L二甲亚砜(DMSO)作用3-4 d, 均可诱导WB及WB-EGFP细胞向肝细胞分化, 卵圆细胞开始减少, 出现核大、核仁清晰、胞质丰富的多角上皮细胞. 更高浓度的丁酸钠或二甲亚砜导致细胞存活受损. 分化的细胞形态向肝细胞转变, 免疫细胞化学染色c-kit(-), AFP(-), ck19(-), a1-ATT(+)(图4). 表明WB-EGFP细胞仍具有向特异性细胞分化的干细胞特性, 腺病毒转染并不影响干细胞的生物特性.
胚胎或成体肝干细胞被认为是一种理想的替代治疗细胞和外源基因转移的靶细胞, 而成体肝干细胞因不涉及伦理问题, 目前是干细胞研究的热点[16-20]. 成体肝干细胞移植不但可替代坏死肝组织, 还可刺激肝组织再生修复. 同时通过体外基因修饰肝干细胞作为基因治疗的良好载体为基因治疗提供新的可能[21-22]. 其在体内如何分布、分化、转归, 如何参与肝损伤修复中肝小叶的重建, 机体的微环境对移植肝干细胞的调控, 以及肝干细胞在相关肝病中的作用均未阐明.
增强型绿色荧光蛋白(enhanced green fluorescent protein, EGFP)基因是一种较好的报告基因, 在紫外光或荧光的照射下能自发发出绿色荧光, 观察直观, 易于追踪[23-24]. 我们采用EGFP标记成体肝干细胞, 可较好的在体内、外对其追踪、鉴定, EGFP表达的强度高、时间较长, 同时肝干细胞增生能力、特有的生物特性并未受到影响.
腺病毒不仅对分裂期细胞能进行基因转染, 而且对一般非分裂细胞或增生缓慢的细胞也可进行有效的基因转染, 转移的基因并不整合到细胞基因组中, 而目的基因的表达水平高, 持续时间也较长, 近年来腺病毒载体在体内基因治疗中日益受到重视[25].与肿瘤细胞等分裂增生快的细胞相比, 成体肝干细胞分裂增生速度相对更慢, 一般的物理或化学转染的方法转移基因较为困难[26]. 转染后使成体肝干细胞较稳定表达转移基因产物而不影响干细胞的特性, 也有待进一步研究. 因而我们采用腺病毒作为基因转移的载体, 观察到腺病毒载体Ad-EGFP对肝干细胞的转导效率高, MOI=1-100的重组病毒即可有效的转染, 转染率在40-70%, 稳定转染的肝干细胞连续传代8-9代仍有转移基因的表达, 细胞增生、分化能力保持稳定. 只有当MOI大于100时, 可导致细胞出现病态改变. 表明在一定的感染复数范围腺病毒可有效的对成体肝干细胞进行基因转导, 重组腺病毒是一种有效转染肝干细胞的载体工具.
本转基因方法简单、高效, 所克隆转染细胞具有连续传代、稳定性好、高水平表达EGFP, 并可进行活细胞直接观察的优点, 同时转染的肝干细胞仍具有干细胞的基本生物学特性, 为肝干细胞体内外增生、分化调控、信号转导、基因修饰的研究提供一个有力的工具.
| 1. | Alison MR, Vig P, Russo F, Bigger BW, Amofah E, Themis M, Forbes S. Hepatic stem cells: from inside and outside the liver? Cell Prolif. 2004;37:1-21. [PubMed] [DOI] |
| 2. | Qin AL, Zhou XQ, Zhang W, Yu H, Xie Q. Characterization and enrichment of hepatic progenitor cells in adult rat liver. World J Gastroenterol. 2004;10:1480-1486. [PubMed] [DOI] |
| 3. | Strain AJ, Crosby HA, Nijjar S, Kelly DA, Hubscher SG. Human liver-derived stem cells. Semin Liver Dis. 2003;23:373-384. [PubMed] [DOI] |
| 4. | Wang X, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Grompe M. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100 Suppl 1:11881-11888. [PubMed] [DOI] |
| 5. | Le LP, Everts M, Dmitriev IP, Davydova JG, Yamamoto M, Curiel DT. Fluorescently labeled adenovirus with pIX-EGFP for vector detection. Mol Imaging. 2004;3:105-116. [PubMed] [DOI] |
| 6. | Magness ST, Jijon H, Van Houten Fisher N, Sharpless NE, Brenner DA, Jobin C. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-kappa B activation using a novel gene-targeted enhanced GFP reporter gene mouse. J Immunol. 2004;173:1561-1570. [PubMed] [DOI] |
| 7. | Magin-Lachmann C, Kotzamanis G, D'Aiuto L, Cooke H, Huxley C, Wagner E. In vitro and in vivo delivery of intact BAC DNA -- comparison of different methods. J Gene Med. 2004;6:195-209. [PubMed] [DOI] |
| 8. | Muller-Borer BJ, Cascio WE, Anderson PA, Snowwaert JN, Frye JR, Desai N, Esch GL, Brackham JA, Bagnell CR, Coleman WB. Adult-derived liver stem cells acquire a cardiomyocyte structural and functional phenotype ex vivo. Am J Pathol. 2004;165:135-145. [PubMed] [DOI] |
| 9. | Chramostová K, Vondrácek J, Sindlerová L, Vojtesek B, Kozubík A, Machala M. Polycyclic aromatic hydrocarbons modulate cell proliferation in rat hepatic epithelial stem-like WB-F344 cells. Toxicol Appl Pharmacol. 2004;196:136-148. [PubMed] [DOI] |
| 10. | Pan X, Li ZS, Xu GM, Cui L, Tu ZX. Adenovirus-mediated gene transfer in the treatment of pancreatic cancer. Pancreas. 2003;26:274-278. [PubMed] [DOI] |
| 11. | Zheng Q, Huang ZH, Tang FX, Huang YY, Che XY. [Highly efficient construction of recombinant adenovirus containing double suicide gene driven by cytomegalovirus promoter using two-step CaCl2 transformation method]. Diyi Junyi Daxue Xuebao. 2003;23:575-577. [PubMed] |
| 12. | Green AP, Huang JJ, Scott MO, Kierstead TD, Beaupré I, Gao GP, Wilson JM. A new scalable method for the purification of recombinant adenovirus vectors. Hum Gene Ther. 2002;13:1921-1934. [PubMed] [DOI] |
| 13. | Masamune A, Satoh M, Kikuta K, Suzuki N, Shimosegawa T. Establishment and characterization of a rat pancreatic stellate cell line by spontaneous immortalization. World J Gastroenterol. 2003;9:2751-2758. [PubMed] [DOI] |
| 14. | Rambhatla L, Chiu CP, Kundu P, Peng Y, Carpenter MK. Generation of hepatocyte-like cells from human embryonic stem cells. Cell Transplant. 2003;12:1-11. [PubMed] [DOI] |
| 15. | Azuma H, Hirose T, Fujii H, Oe S, Yasuchika K, Fujikawa T, Yamaoka Y. Enrichment of hepatic progenitor cells from adult mouse liver. Hepatology. 2003;37:1385-1394. [PubMed] [DOI] |
| 16. | Suzuki A, Nakauchi H, Taniguchi H. In vitro production of functionally mature hepatocytes from prospectively isolated hepatic stem cells. Cell Transplant. 2003;12:469-473. [PubMed] [DOI] |
| 17. | Poulsom R, Alison MR, Forbes SJ, Wright NA. Adult stem cell plasticity. J Pathol. 2002;197:441-456. [PubMed] [DOI] |
| 18. | Yin L, Sun M, Ilic Z, Leffert HL, Sell S. Derivation, characterization, and phenotypic variation of hepatic progenitor cell lines isolated from adult rats. Hepatology. 2002;35:315-324. [PubMed] [DOI] |
| 19. | Zheng YW, Taniguchi H. Diversity of hepatic stem cells in the fetal and adult liver. Semin Liver Dis. 2003;23:337-348. [PubMed] [DOI] |
| 21. | Weber A. Immortalization of hepatic progenitor cells. Pathol Biol (Paris). 2004;52:93-96. [PubMed] [DOI] |
| 23. | Pierce EA, Liu Q, Igoucheva O, Omarrudin R, Ma H, Diamond SL, Yoon K. Oligonucleotide-directed single-base DNA alterations in mouse embryonic stem cells. Gene Ther. 2003;10:24-33. [PubMed] [DOI] |
| 24. | Del Rio M, Larcher F, Serrano F, Meana A, Muñoz M, Garcia M, Muñoz E, Martin C, Bernad A, Jorcano JL. A preclinical model for the analysis of genetically modified human skin in vivo. Hum Gene Ther. 2002;13:959-968. [PubMed] [DOI] |
| 25. | Cheng K, Fraga D, Zhang C, Kotb M, Gaber AO, Guntaka RV, Mahato RI. Adenovirus-based vascular endothelial growth factor gene delivery to human pancreatic islets. Gene Ther. 2004;11:1105-1116. [PubMed] [DOI] |
| 26. | Lakshmipathy U, Pelacho B, Sudo K, Linehan JL, Coucouvanis E, Kaufman DS, Verfaillie CM. Efficient transfection of embryonic and adult stem cells. Stem Cells. 2004;22:531-543. [PubMed] [DOI] |