修回日期: 2002-11-10
接受日期: 2002-11-13
在线出版日期: 2003-06-15
用反义环氧合酶-2(COX-2)重组载体转染COX-2高表达人胆管癌细胞QBC939, 观察其对QBC939细胞的生长抑制作用, 并探讨其作用机制.
通过脂质体介导将COX-2反义核酸质粒转入QBC939细胞, 经G418筛选获得稳定表达COX-2反义核酸的胆管癌转染细胞模型. 采用逆转录聚合酶链式反应(RT-PCR)技术检测转染前后QBC939细胞COX-2 mRNA表达的变化, 应用免疫细胞化学链霉亲合素-生物素复合物(SABC)技术检测转染前后QBC939细胞COX-2蛋白表达水平的变化, 应用四唑氮蓝(MTT)比色法检测COX-2反义核酸对QBC939细胞增生的影响, 应用流式细胞仪测定细胞周期和细胞凋亡.
反义COX-2基因转染后胆管癌细胞中COX-2 mRNA表达水平明显下调, COX-2蛋白表达减弱. 转染反义COX-2重组载体的QBC939细胞生长速率明显下降(P<0.01), 细胞周期分析转染后细胞比转染前细胞增生指数显著下降(P<0.01), S期细胞比例为9.27±1.91%, 比转染前(16.35±2.87%)明显降低(P<0.05), G0/G1期细胞比例为75.16±4.13%, 比转染前(57.31±10.16%) 明显上升(P<0.05), 细胞被抑制在G0/G1期; COX-2反义核酸转染对QBC939细胞凋亡率无明显影响(P>0.05).
COX-2反义核酸导入可抑制QBC939细胞增生.
引文著录: 吴高松, 武小勇, 邹声泉, 裘法祖. 环氧合酶-2反义核酸对人胆管癌细胞增生的影响. 世界华人消化杂志 2003; 11(6): 733-736
Revised: November 10, 2002
Accepted: November 13, 2002
Published online: June 15, 2003
To transfect antisense vector of human COX-2 gene into COX-2 highly expressing cholangiocarcinoma cell line QBC939 and to explore its biological activities and role in carcinogenesis.
QBC939 cells were transfected with antisense vector of human COX-2 gene using LipoVecTM transfecting technique. Transfected cells were selected with G418; COX-2 mRNA was examined by using reverse transcription polymerase chain reaction (RT-PCR) and COX-2 protein expression was detected by immunocytochemistry using isozyme selective antibodies. The proliferative status of transfected cells was measured by using methabenzthiazuron (MTT) assay; Cell cycle and apoptosis was analyzed by using flow cytometry (FCM).
RT-PCR showed a lower COX-2 mRNA level in transfected cells and immunocytochemistry showed weaker COX-2 protein expression in transfected cells. The proliferative index of the transfected cells decreased significantly(P<0.01), the percentage of S phase decreased remarkably in transfected cells(9.27±1.91%) compared with that in QBC939 cells without transfection (16.35±2.87%)(P<0.05), and the percentage of G0/G1 phase increased remarkably in transfected cells (75.16±4.13%) compared with that in QBC939 cells without transfection (57.31±10.16%)(P<0.05). Transfection with antisense vector of human COX-2 gene had no significant influence on the apoptosis in QBC939 cells (P>0.05).
Transfection with antisense vector of human COX-2 gene is able to inhibit the proliferation of human cholangiocarcinoma QBC939 cells.
- Citation: Wu GS, Wu XY, Zou SQ, Qiu FZ. Effects of cyclooxygenase-2 antisense vector on proliferation of human cholan-giocarcinoma cells. Shijie Huaren Xiaohua Zazhi 2003; 11(6): 733-736
- URL: https://www.wjgnet.com/1009-3079/full/v11/i6/733.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i6.733
COX-2除了在炎症反应中起作用外, 还与癌症的发生有关[1-3]. COX-2表达上调与胆管癌的形成关系密切[4]. 我们通过脂质体介导将反义COX-2重组载体转染COX-2高表达人胆管癌细胞QBC939, 观察COX-2反义核酸对QBC939细胞的生长抑制作用, 并探讨其作用机制, 为 COX-2反义核酸用于胆管癌的治疗提供实验依据.
人胆管癌细胞系QBC939由第三军医大学西南医院王曙光教授[5]建系并惠赠, 我室保存; RPMI1640培养基及胎牛血清购自Gibco公司; 胆管癌QBC939细胞在含10 mL/L胎牛血清的培养基中置于37 °C, 50 mL/L CO2孵育箱常规培养; 人COX-2反义重组质粒载体pcDNA3.1/hCOX2(-)由第四军医大学西京医院吴开春教授[6]构建并惠赠; 空载体pcDNA3.1由我室保存. LipoVecTM脂质体购自美国InvivoGen公司; RNA提取试剂盒RNA-SOLV为Omega公司产品; PBR322 marker 购自晶美公司; 引物由上海生工公司合成; 兔抗人COX-2多克隆抗体购自北京中山生物技术有限公司(Santa Cruz公司产品); SABC试剂盒和DAB显色剂购自武汉博士德公司.
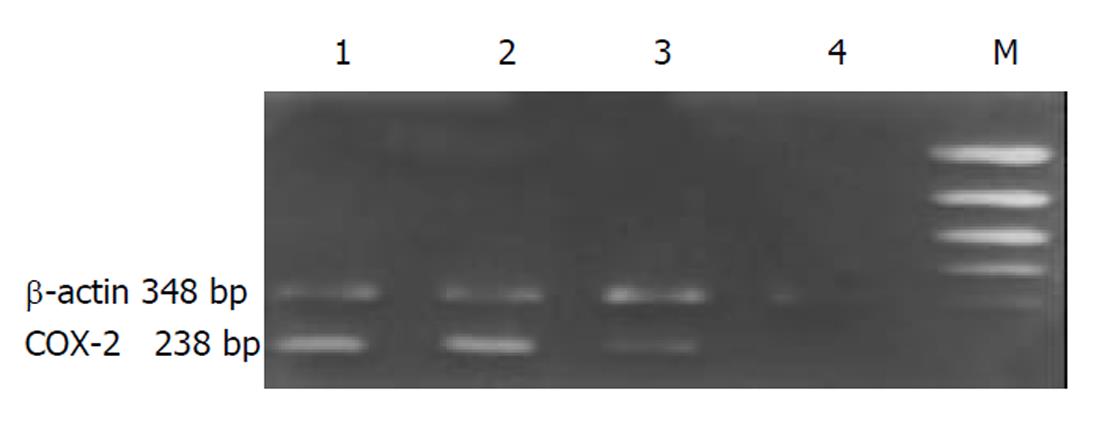
取pcDNA3.1/hCOX2(-)质粒或pcDNA3.1质粒3 μg加入(或不加任何质粒作空白对照)LipoVecTM脂质体100 μL中混匀, 室温下作用30 min配置脂质体与质粒复合物. 以每孔5×105个细胞接种在6孔板中, 贴壁后用20倍体积含血清培养基稀释的上述复合物常规培养3 d. 将各组细胞倍比稀释(1:10, 1:50, 1:250)至24孔板, 换用含G418(300 mg/L)的筛选液继续培养2 wk后, 随机挑选转染组细胞克隆及对照组克隆, 扩大培养, 分别命名为QBC-AS和QBC-P. 采用RT-PCR方法检测转染细胞COX-2 mRNA表达水平的变化. COX-2上游引物: 5'-ACAATGCTGACTATGGCTAC-3', 下游引物: 5'-AACTGATGCGTGAAGTGCTG-3', 扩增产物238 bp; 内参照β-actin上游引物: 5'-CATCACCATTGGCAATGAGCG-3', 下游引物: 5'-CTAGAAGCATTTGCGGTCGGAC-3', 扩增产物348 bp. 对数生长期细胞传代培养3 d后收集细胞. 细胞总RNA的提取按一步法RNA提取试剂RNA-SOLV说明书进行. 取1/10样品测定RNA浓度及纯度后用于RT-PCR扩增. cDNA的合成: 冰上混合RNA样品2 μL及随机六聚体引物1.25 μL, 70 °C 5 min后, 加入逆转录酶缓冲液4 μL, RNAsin 0.5 μL及10 mmol/L dNTP 1.25 μL, 25 °C 5 min, 37 °C 60 min, 70 °C加热10 min终止反应. PCR反应体系: cDNA 10 μL, 10×Taq酶缓冲液2 μL, 10 mmol/L dNTP 0.4 μL, 25 mmol/L MgCl2 0.8 μL, 上下游引物各1 μL及Taq酶1 μL, 补水至50 μL. PCR反应条件: 94 °C 45 s, 58 °C 30 s, 72 °C 60 s, 共30次循环. 最后, 72 °C 3 min. 取扩增产物10 μL, 在15 g/L琼脂糖凝胶上进行电泳分离, 紫外光照下以计算机图像分析系统成像和定量分析, 以COX-2/β-actin蛋白的吸光度比值表示COX-2 mRNA表达水平. 用2.5 g/L胰蛋白酶消化对数生长期肿瘤细胞, 制成单细胞悬液, 接种在盖玻片上, 置孵育箱中培养, 待细胞贴壁后取出, 用PBS清洗2次, 4 °C冷丙酮固定10 min后取出自然干燥, -20 °C冰箱保存, 进行免疫细胞化学SABC染色. 具体方法参见文献[4]. 每次实验均设阴性和阳性对照. 用PBS代替一抗作阴性对照, 用已知阳性白片人结肠癌作阳性对照. MTT比色试验: 用2.5 g/L胰蛋白酶消化对数生长期肿瘤细胞, 制成单细胞悬液. 以每孔1.0×104个细胞接种于96孔培养板. 置于37 °C 50 mL/L CO2 孵育箱中分别培养1, 2, 3或4 d后每孔加入MTT(5 g/L)20 μL, 孵育4 h, 弃上清, 加入150 μL DMSO, 振荡5 min使结晶物充分溶解, 在酶联免疫检测分析仪上测定A490 nm 值. 以只加培养液不加细胞的阴性对照孔调零, 每种样品设3个复孔, 实验重复3次. 收集培养2 d的QBC-AS和QBC-P肿瘤细胞, 胰酶充分消化, 800 r/m 离心5 min, -20 °C预冷的80%乙醇固定. 在流式细胞仪上作细胞周期和凋亡分析, 实验重复3次.
统计学处理 计量数据以mean±SD表示. 采用SPSS统计软件包的t检验, P<0.05被认为有显著性意义.
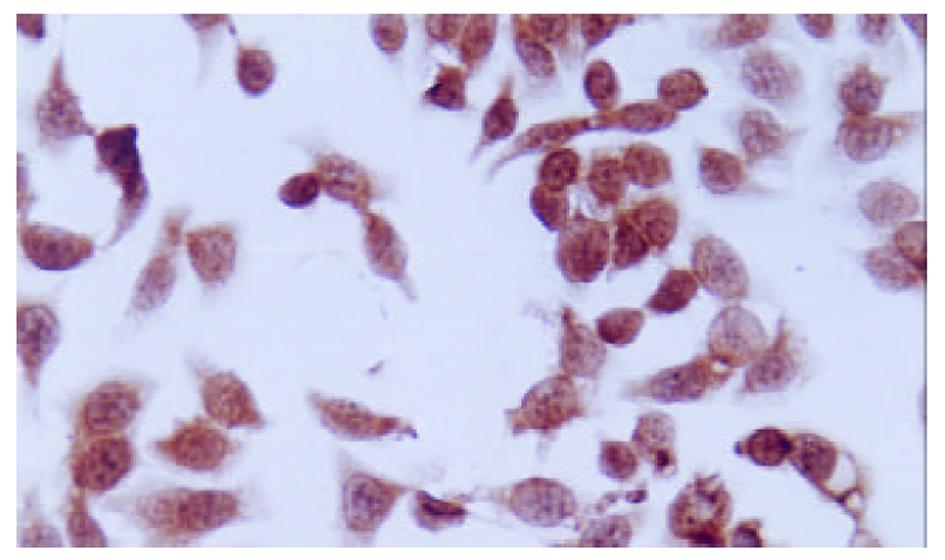
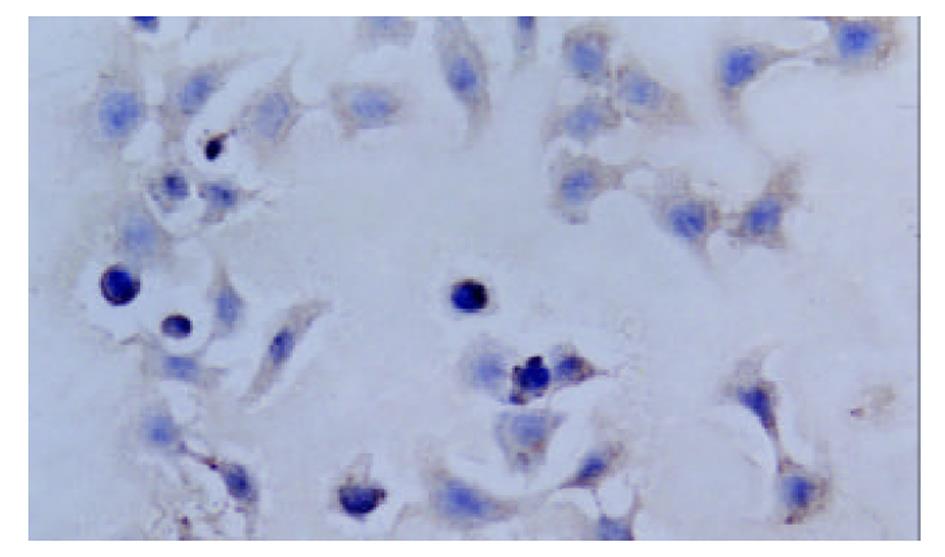
QBC-AS细胞COX-2 mRNA表达水平与QBC-P细胞及QBC939细胞相比明显下调, QBC-P细胞和QBC939细胞中COX-2 mRNA表达水平无明显变化(图1). COX-2阳性表达表现为胞质或胞核出现棕黄色颗粒. QBC939细胞表达呈强阳性, 胞质与胞核均有表达, COX-2反义基因转染后的QBC-AS细胞表达呈弱阳性, 主要为胞质着色(图2, 图3).
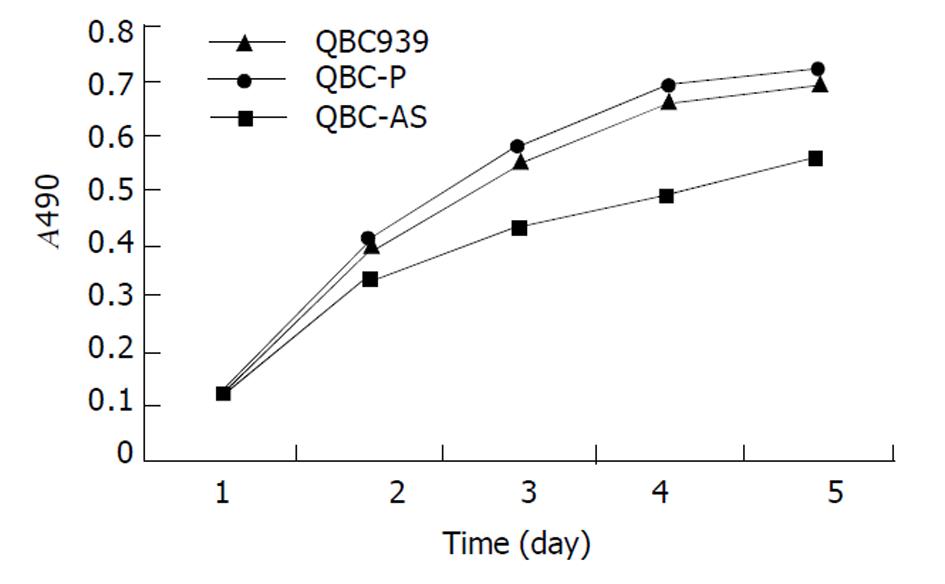
分别在培养1, 2, 3, 4和5 d测细胞密度, 绘制生长曲线(图4). QBC-AS细胞生长速率明显低于对照组QBC-P细胞和QBC939细胞, 而QBC-P细胞和QBC939细胞生长速率无明显变化. QBC-AS组S期细胞为0.09±0.02, 比对照QBC939组(0.16±0.03)明显降低(P<0.05), QBC-AS组G0/G1期细胞为0.75±0.04, 比QBC939组(0.57±0.10) 显著增高(P<0.01), 增生指数明显降低(P<0.01), 增生指数(PI)用(S+G2/M)%×100表示, QBC-AS组和QBC939组细胞增生指数分别为22.6±9.0和41.8±10.1; 细胞凋亡分析显示COX-2反义基因转染不影响细胞凋亡(P>0.05), QBC-AS组和QBC939组细胞凋亡率分别为4.0±0.9和2.8±0.2.QBC-P组与QBC939组细胞周期和细胞凋亡无明显差异(P>0.05).
近年研究表明, COX-2不仅是炎症反应的中心环节, 而且在很多肿瘤组织中表达上调, 与肿瘤的发生与发展关系密切[7-22]. COX-2基因表达的蛋白被认为是一种原癌蛋白(prooncogenic protein)[23]. Uefuji et al[24]用RT-PCR方法检测了37例胃腺癌的COX-2 mRNA水平, 他们的研究结果表明mRNA水平与肿瘤大小有关, pT2-pT4期胃癌COX-2 mRNA水平明显高于pT1期胃癌, 提示COX-2可能促进胃腺癌的进展. COX-1则普遍被认为与肿瘤的发生与发展无关, 只有极少数报道提示COX-1有与COX-2类似的作用. Narko et al[25]报道通过上调COX-1表达水平可使在体外培养的永生内皮细胞ECV向肿瘤细胞转化; Marks et al[26]在小鼠皮肤鳞状细胞癌模型的研究中发现COX-1表达缺陷鼠的致瘤率和形成肿瘤的体积比野生型小鼠明显降低. COX-2促进肿瘤的发生、发展的作用机制包括: 提高促进肿瘤血管生长的因子VEGF的表达水平; 通过催化花生四烯酸代谢的产物血栓素A2、PGE2和PGI2直接刺激诱导肿瘤血管形成; 通过激活Bcl-2或Akt 而阻止内皮细胞的凋亡[27]; Tsujii et al[28]报道, 结构性COX-2的表达能导致结直肠癌细胞表型的变化, 从而引起结直肠癌细胞转移潜能的改变. 流行病学研究表明, 长期服用阿司匹林或其他非甾体类抗炎药的人群结肠癌、直肠癌、胃肠癌及肺癌的发生率较低[29-31].
胆管癌被称为21世纪癌王, 目前还没有有效的治疗方法. 胆管癌发病的高危因素有: 胆管结石; 原发性硬化性胆管炎; 先天性胆管扩张症, 特别是行囊肿肠管吻合术后以及中华支睾吸虫感染、慢性炎性肠病等, 这些因素中有一个共同的环节: 慢性损伤和炎症. COX-2是炎症反应的中心环节, 因此我们提出一个假说: 胆管癌的发生可能通过COX-2途径. 我们以前的实验表明COX-2在胆管癌组织中为诱导性表达, 其表达上调与胆管癌的形成关系密切[4]. 本实验通过脂质体介导将反义COX-2重组载体转染COX-2高表达人胆管癌细胞QBC939, 观察COX-2反义核酸对QBC939细胞的生长抑制作用. 我们的实验表明, 反义COX-2基因转染后胆管癌细胞中COX-2 mRNA表达水平明显下调, COX-2 蛋白表达减弱.转染反义COX-2重组载体的QBC939细胞生长速率明显下降. 细胞周期分析表明, 反义基因转染后胆管癌细胞被抑制在G0/G1期, G0/G1期细胞比例比转染前明显上升; 反义基因转染后比转染前增生指数显著下降, S期细胞比例比转染前明显降低, 但COX-2反义核酸转染对QBC939细胞凋亡率无明显影响.提示COX-2反义基因转染能改变QBC939细胞的增生状态, 其抑制胆管癌细胞增生作用是通过改变细胞周期而不是通过影响细胞凋亡机制实现的.
| 1. | Yoon JH, Higuchi H, Werneburg NW, Kaufmann SH, Gores GJ. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985-93. [PubMed] [DOI] |
| 2. | Tian G, Yu JP, Luo HS, Yu BP, Yue H, Li JY, Mei Q. Effect of Nimesulide on proliferation and apoptosis of human hepatoma SMMC-7721 cells. World J Gastroenterol. 2002;8:483-487. [PubMed] [DOI] |
| 3. | Cheng J, Imanishi H, Amuro Y, Hada T. NS-398, a selective cyclooxygenase 2 inhibitor, inhibited cell growth and induced cell cycle arrest in human hepatocellular carcinoma cell lines. Int J Cancer. 2002;99:755-761. [PubMed] [DOI] |
| 4. | Wu GS, Wang JH, Liu ZY, Zou SQ. Expression of cyclooxygenase-1 and -2 in extra-hepatic cholangiocarcinoma. HBPD INT. 2002;1:429-433. [PubMed] |
| 6. | 吴 汉平, 吴 开春, 李 玲, 幺 立萍, 兰 梅, 王 新, 樊 代明. 人环氧合酶-2(hCOX-2)编码基因的克隆及其反义核酸转染胃癌细胞的初步研究. 世界华人消化杂志. 2000;8:1211-1217. |
| 7. | Kisley LR, Barrett BS, Bauer AK, Dwyer-Nield LD, Barthel B, Meyer AM, Thompson DC, Malkinson AM. Genetic ablation of inducible nitric oxide synthase decreases mouse lung tumorigenesis. Cancer Res. 2002;62:6850-6856. [PubMed] |
| 8. | Sonoshita M, Takaku K, Oshima M, Sugihara K, Taketo MM. Cyclooxygenase-2 expression in fibroblasts and endothelial cells of intestinal polyps. Cancer Res. 2002;62:6846-6849. [PubMed] |
| 9. | Wu YL, Sun B, Zhang XJ, Wang SN, He HY, Qiao MM, Zhong J, Xu JY. Growth inhibition and apoptosis induction of Sulindac on Human gastric cancer cells. World J Gastroenterol. 2001;7:796-800. [DOI] |
| 10. | Cheng J, Imanishi H, Iijima H, Shimomura S, Yamamoto T, Amuro Y, Kubota A, Hada T. Expression of cyclooxygenase 2 and cytosolic phospholipase A (2) in the liver tissue of patients with chronic hepatitis and liver cirrhosis. Hepatol Res. 2002;23:185-195. [DOI] |
| 11. | Davies G, Martin LA, Sacks N, Dowsett M. Cyclooxygenase-2 (COX-2), aromatase and breast cancer: a possible role for COX-2 inhibitors in breast cancer chemoprevention. Ann Oncol. 2002;13:669-678. [DOI] |
| 12. | Seno H, Oshima M, Ishikawa TO, Oshima H, Takaku K, Chiba T, Narumiya S, Taketo MM. Cyclooxygenase 2- and prostaglandin E (2) receptor EP (2)-dependent angiogenesis in Apc(Delta716) mouse intestinal polyps. Cancer Res. 2002;62:506-511. [PubMed] |
| 13. | Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999;59:198-204. [PubMed] |
| 14. | Gupta S, Srivastava M, Ahmad N, Sakamoto K, Bostwick DG, Mukhtar H. Lipoxygenase-5 is overexpressed in prostate adenocarcinoma. Cancer. 2001;91:737-743. [DOI] |
| 15. | Weddle DL, Tithoff P, Williams M, Schuller HM. Beta-adrenergic growth regulation of human cancer cell lines derived from pancreatic ductal carcinomas. Carcinogenesis. 2001;22:473-479. [DOI] |
| 16. | Ristimaki A, Nieminen O, Saukkonen K, Hotakainen K, Nordling S, Haglund C. Expression of cyclooxygenase-2 in human transitional cell carcinoma of the urinary bladder. Am J Pathol. 2001;158:849-853. [DOI] |
| 17. | Kulkarni S, Rader JS, Zhang F, Liapis H, Koki AT, Masferrer JL, Subbaramaiah K, Dannenberg AJ. Cyclooxygenase-2 is overexpressed in human cervical cancer. Clin Cancer Res. 2001;7:429-434. [PubMed] |
| 18. | Tong BJ, Tan J, Tajeda L, Das SK, Chapman JA, DuBois RN, Dey SK. Heightened expression of cyclooxygenase-2 and peroxisome proliferator-activated receptor-delta in human endometrial adenocarcinoma. Neoplasia. 2000;2:483-490. [DOI] |
| 19. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Oncol. 2001;76:26-30. [DOI] |
| 20. | Kokawa A, Kondo H, Gotoda T, Ono H, Saito D, Nakadaira S, Kosuge T, Yoshida S. Increased expression of cyclooxygenase-2 in human pancreatic neoplasms and potential for chemoprevention by cyclooxygenase inhibitors. Cancer. 2001;91:333-338. [DOI] |
| 21. | Fantappie O, Masini E, Sardi I, Raimondi L, Bani D, Solazzo M, Vannacci A, Mazzanti R. The MDR phenotype is associated with the expression of COX-2 and iNOS in a human hepatocellular carcinoma cell line. Hepatology. 2002;35:843-852. [PubMed] [DOI] |
| 22. | Kakiuchi Y, Tsuji S, Tsujii M. Cyclooxygenase-2 activity altered the cell-surface carbohydrate antigens on colon cancer cells and enhanced liver metastasis. Cancer Res. 2002;62:1567-1572. [PubMed] |
| 23. | Oshima M, Murai N, Kargman S, Arguello M, Luk P, Kwong E, Taketo MM, Evans JF. Chemoprention of intestinal polyposis in the Apcdelta716 mouse by rofecoxib, a specific cyclooxygenase-2 inhibitor. Cancer Res. 2001;61:1733-1740. [PubMed] |
| 24. | Uefuji K, Ichikura T, Mochizuki H. Expression of cyclooxygenase-2 in human gastric adenomas and adenocarcinomas. J Surg Onco. 2001;76:26-30. [DOI] |
| 25. | Narko K, Ristimaki A, MacPhee M, Smith E, Haudenschild CC, Hla T. Tumorigenic transformation of immortalized ECV endothelial cells by cyclooxygenase-1 overexpression. J Biol Chem. 1997;101:95-98. [DOI] |
| 26. | Marks F, Furstenberger G. Proliferative responses of the skin to external stimuli. Environ Health Perspect. 1993;101:95-97. [PubMed] [DOI] |
| 27. | Gately S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metasis Rev. 2000;19:19-27. [DOI] |
| 28. | Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci USA. 1997;94:3336-3340. [DOI] |
| 30. | Saha D, Roman C, Beauchamp RD. New strategies for colorectal cancer prevention and treatment. World J Surg. 2002;26:26-30. [PubMed] [DOI] |