修回日期: 2002-08-21
接受日期: 2002-08-31
在线出版日期: 2003-03-15
目的: 构建抗人大肠癌重组单链抗体ND-1scFv与酵母胞嘧啶脱氨酶CD的融合基因, 并在大肠杆菌中表达.
方法: 采用基因重组技术借助GSGGSG Linker序列将酵母胞嘧啶脱氨酶基因融合于ND-1scFv基因的3'端, 构建pET-28a(+)ND-1scFv-CD载体, 转化E. coli BL21, 经IPTG诱导, 表达二者的融合蛋白. 表达产物用Ni-NTA resin亲和层析方法纯化, 并采用ELISA检测其免疫活性, MTT法测定由ND-1scFv-CD/5FC构成的ADEPT系统对大肠癌细胞的体外杀伤作用.
结果: 序列测定表明: ND-1scFv-CD基因全长1 269 bp, 包含了732 bp的scFv基因和477 bp的CD基因. SDS-PAGE检测显示, 融合蛋白相对分子质量47 KDa, 与预测值一致. 光密度扫描结果表明, 重组蛋白占菌体蛋白总量的27%, ELISA检测证实, ND-1scFv-CD保留了与ND-1scFv相近的免疫活性. MTT实验检测结果显示, 由scFv-CD/5FC构成的ADEPT体系对大肠癌细胞具靶向杀伤作用.
结论: 成功地构建了抗人大肠癌单链抗体ND-1scFv与酵母胞嘧啶脱氨酶CD的融合蛋白, 并在大肠杆菌中高效表达. 融合蛋白具有良好的免疫活性和一定的酶活性.
引文著录: 方瑾, 宋今丹. 抗人大肠癌单链抗体-胞嘧啶脱氨酶融合基因的构建及表达. 世界华人消化杂志 2003; 11(3): 289-293
Revised: August 21, 2002
Accepted: August 31, 2002
Published online: March 15, 2003
AIM: To explore the construction of the fusion gene of recombinant ND-1scFv against human colorectal carcinoma and yeast cytosine deaminase and the expression of the fusion protein in E. coli.
METHODS: Yeast cytosine deaminase gene was fused with the 3'terminus of gene of ND-1scFv against human colorectal carcinoma by a 18 bp linker with sequences encoding GSGGSG. To construct ND-1scFv-CD gene, plasmid pET 28 a(+)-ND-1scFv-CD was transformed into E. coli BL-21, and induced by IPTG to express the ND-1scFv-CD fusion protein. The expressed product was purified by affinity chromatography using NI-NTA resin, and its immunity was analyzed by ELISA. The cytotoxic activity of the ADEPT system containing ND-1 scFv-CD/5FC against human colon carcinoma cell line was evaluated by MTT assay.
RESULTS: Sequencing results showed that the ND-1scFv-CD gene consisted of 1 269 bp, including ND-1scFv 732 bp and CD 477 bp. SDS-PAGE analysis showed that the expected molecular weight of fusion protein was 47 Kd. Optical density scanning showed that fusion protein expressed in E. coli accounted to 27% of the total bacterial proteins. ELISA analysis revealed that ND-1scFv-CD reserved similar immunity of ND-1scFv. MTT assay showed scFv-CD/5FC was cytotoxic to human colorectal carcinoma cells.
CONCLUSION: ND-1scFv-CD gene against human colorectal carcinoma was constructed and expressed successfully in E. coli. The fusion protein exhibited good immunity and enzymatic activity.
- Citation: Fang J, Song JD. Construction and expression of fusion gene of single chain Fv against human colorectal carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(3): 289-293
- URL: https://www.wjgnet.com/1009-3079/full/v11/i3/289.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i3.289
对肿瘤细胞实施特异性的杀伤作用一直是肿瘤导向治疗的关键所在. 抗体介导的酶解前药疗法(antibody directed enzyme prodrug therapy, ADEPT)是近年发展起来的颇具应用前景的肿瘤导向治疗新途径[1-13], 他以巧妙的设计克服了以往单抗作为药物载体的许多缺陷, 日益受到广泛关注, 并已有效应用于临床. 其基本思想是将单克隆抗体与一种活化酶交联, 制备抗体-酶偶联物, 借助抗体识别瘤细胞表面抗原的特性, 把酶带到靶部位, 某些无抗癌活性或低活性的前体药在酶的催化下可转化为细胞毒性药物, 实现抗癌药物在靶部位的特异性释放, 提高疗效.
ND-1是本室以人大肠癌细胞系CCL-187为免疫源制备的鼠抗人大肠癌单克隆抗体, 体内外一系列实验显示, 该单抗特异性强, 亲和力高, 并优于目前广泛采用的美国商业产品抗CEA 单抗. 以该单抗构建的基因工程抗体ND-1scFv亦显示了良好的体内外特异结合活性. 本研究在此基础上, 将ND-1单链抗体基因与酵母胞嘧啶脱氨酶(cytosine deaminase, CD)基因融合, 在大肠杆菌中成功地表达了单链抗体与酶的融合蛋白, 并考察了其与5-氟尿嘧啶(5-FU)的前体药5-氟胞嘧啶(5-FC)构成的ADEPT系统对大肠癌细胞的体外杀伤作用.
CCL-187人大肠癌细胞株由美国哈佛大学医学院肿瘤所惠赠, HeLa人宫颈癌上皮细胞株为本室保存. pET-28a(+)-ND-1scFv表达载体及pMD18-T-CD由本室构建. T4 DNA连接酶为Pharmacia公司产品. Pyrobest DNA聚合酶、HindⅢ、EcoRⅠ、SalⅠ限制性内切酶、IPTG、卡那霉素、DNA片段回收试剂盒为宝生物工程有限公司产品. Ni-NTA亲和层析填料为Qiagen公司产品. FITC荧光标记羊抗鼠抗体、5-氟胞嘧啶(5FC)为 Sigma公司产品, 辣根过氧化物酶标记的羊抗鼠抗体为北京中山生物技术有限公司产品. 抗His-Tag单抗为Invitrogen公司产品. MTT购自Fluka公司. 引物C1、C2由大连宝生物工程有限公司合成. 其余试剂均为分析纯或分子生物学纯级.
1.2.1 ND-1scFv-CD载体的构建: 引物设计: 根据CD序列设计5'和3'引物, 使其包含有能够与ND-1scFv基因连接的Linker序列GSGGSG, 同时引入酶切位点. 具体序列如下: 引物C1: 5'AGCAAGCTTACAGCGTGCTGCGTGCTCTACTCAC 3'; Hind Ⅲ引物C2: 5'TATGTCGACGGTAGCGGCGGTTCTGGTATGGTGACAGGGGGA 3'; SalⅠ Linke引物C1为酵母CD带有HindⅢ位点的3'引物, 引物C2为酵母CD的5'引物, 带有SalⅠ位点和编码6个氨基酸GSGGSG的Linker序列.
载体构建: 以质粒pMD18-T-CD为模板, 以引物C1、C2进行PCR, 扩增出5'端带有SalⅠ和Linker序列, 3'端带有HindⅢ位点的CD片段, 产物经1.5%琼脂糖电泳, DNA片段回收试剂盒回收纯化后, 用SalⅠ/HindⅢ双酶切, 与相同酶切的pET-28a(+)-ND-1 scfv载体于16℃连接过夜, 转化宿主菌BL21, 分别用EcoRⅠ/ HindⅢ、EcoRⅠ/SalⅠ和SalⅠ/ HindⅢ酶切鉴定插入片段并测序.
1.2.2 ND-1scFv-CD基因序列测定: 采用Sanger双脱氧末端终止法用ABI PRJSMTM377 DNA测序仪进行DNA序列测定, 确定CD 5'与ND-1scFv 3'连接处及CD再次PCR后序列的正确性. 每个基因测三个独立克隆.
1.2.3 ND-1scFv-CD融合基因的诱导表达: 挑取鉴定正确的阳性克隆接种于2 ml LB(含50 μg/mL卡那霉素)液体培养基中, 37℃振荡培养过夜, 并以1%接种量转种到100 ml相同 LB培养基中, 37℃振荡培养至OD600为0.6左右, 加入IPTG至终浓度1 mmol/L, 继续培养3 h, 获得诱导培养物, 表达产物以SDS-PAGE鉴定, 并用凝胶灰度扫描测定蛋白表达量.
1.2.4 包涵体蛋白的变性、复性及纯化: 将诱导的菌体用裂解缓冲液冰上裂解30 min, 并于冰浴上超声破碎, 4℃, 10 000 g离心20 min得到包涵体. 将包涵体重悬于原培养物0.1倍体积的变性液中(0.1 mol/L NaH2PO4, 0.01 mol/L Tris, 6 mol/L盐酸胍, pH8.0), 待包涵体全部溶解后, 20 000 g离心20 min, 取上清加至Ni-NTA resin亲和层析柱中, 按顺序分别用10倍柱体积的6 mol/L盐酸胍(pH8.0)→8mol/L 尿素(pH8.0)→8 mol/L尿素(pH 6.5)→8mol/L尿素(pH 4.2)洗脱镍柱, 收集8 mol/L 尿素(pH 4.2)流出液. 用变性液调整蛋白浓度至100 mg/mL, 加入4倍体积复性液(20 mmol/L Tris, 0.25 mol/L NaCl, 0.5% NP-40, 0.4 mmol/L PMSF, 2 mmol/L还原型谷胱甘肽, 0.2 mmol/L氧化型谷胱甘肽, 100mmol/L EDTA)4℃ 复性24 h, 然后将复性样品转移至截留分子量为8-10 kDa的透析袋中用透析液(20 mmol/L Tris, 500 mmol/L NaCl, 5%蔗糖)透析48 h, 超滤浓缩样品至适当体积, -20℃分装保存.
1.2.5 ELISA检测ND-1scFv-CD免疫活性: 取处于对数生长期的人大肠癌细胞CCL-187和人宫颈癌上皮细胞HeLa, 以5×104接种于96孔培养板中, 100 μL/孔, 于37℃培养24 h, 分别以ND-1scFv和ND-1scFv-CD作为一抗, 抗His6 mAb作为二抗, HRP-羊抗鼠IgG作为三抗, 于37℃分别孵育2 h, 以底物TMB显色, 终止反应后, 用酶标仪于波长450 nm测定光吸收(A)值.
1.2.6 MTT法检测ND-1scFv-CD/5FC系统的细胞杀伤活性: 取对数生长期人大肠癌细胞CCL-187, 人宫颈癌上皮细胞HeLa, 将密度为3×105/mL的细胞悬液接种于96孔培养板100 μL/孔, 37℃培养24 h后, 加入ND-1scFv-CD融合蛋白(10 μg/mL), 37℃培养2 h, 弃去培养液, 用PBS涮洗3次, 加入新鲜培养液100 μL/孔, 同时加入5FC 10 μL(4 mmol/L, 该浓度下5FC对CCL-187细胞无杀伤作用), 37℃培养48 h后, 每孔加入10 μL MTT(5mg/mL), 37℃孵育4 h, 吸去上清, 每孔加入100 μL DMSO, 振荡2 min, 在酶标仪上测定A540 nm, 同时用PBS作空白对照, 5FC作阴性对照.
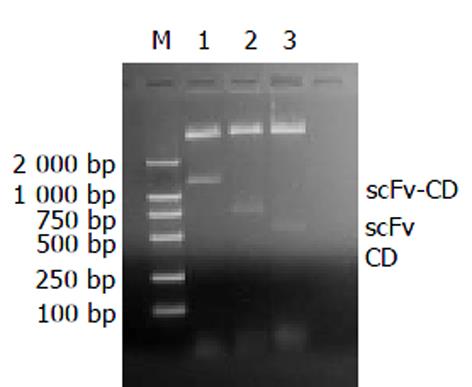
将克隆至pMD18-T Vector 中具有正确序列的CD基因经过再次PCR, 引入酶切位点及Linker序列, 将该序列与载有ND-1scFv基因的PET-28a(+)-ND-1scFv同时进行SalⅠ/Hind Ⅲ 双酶切, 连接后, ND-1scFv与CD通过一柔性Linker拼接为ND-1scFv-CD基因, 连接体转化E. coli BL21, 随机挑取菌落, 提取质粒以酶切鉴定. 1%琼脂糖电泳显示, 经Sal Ⅰ/Hind Ⅲ 双酶切, 在500 bp左右呈现一明显条带, 提示CD片段被克隆入pET-28a(+)-ND-1scFv载体, 且插入方向正确. 同时经EcoRⅠ/SalⅠ和EcoRⅠ/ Hind Ⅲ 酶切, 分别在750 bp和1 200 bp处呈现一单一条带, 表明ND-1scFv-CD基因已构建到pET-28a(+)载体, 且ND-1scFv和CD连接方向及顺序正确(图1). 序列测定表明, ND-1scFv-CD基因全长1 269 bp, scFv基因在上游, 片段长度732 bp, CD 基因在下游, 片段长度477 bp, 中间为18个bp的柔性序列, 两侧为Hind Ⅲ 和EcoRⅠ位点.
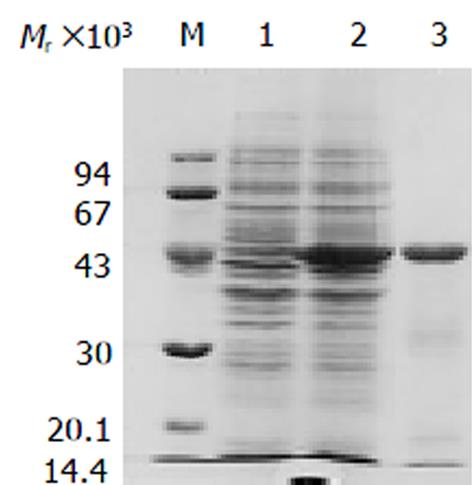
将构建正确的表达质粒pET-28a(+)-ND-1scFv-CD转化宿住菌BL21, 以1 mmol/L IPTG诱导表达, SDS-PAGE检测显示, 经诱导的菌体裂解物在Marker 43KD附近有一新的条带, 且呈高表达. pET-28a(+)载体在外源基因插入部位的上游有一编码His-Tag小肽的基因, 因而, 在IPTG诱导下表达的是ND-1scFv-CD蛋白与His-Tag及其上游序列的融合蛋白, 相对分子量47 KD(ND-1scFv 26KD, His-tag +上游序列4 KD, CD 17 KD), 电泳结果所示与该融合蛋白的理论推算值相符(图2). 凝胶灰度扫描结果显示表达量占菌体总蛋白量的27%.
SDS-PAGE检测显示, 经IPTG诱导后的菌体裂解物上清中未见可溶性蛋白条带, 故推断ND-1scFv-CD蛋白主要以包涵体形式存在. 通过盐酸胍变性处理使包涵体溶解, 借助能与His序列特异结合的Ni-NTA亲和层析柱对变性的包涵体蛋白进行纯化, 获得了高纯度的ND-1scFv-CD(图2).
ELISA定量检测显示, ND-1scFv与CD重组而表达的融合蛋白仍具有与靶细胞的结合活性, 其免疫活性未见明显下降(表1).
| Sample | OD450nm (mean±SD) | |
| CCL-187 | HeLa | |
| ND-1scFv | 0.95±0.16 | 0.19±0.03 |
| ND-1scFv-CD | 0.74±0.15 | 0.17±0.03 |
| PBS | 0.14±0.03 | 0.13±0.01 |
将ND-1scFvCD融合蛋白与细胞孵育2 h后, 加入CD的前体药5FC, 同时以单独加入5FC作为对照, 测定对细胞的杀伤活性. 表2结果显示, 在4 mmol/L使用浓度下, 单独加入5FC对CCL-187及HeLa细胞均无明显杀伤作用, 而预先与ND-1scFv-CD融合蛋白作用后, 5FC表现出对细胞的杀伤活性, 且对表达有ND-1相应抗原的CCL-187的杀伤活性明显优于非靶向的HeLa细胞. 提示scFv-CD融合蛋白具有一定的酶活性, 可将5FC转化为5FU而杀伤细胞, 并具明显靶向作用.
| Sample | Inhibition rate (mean±SD) | |
| CCL-187 | HeLa | |
| ND-1scFv-CD+5FC | 24.5±2.78 | 4.5±0.31 |
| 5FC | - | 1.5±0.05 |
将抗体与其他效应分子结合构建具有导向性的双功能分子是目前抗体应用研究的热点[14,15], 抗体基因与酶基因融合表达融合蛋白是ADEPT策略的一个新思路, 其优势不仅在于可对抗体及具有催化功能的部分进行改造, 改变体内动力学性质, 还在于可获得高效均一的产物, 避免了常规化学偶联方法导致的产物异质性的问题[16]. 本实验首次将抗人大肠癌单克隆抗体ND-1的单链抗体基因与酵母胞嘧啶脱氨酶基因融合, 构建了高效表达的pET-28a(+)-ND-1scFv-CD融合蛋白表达载体, 在大肠杆菌中的表达量高达27%, 经纯化后纯度达87%, 而且整个体系具有操作简单, 重复性好的特点, 不仅可有效克服抗体-酶直接偶联带来的产物异质性等弊病, 提高产物的特异性, 并可为相应药物制剂的规范化和产业化提供可能, 同时也为其他双功能效应分子的构建提供了简洁、高效的方法.
活化酶在肿瘤组织的选择性积聚是ADEPT策略成功的关键因素之一. 人体组织中广泛存在的酶通常缺乏特异性, 会引起肿瘤外组织的毒副作用, 而胞嘧啶脱氨酶在哺乳动物组织中不存在, 他来源于细菌和真菌, 是嘧啶合成补救代谢途径的关键酶, 可将5FC水解为抗癌药5-FU, 将其用于ADEPT可避免药物在其他组织的激活[17-21]. 人们最初是将细菌CD用于ADEPT系统, 但在随后的体内实验中发现, 哺乳动物肠道系统存在有相同细菌, 可将5FC转化为5FU, 这样在治疗中就难以保证产生足够的5FU而对正常细胞无损, 因而, 1997年Erbs et al[22,23]首次从啤酒酵母中克隆出CD基因, 随后在真核系统中获得表达, 并广泛用于基因介导的酶解前药疗法GDEPT[24,25], 相关实验同时证实了酵母胞嘧啶脱氨酶在该系统中优于细菌胞嘧啶脱氨酶的体内抑瘤作用[26-28]. 本实验首次将克隆的酵母基因与单链抗体基因融合用于ADEPT系统. 免疫活性检测显示, ND-1scFv-CD融合蛋白保持了良好的免疫活性, 同时CD组成的ADEPT系统对表达有相应抗原的CCL-187产生了一定的杀伤作用, 而且这种杀伤作用具有明显的靶向专一性.
ADEPT系统的有效性在于高特异性地激活前体药, 使其定向释放以杀伤靶细胞. 本实验中ND-1scFv与CD基因融合后, 其表达蛋白仍保留了与原scFv相似的免疫活性, 与5FC构成的ADEPT系统亦表现出对表达相应抗原细胞的靶向杀伤作用, 同时在光镜下也可观察到被作用细胞呈现的细胞凋亡特征[29], 与5FU游离药物的作用相似, 这说明该融合蛋白具有酶活性, 可使5FC转化为5FU, 从而杀伤细胞, 但其作用强度尚不如5FU游离药物, 只有24.5%, 提示ND-1scFv-CD中的CD部分未获得最佳的酶活性. 有研究指出, scFv与效应分子连接后产生的空间位阻有可能影响分子活性, 已有报道通过加长二者之间Linker序列的长度而优化蛋白结构域的有效形成[30]. 本实验在两效应分子间采用了广为接受的由6个氨基酸组成的Linker, 是否可通过加长该序列而获得更为理想的双功能效应分子将有待进一步探讨.
本实验通过基因工程技术成功地构建了抗人大肠癌单链抗体与胞嘧啶脱氨酶的融合基因, 并在大肠杆菌中进行了高效表达, 融合蛋白的抗体部分显示了良好的免疫活性, 所连接的酶结构也表现出初步的功能, 为该体系最终的实际应用奠定了基础, 经进一步提高酶活性, 可望成为良好的大肠癌治疗试剂.
编辑: N/A
| 1. | Chen BM, Cheng TL, Tzou SC, Roffler SR. Potentiation of antitumor immunity by antibody-directed enzyme prodrug therapy. Int J Cancer. 2001;94:850-858. [PubMed] [DOI] |
| 2. | Xu G, McLeod HL. Strategies for enzyme/prodrug cancer therapy. Clin Cancer Res. 2001;7:3314-3324. [PubMed] |
| 3. | Houba PH, Boven E, van der Meulen-Muileman IH, Leenders RG, Scheeren JW, Pinedo HM, Haisma HJ. Pronounced antitumor efficacy of doxorubicin when given as the prodrug DOX-GA3 in combination with a monoclonal antibody beta-glucuronidase conjugate. Int J Cancer. 2001;91:550-554. [PubMed] [DOI] |
| 4. | Napier MP, Sharma SK, Springer CJ, Bagshawe KD, Green AJ, Martin J, Stribbling SM, Cushen N, O'Malley D, Begent RH. Antibody-directed enzyme prodrug therapy: efficacy and mechanism of action in colorectal carcinoma. Clin Cancer Res. 2000;6:765-772. [PubMed] |
| 5. | Syrigos KN, Epenetos AA. Antibody directed enzyme prodrug therapy (ADEPT): a review of the experimental and clinical considerations. Anticancer Res. 1999;19:605-613. [PubMed] |
| 6. | Cheng TL, Wei SL, Chen BM, Chern JW, Wu MF, Liu PW, Roffler SR. Bystander killing of tumour cells by antibody-targeted enzymatic activation of a glucuronide prodrug. Br J Cancer. 1999;79:1378-1385. [PubMed] [DOI] |
| 7. | Dubowchik GM, Walker MA. Receptor-mediated and enzyme-dependent targeting of cytotoxic anticancer drugs. Pharmacol Ther. 1999;83:67-123. [PubMed] [DOI] |
| 8. | Senter PD, Springer CJ. Selective activation of anticancer prodrugs by monoclonal antibody-enzyme conjugates. Adv Drug Deliv Rev. 2001;53:247-264. [PubMed] [DOI] |
| 9. | Syrigos KN, Deonarian DP, Epenetos AA. Use of monoclonal antibodies for the diagnosis and treatment of bladder cancer. Hybridoma. 1999;18:219-224. [PubMed] [DOI] |
| 10. | Sperker B, Mürdter TE, Backman JT, Fritz P, Kroemer HK. Expression of active human beta-glucuronidase in Sf9 cells infected with recombinant baculovirus. Life Sci. 2002;71:1547-1557. [PubMed] [DOI] |
| 11. | Monks NR, Blakey DC, East SJ, Dowell RI, Calvete JA, Curtin NJ, Arris CE, Newell DR. DNA interstrand cross-linking and TP53 status as determinants of tumour cell sensitivity in vitro to the antibody-directed enzyme prodrug therapy ZD2767. Eur J Cancer. 2002;38:1543-1552. [PubMed] [DOI] |
| 12. | de Graaf M, Boven E, Scheeren HW, Haisma HJ, Pinedo HM. Beta-glucuronidase-mediated drug release. Curr Pharm Des. 2002;8:1391-1403. [PubMed] [DOI] |
| 13. | Tietze LF, Herzig T, Fecher A, Haunert F, Schuberth I. Highly selective glycosylated prodrugs of cytostatic CC-1065 analogues for antibody-directed enzyme tumor therapy. Chembiochem. 2001;2:758-765. [PubMed] [DOI] |
| 14. | Helfrich W, Haisma HJ, Magdolen V, Luther T, Bom VJ, Westra J, van der Hoeven R, Kroesen BJ, Molema G, de Leij L. A rapid and versatile method for harnessing scFv antibody fragments with various biological effector functions. J Immunol Methods. 2000;237:131-145. [PubMed] [DOI] |
| 15. | Matthey B, Engert A, Klimka A, Diehl V, Barth S. A new series of pET-derived vectors for high efficiency expression of Pseudomonas exotoxin-based fusion proteins. Gene. 1999;229:145-153. [PubMed] [DOI] |
| 16. | Kerr DE, Vrudhula VM, Svensson HP, Siemers NO, Senter PD. Comparison of recombinant and synthetically formed monoclonal antibody-beta-lactamase conjugates for anticancer prodrug activation. Bioconjug Chem. 1999;10:1084-1089. [PubMed] [DOI] |
| 17. | O'Boyle KP, Senter PD, Bhargava K, Chun S, Anthony G, Markowitz AL, Wadler S. Effects of a hybrid recombinant human alpha interferon (A/D) on in vitro cytotoxicity and in vivo localization of monoclonal antibody L6-cytosine deaminase conjugate in a colon cancer model. Cancer Biother Radiopharm. 1998;13:33-42. [PubMed] [DOI] |
| 18. | Aboagye EO, Artemov D, Senter PD, Bhujwalla ZM. Intratumoral conversion of 5-fluorocytosine to 5-fluorouracil by monoclonal antibody-cytosine deaminase conjugates: noninvasive detection of prodrug activation by magnetic resonance spectroscopy and spectroscopic imaging. Cancer Res. 1998;58:4075-4078. [PubMed] |
| 19. | King I, Bermudes D, Lin S, Belcourt M, Pike J, Troy K, Le T, Ittensohn M, Mao J, Lang W. Tumor-targeted Salmonella expressing cytosine deaminase as an anticancer agent. Hum Gene Ther. 2002;13:1225-1233. [PubMed] [DOI] |
| 20. | Shen LZ, Wu WX, Xu DH, Zheng ZC, Liu XY, Ding Q, Hua YB, Yao K. Specific CEA-producing colorectal carcinoma cell killing with recombinant adenoviral vector containing cytosine deaminase gene. World J Gastroenterol. 2002;8:270-275. [PubMed] [DOI] |
| 21. | Akimoto M, Miyahara T, Arai J, Akimoto A, Hamada H, Yoshida Y, Yoshimura N. A new delivery system for 5-fluorouracil using prodrug and converting enzyme. Br J Ophthalmol. 2002;86:581-586. [PubMed] [DOI] |
| 22. | Erbs P, Exinger F, Jund R. Characterization of the Saccharomyces cerevisiae FCY1 gene encoding cytosine deaminase and its homologue FCA1 of Candida albicans. Curr Genet. 1997;31:1-6. [PubMed] [DOI] |
| 23. | Hayden MS, Linsley PS, Wallace AR, Marquardt H, Kerr DE. Cloning, overexpression, and purification of cytosine deaminase from Saccharomyces cerevisiae. Protein Expr Purif. 1998;12:173-184. [PubMed] [DOI] |
| 24. | Nyati MK, Symon Z, Kievit E, Dornfeld KJ, Rynkiewicz SD, Ross BD, Rehemtulla A, Lawrence TS. The potential of 5-fluorocytosine/cytosine deaminase enzyme prodrug gene therapy in an intrahepatic colon cancer model. Gene Ther. 2002;9:844-849. [PubMed] [DOI] |
| 25. | Zhang Y, Wang J, Zhou H, Zhai Y. [Study on the in vivo killing activity of YCD/5-FC gene therapy system on K562B cells]. Zhonghua Xue Ye Xue Za Zhi. 2002;23:173-175. [PubMed] |
| 26. | Kievit E, Nyati MK, Ng E, Stegman LD, Parsels J, Ross BD, Rehemtulla A, Lawrence TS. Yeast cytosine deaminase improves radiosensitization and bystander effect by 5-fluorocytosine of human colorectal cancer xenografts. Cancer Res. 2000;60:6649-6655. [PubMed] |
| 27. | Kievit E, Bershad E, Ng E, Sethna P, Dev I, Lawrence TS, Rehemtulla A. Superiority of yeast over bacterial cytosine deaminase for enzyme/prodrug gene therapy in colon cancer xenografts. Cancer Res. 1999;59:1417-1421. [PubMed] |
| 28. | Hamstra DA, Rice DJ, Fahmy S, Ross BD, Rehemtulla A. Enzyme/prodrug therapy for head and neck cancer using a catalytically superior cytosine deaminase. Hum Gene Ther. 1999;10:1993-2003. [PubMed] [DOI] |
| 29. | Zhang SN, Yuan SZ, Zhu ZH, Wen ZF, Huang ZQ, Zeng ZY. Apoptosis induced by 5-flucytosine in human pancreatic cancer cells genetically modified to express cytosine deaminase. Acta Pharmacol Sin. 2000;21:655-659. [PubMed] |
| 30. | Liu ZG, Yu WY, Wang X. [The construction and expression of two humanized scFv-urokinase fusion genes]. Sheng Wu Gong Cheng Xue Bao. 2000;16:514-516. [PubMed] |