修回日期: 2002-11-20
接受日期: 2002-12-07
在线出版日期: 2003-10-15
评价奥沙利铂(Oxaliplatin, L-OHP)对人胃癌的疗效, 探讨其治疗胃癌的作用机制.
IV期胃癌患者22例接受包含L-OHP的联合化疗方案(L-OHP 85 mg/m2, 静脉点滴, 1 h, 第 1 d; 四氢叶酸钙 200 mg/m2, 静脉点滴, 1 h, 第 1-5 d; 5-FU 300 mg/m2, 静脉注射, 第 1-2 d, 5-FU, 持续静脉点滴, 48 h; 2 wk 1疗程)4-6(平均4.6)个疗程. 观察有效率, 无进展生存时间(progression-free survival, PFS), 总体生存时间及毒副作用. 体外培养人中分化胃癌细胞株SGC-7901, 应用MTT法检测L-OHP对细胞生长的抑制作用, 并计算50%抑制浓度(IC50); 将不同浓度梯度的L-OHP与细胞株作用后, 应用流式细胞仪(ANEXIN-V标记)及TUNEL检测细胞凋亡情况. 应用RT-PCR检测caspase-3m-RNA的表达.
有效(完全有效及部分有效)9例(40.9%). 平均PFS 4. 2 mo, 总体生存时间7.2 mo. 蓄积性神经毒性(全部为I-II级), 呕吐及腹泻(1例III级腹泻), 骨髓抑制发生率分别为93.5%, 20%, 32.9%. 浓度为1 mmol/L的L-OHP与细胞株作用30 min, 流式细胞仪即可检测出细胞凋亡水平升高, 但无统计学差异(P>0.05), 1 mmol/L作用2 d, 流式细胞仪及TUNEL均可检测到细胞凋亡水平显著性升高(P<0.05). L-OHP作用后的细胞caspase-3 m-RNA表达升高, 并与药物诱导的凋亡相关.
L-OHP治疗进展期胃癌安全有效. L-OHP在体外可明显抑制胃癌细胞株SGC-7901的生长, 诱导细胞caspase-3m-RNA表达及凋亡.
引文著录: 林万隆, 李定国, 陈强, 陆汉民, 马小明, 孙培龙. 奥沙利铂综合治疗胃癌的疗效及机制. 世界华人消化杂志 2003; 11(10): 1535-1539
Revised: November 20, 2002
Accepted: December 7, 2002
Published online: October 15, 2003
To evaluate the therapeutic effect of oxaliplatin on human gastric carcinoma and to explore the mechanisms.
22 cases of stage IV gastric carcinoma patients received 4-6 (mean 4.6) cycles of first line combined chemotherapy with oxaliplatin (oxaliplatin 85 mg/m2, ivgtt, 1 h, d 1; leukovorin 200 mg/m2, iv, gtt, 1 h, d 1-5; 5-FU 300 mg/m2, iv, d 1-2; 5-FU, continuously iv, gtt, 48 h; 1 cycle/2w). Response rate, progression-free survival (PFS), total survival time, toxic side effects were evaluated. The inhibitory effect of oxaliplatin on human gastric cell line SGC-7901 was calculated by MTT and IC50 was measured. Flow cytometry and TUNEL were applied to evaluate the apoptosis of cell line induced by the drug. The expression of caspase-3 mRNA was detected by RT-PCR.
Total response (complete and partial) occurred in 9 (40.9%) patients. Mean PFS was 4.2 months and mean total survival time was 7.2 months. Cumulative neurotoxicity (all grade I-II), vomiting and diarrhea, myelosuppression appeared in 93.5%, 20%, 32.9% of the patients, respectively. Apoptosis index was elevated after incubating with 1 mmol/L oxaliplatin for 30 min, but without statistic significance (P>0.05), but was much higher both by flowcytometry and TUNEL with statistical significance (P<0.05) after incubating with 1 mmol/L oxaliplatin for 2 days. Caspase-3 mRNA expression was elevated in oxaliplatin treated cells and correlated with apoptosis induced by the drug.
Oxaliplatin is effective and well-tolerated on human advanced gastric carcinoma. Oxaliplatin could significantly inhibit the growth of human gastric cell line SGC-7901, inducing caspase-3 mRNA expression and cell apoptosis.
- Citation: Lin WL, Li DG, Chen Q, Lu HM, Ma XM, Sun PL. Clinical efficacy and mechanism of oxaliplatin in treating human gastric carcinoma. Shijie Huaren Xiaohua Zazhi 2003; 11(10): 1535-1539
- URL: https://www.wjgnet.com/1009-3079/full/v11/i10/1535.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i10.1535
胃癌是危害人民健康的主要恶性肿瘤之一, 其发病原因尚未明了. 近年来药物治疗作为胃癌综合治疗的重要组成部分日益受到重视. 在消化道恶性肿瘤中, 胃癌属于对药物治疗较敏感的肿瘤, 目前普遍认为药物治疗有可能减少复发, 延长生存期. 奥沙利铂(oxaliplatin, L-OHP)为第三代的铂类药物, 抗瘤活性强, 与CDDP无交叉耐药, 与5-Fu有协同作用, 对多种肿瘤有效, 毒副作用较低. 目前用L-OHP治疗胃癌的临床及基础研究较少, 我们总结包含L-OHP的联合化疗方案一线治疗IV期胃癌22例的疗效及毒副作用, 并试图通过检测L-OHP诱导胃癌细胞株SGC-7901caspase-3 m-RNA的表达及凋亡的作用, 探讨其治疗胃癌的作用机制.
1999/2002年新华医院肿瘤中心住院IV期胃癌患者22例, 男17例, 女5例, 年龄25-70(平均60.3)岁. 均经术后病理或胃镜病理证实为低分化腺癌16例, 印戒细胞癌6例. 至少有一个可测量病灶直径大于或等于2 cm; WHO活动能力评分小于或等于2; 预计生存期大于或等于3 mo; 从未接受过化疗; 无外周神经疾病; 血液学, 心脏, 肝肾功能正常; 无其他抗肿瘤治疗.
化疗方案: L-OHP 85 mg/m2, 静脉点滴, 1 h, 第1 d; 四氢叶酸钙 200 mg/m2, 静脉点滴, 1 h, 第1-5 d; 5-FU 300 mg/m2, 静脉注射, 第1-2 d, 5-FU, 持续静脉点滴, 48 h; 2 wk 1疗程. 4-6 (平均4.6)个疗程. 化疗前检查: 体质评分, 身体质量, 身高, 体表面积, 全身系统检查, 神经系统检查, 心电图, 血象, 血糖, 肝肾功能, 电解质, 血CEA; 化疗后每周复查一次各项生物学检查(同化疗前); 按NCI标准作前一疗程耐受性评价; 神经系统检查; 不良反应记录. 治疗前及4, 6周期后肿瘤病灶评价评定. 入组患者4个周期化疗后进行全面疗效评估, 1 mo后再次确认. 如果病情稳定或有效则继续用药至第6个周期.
按WHO和UICC标准分为完全有效(complete response, CR), 部分有效(partial response, PR), 稳定(stable disease, SD), 病情进展(progress disease, PD); 按NCI毒性评定标准评定毒性; 按神经毒副反应乐沙定专用分级法评定神经毒性.
有效率40.9%. 无进展生存期(progression free survival, PFS)1-12(平均4.2 mo), 总体生存时间4-12(平均7.2 mo). 值得指出的是有1例PR和1例SD患者病情至今依然无进展, PFS分别达10 mo和12 mo. L-OHP主要不良反应是消化道反应, 骨髓抑制, 神经感觉障碍. 消化道反应表现为呕吐(20%)和腹泻(20%), 其中III级腹泻1例. 骨髓抑制以轻中度贫血为特点, 发生率32.9%, 而对白细胞及血小板抑制较轻, 分别为22.9%和10%, 全部为I-II级. 神经感觉障碍是L-OHP最主要的不良反应, 发生率达93.5%, 以I级为主, II级3例, 化疗间歇期可完全缓解.
人中分化胃癌细胞株SGC-7901, 购自中国科学院上海细胞所, 用含100 ml/L小牛血清的RPMI 1640培养基, 在37 °C, 50 ml/L CO2条件下培养, 生长至单层后, 以2.5 g/L的胰蛋白酶消化贴壁细胞, 调整细胞数至1×105/L.
2.2.1 MTT法及IC50 将收集的细胞接种于96孔培养板中, 5×103/孔, 37 °C, 50 ml/L CO2条件下培养24 h. 设置6组浓度梯度, 每组3个孔, 加入L-OHP, 使终浓度分别为10, 1, 0.5, 0.1, 0.05, 0.01 mmol/L, 对照组加入相同体积的培养液. 37 °C、50 ml/L CO2条件下培养48 h, 离心弃上清后, 每孔加入0.5 g/L的MTT 150 μL, 37 °C、50 ml/L CO2条件下避光孵育4 h; 离心弃上清后, 加入DMSO 150 μl/孔, 置水平摇床上15 min, 在525 nm波长下测A值, 细胞生长抑制率 = (对照组的A值-实验组的A值)/对照组的A值100%.
2.2.2 流式细胞仪 取对数生长期的细胞分为4组, 前3组加入L-OHP, 使终浓度分别为10, 1, 0.5 mmol/L, 第4组加入相同体积的培养液作为对照. 药物作用30 min后, 用2.5 g/L的胰蛋白酶消化, 离心后弃上清, 每组细胞数大于或等于1×105/L, 参照ANEXIN-V (Roche)说明书操作, 于EPICS流式细胞仪(beckman-coulter)上进行分析.
2.2.3 TdT酶介导的dUTP缺口末端标记(TUNEL) 应用6孔板培养细胞, 取对数生长期的细胞, 细胞爬片. 试验组加入L-OHP, 对照组加入相同体积的培养液作为对照. 药物作用后, 弃上清后用培养液洗3次(图1), 所有标本均应用40 g/L多聚甲醛固定30 min, 用TdT酶将生物素标记的dUTP连接到DNA断裂的3'OH端, 加入ABC反应剂作用数分钟, 再加入DAB/H2O2溶液显色, 苏木精复染, 中性树胶封固.
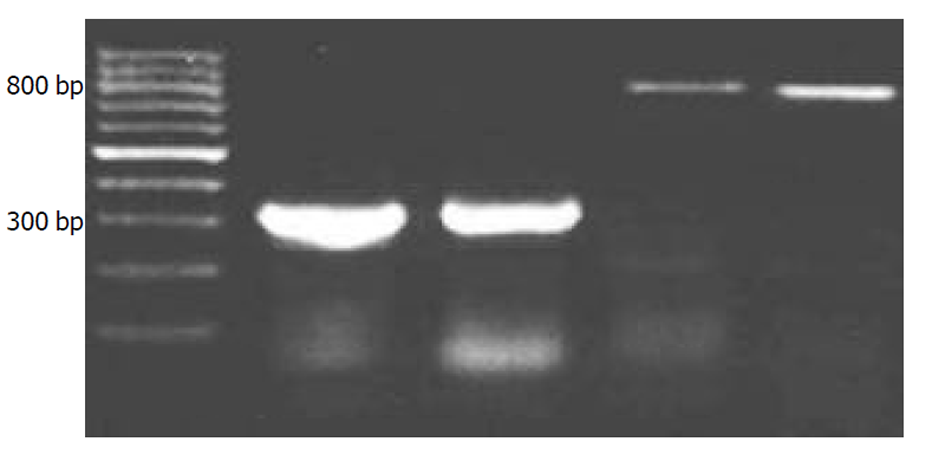
2.2.4 RT-PCR取对数生长期的细胞分为2组, 每组3个样本, 1组加入L-OHP, 使终浓度分别为1 mmol/L, 第2组加入相同体积的培养液作为对照. 药物作用30 min后, 用2.5 g/L的胰蛋白酶消化, 离心后弃上清, 每样本细胞数大于或等于1×105/L, PBS洗3次. 采用Trizol一步法提取RNA, 具体步骤见GibcoBrl公司的Protocol; RT步骤参见AMV reverse transcriptase Protocol; PCR步骤参见Taq DNA Polymerase Protocol; PCR产物经20 g/L琼脂糖电泳, 电泳图谱由VDS凝胶图像分析系统测定条带的积分吸光度(IOD). Caspase-3基因相对表达值=caspase-3 PCR产物条带IOD值/β-Actin PCR产物条IOD值. 引物序列如下.β-actin forward 5'-TGG AGG GGC CGG ACT CGT CA-3'; β-actin reverse 5'-CTT CCT TCC TGG GCA TGG AG-3'; 扩增DNA片段: 315 bp, caspase-3; Sense 5'-ATGGAGAACACTGAAAACTCA-3'; Antisense 5'-TTAGTGATAAAAATAGAGTTC-3'; 扩增DNA片段: 834 bp
统计学处理 采用t检验.
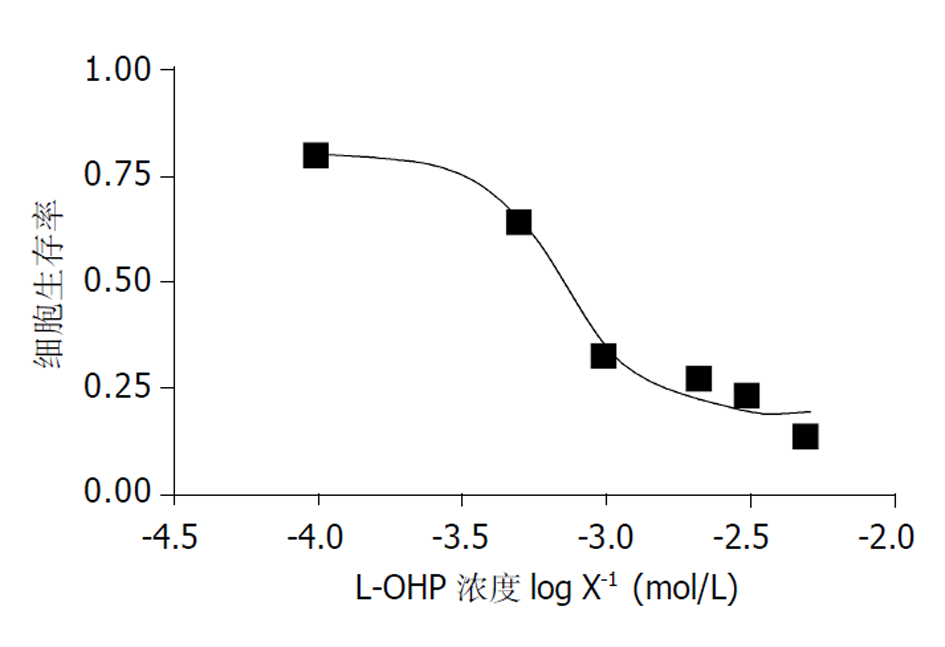
2.3.1 L-OHP对胃癌细胞株SGC-7901的抑制作用 根据3次MTT实验结果计算平均值, 由Graphpaol Prism 软件绘制, 并求得IC50值为0.71 mmoL/L. 由此可见, 曲线呈典型的倒S形, 较为平坦, 较好地反映了细胞生存率随药物浓度的变化情况(图2), 说明L-OHP有效地抑制SGC-7901细胞株的生长.
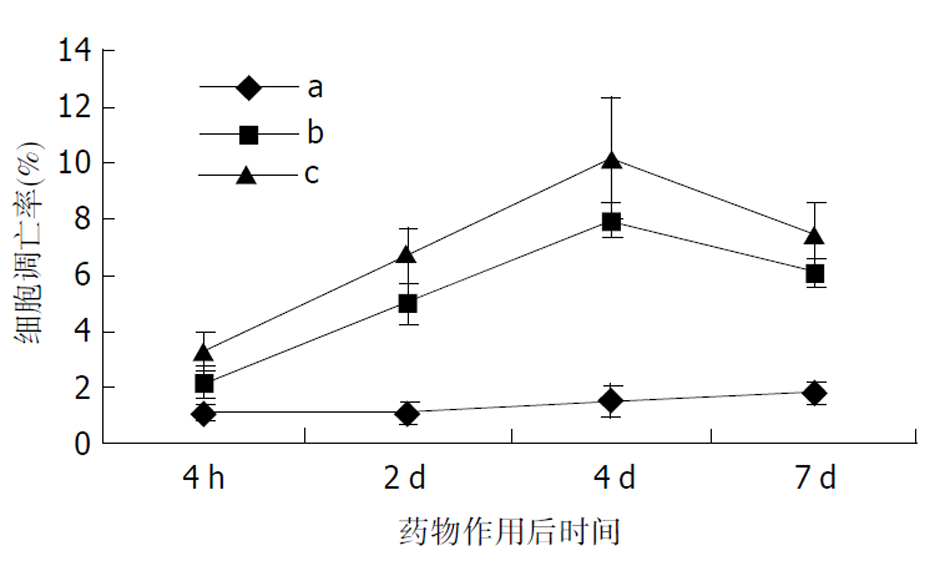
2.3.2 L-OHP诱导凋亡检测结果 浓度为1 mmoL/L的L-OHP与细胞株作用30 min后, 流式细胞仪即可检测出细胞凋亡水平升高, 但无统计学差异(P>0.05). L-OHP (10 mmoL/L, 30 min)诱导胃癌细胞株SGC-7901凋亡为细胞凋亡明显增加(表1, 图3). TUNEL实验空白对照组凋亡率为0.38%, 0.5 mmoL/L组为4.63%, 1.0 mmoL/L组为7.35%, 5.0 mmoL/L组为14.35%. 随药物浓度的增加, 细胞凋亡率显著上升(P<0.01)随时间变化, 对照组凋亡率无明显改变(P >0.05); 加药组凋亡率随时间延长明显上升(P<0.01), 第4 d达到峰值7.93%和10.15%, 第7 d分别下降至6.08%和7.55%(图5). 图3-1为典型镜像.
| 分组 | R1 | R2 | R3 | R4 | AI |
| L-OHP组 | 0.6 | 34.1 | 39.9 | 25.4 | 59.5 |
| 空白对照组 | 4.6 | 0.8 | 89.6 | 4.9 | 5.7 |
2.3.3 RT-PCR实验结果 图5为L-OHP诱导细胞表达caspase-3 m-RNA的琼脂糖电泳图谱, L-OHP作用后的细胞caspase-3 m-RNA表达明显增强(图5, 0.48±0.47 vs 0.18±0.20, P<0.05).
胃癌是危害人民健康的主要恶性肿瘤之一, 他的转移及复发是导致治疗失败及死亡的主要原因. 目前在临床上对于已经有转移的患者主要应用以药物治疗为主的综合治疗, 虽然胃癌对药物治疗较为敏感, 但联合化疗方案疗效仅为40%左右, 且药物副作用较大[1-8], 化疗药物的毒性与疗效是一对矛盾. 临床常用方案中以EAP方案(表阿霉素, 鬼臼乙叉甙, 顺铂)疗效较高, 最高达70%, 完全有效率达15%, 但治疗相关死亡率亦高达11%, 从而限制了临床推广. 顺铂为胃癌化疗的常用药物之一, 对胃癌具有确切的疗效, 但其严重的肾脏毒性亦限制了临床使用. 因此临床需要一种相对低毒高效药物的问世.
L-OHP是第三代铂类化合物, 化学名为左旋反式二氨基环己烷草酸铂, L-OHP的药理学特性与其他铂类药物相似, 均以DNA为靶点, L-OHP易与DNA链上的G共价结合, 并可能形成链间交联, 从而阻断DNA的复制及转录. 目前已知肿瘤细胞通过错误配对修复(mismatch repair)功能移去有铂类结合DNA的复制产物, 而这一过程将诱发细胞凋亡. 我们总结L-OHP一线治疗22例IV期胃癌, 有效率40.9%. 平均PFS4. 2 mo, 总体生存时间7.2 mo. 提示L-OHP对胃癌可能具有较高的抗瘤活性. 国外研究报道L-OHP毒性较小, 常规剂量下绝大多数患者均可耐受. 外周神经毒性是其剂量限制性毒性, 当累计剂量超过600 mg/m2时易出现感觉异常和肢端麻木, 常由寒冷触发, 但治疗期间注意保暖可以减轻症状, 且停药后可逐渐恢复. L-OHP优于CDDP的特点之一为其无明显的肾脏毒性, 血液系统及消化系统毒性亦较轻. 目前普遍认为胃癌的发生及发展是一个多基因, 多步骤的长期过程. 细胞凋亡与肿瘤的发生, 发展, 治疗和预后均密切相关[9-28]. 1980年发现bcl-2基因家族与细胞凋亡及肿瘤存在密切关系以来, 多数认为细胞凋亡在分子水平是受到诸因素调控的. 目前一组门冬氨酸特异性的胱氨酸蛋白酶-caspase家族渐成为细胞凋亡及肿瘤研究的热点之一. 目前认为在细胞色素C和dATP存在的条件下, Apaf-1与caspase-9通过他们与CED-3相似的氨基端相结合, 从而导致caspase-9的活化, 而活化的caspase-9又继而激活caspase-3从而导致的细胞内重要蛋白的溶解和细胞死亡, 因此caspase-3的激活可能是导致细胞凋亡最后的共同通路[29,30]
细胞凋亡检测方法多种多样, 如光镜、电镜下形态学观察; DNA末端原位标记染色法(TUNEL); DNA片断凝胶电泳以及碘化丙啶(PI)染色后流式细胞仪, 等. TUNEL应用较广泛, 但是无法区分死亡细胞与凋亡细胞. 而PI染色后流式细胞仪仅能检出已死亡的细胞. 目前发现细胞凋亡早期仅存于胞膜内侧的磷脂酰丝氨酸会翻转到胞膜外侧, 而AnnexinV能特异地与磷脂酰丝氨酸结合从而通过流式细胞仪检出凋亡早期的细胞. 故本文选用联合TUNEL法及AnnexinV标记的流式细胞仪法检测细胞凋亡, 发现L-OHP在体外可以明显抑制胃癌细胞株SGC-7901的生长. 浓度为1 mmoL/L的L-OHP与细胞株作用30min, 流式细胞仪即可检测出细胞凋亡水平升高, 提示药物作用早期以细胞凋亡为主; 1 mmol/L作用2 d, 流式细胞仪及TUNEL均可检测到细胞凋亡水平显著性升高. 实验结果提示随药物浓度的增加及作用时间的延长, 细胞凋亡率及细胞死亡率均显著上升, 提示此时细胞凋亡与死亡并存. 因此, L-OHP在体外对胃癌细胞株SGC-7901生长的抑制早期主要机制为诱导细胞凋亡, 随着作用时间的延长, 诱导凋亡与细胞毒作用并存. RT-PCR结果提示L-OHP作用后的细胞caspase-3 m-RNA表达明显增强, 与L-OHP诱导凋亡作用相关, 提示药物诱导凋亡过程中有caspase家族的参与.
总之, L-OHP对于进展期胃癌是一种相对低毒高效药物, 包含L-OHP的联合化疗方案临床疗效可与目前其他方案媲美, 但安全性明显提高. 诱导细胞caspase-3基因表达从而导致凋亡可能是其治疗胃癌的机制之一; 在细胞凋亡的早期检测中应用ANEXIN-V标记流式细胞仪检测的灵敏度较高. 然而L-OHP应用于临床时间不久, 对中晚期胃癌的临床疗效及毒副作用还有待大样本的多中心随机双盲临床试验加以验证.
| 1. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] [DOI] |
| 2. | Cao WX, Ou JM, Fei XF, Zhu ZG, Yin HR, Yan M, Lin YZ. Methionine-dependence and combination chemotherapy on human gastric cancer cells in vitro. World J Gastroenterol. 2002;8:230-232. [PubMed] [DOI] |
| 3. | Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol. 2002;8:446-450. [PubMed] [DOI] |
| 4. | Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451-454. [PubMed] [DOI] |
| 5. | Shi XY, Zhao FZ, Dai X, Ma LS, Dong XY, Fang J. Effect of jianpiyiwei capsule on gastric precancerous lesions in rats. World J Gastroenterol. 2002;8:608-612. [PubMed] [DOI] |
| 6. | Zhao AG, Zhao HL, Jin XJ, Yang JK, Tang LD. Effects of Chinese Jianpi herbs on cell apoptosis and related gene expression in human gastric cancer grafted onto nude mice. World J Gastroenterol. 2002;8:792-796. [PubMed] [DOI] |
| 7. | Hu JK, Chen ZX, Zhou ZG, Zhang B, Tian J, Chen JP, Wang L, Wang CH, Chen HY, Li YP. Intravenous chemotherapy for resected gastric cancer: meta-analysis of randomized controlled trials. World J Gastroenterol. 2002;8:1023-1028. [DOI] |
| 8. | Cao WX, Cheng QM, Fei XF, Li SF, Yin HR, Lin YZ. A study of preoperative methionine-depleting parenteral nutrition plus chemotherapy in gastric cancer patients. World J Gastroenterol. 2000;6:255-258. [PubMed] |
| 9. | Liu JR, Chen BQ, Yang YM, Wang XL, Xue YB, Zheng YM, Liu RH. Effect of apoptosis on gastric adenocarcinoma cell line SGC-7901 induced by cis-9, trans-11-conjugated linoleic acid. World J Gastroenterol. 2002;8:999-1004. [PubMed] [DOI] |
| 10. | Liu DH, Zhang XY, Fan DM, Huang YX, Zhang JS, Huang WQ, Zhang YQ, Huang QS, Ma WY, Chai YB. Expression of vascular endothelial growth factor and its role in oncogenesis of human gastric carcinoma. World J Gastroenterol. 2001;7:500-505. [DOI] |
| 11. | Cai L, Yu SZ, Zhang ZF. Glutathione S-transferases M1, T1 genotypes and the risk of gastric cancer: a case-control study. World J Gastroenterol. 2001;7:506-509. [DOI] |
| 12. | He XS, Su Q, Chen ZC, He XT, Long ZF, Ling H, Zhang LR. Expression, deletion [was deleton] and mutation of p16 gene in human gastric cancer. World J Gastroenterol. 2001;7:515-521. [DOI] |
| 13. | Fang DC, Yang SM, Zhou XD, Wang DX, Luo YH. Telomere erosion is independent of microsatellite instability but related to loss of heterozygosity in gastric cancer. World J Gastroenterol. 2001;7:522-526. [DOI] |
| 14. | Liu S, Wu Q, Chen ZM, Su WJ. The effect pathway of retinoic acid through regulation of retinoic acid receptor alpha in gastric cancer cells. World J Gastroenterol. 2001;7:662-666. [DOI] |
| 15. | Xue FB, Xu YY, Wan Y, Pan BR, Ren J, Fan DM. Association of H pylori infection with gastric carcinoma: a Meta analysis. World J Gastroenterol. 2001;7:801-804. [DOI] |
| 16. | Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586-590. [PubMed] [DOI] |
| 17. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS, VEGF, tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] [DOI] |
| 18. | Ren J, Dong L, Xu CB, Pan BR. The role of KDR in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:596-601. [DOI] |
| 19. | Ren J, Dong L, Xu CB, Pan BR. Expression of sphingosine kinase gene in the interactions between human gastric carcinoma cell and vascular endothelial cell. World J Gastroenterol. 2002;8:602-607. [DOI] |
| 20. | Su JM, Gui L, Zhou YP, Zha XL. Expression of focal adhesion kinase and alpha5 and beta1 integrins in carcinomas and its clinical significance. World J Gastroenterol. 2002;8:613-618. [DOI] |
| 21. | Nie YZ, He FT, Li ZK, Wu KC, Cao YX, Chen BJ, Fan DM. Identification of tumor associated single-chain Fv by panning and screening antibody phage library using tumor cells. World J Gastroenterol. 2002;8:619-623. [DOI] |
| 22. | Zhao Y, Wu K, Xia W, Shan YJ, Wu LJ, Yu WP. The effects of vitamin E succinate on the expression of c-jun gene and protein in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:782-786. [DOI] |
| 23. | Fang DC, Luo YH, Yang SM, Li XA, Ling XL, Fang L. Mutation analysis of APC gene in gastric cancer with microsatellite instability. World J Gastroenterol. 2002;8:787-791. [DOI] |
| 24. | Wu K, Li Y, Zhao Y, Shan YJ, Xia W, Yu WP, Zhao L. Roles of Fas signaling pathway in vitamin E succinate-induced apoptosis in human gastric cancer SGC-7901 cells. World J Gastroenterol. 2002;8:982-986. [PubMed] [DOI] |
| 25. | Zhou YN, Xu CP, Han B, Li M, Qiao L, Fang DC, Yang JM. Expression of E-cadherin and beta-catenin in gastric carcinoma and its correlation with the clinicopathological features and patient survival. World J Gastroenterol. 2002;8:987-993. [PubMed] [DOI] |
| 26. | Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002;8:994-998. [DOI] |
| 27. | Jiang YA, Zhang YY, Luo HS, Xing SF. Mast cell density and the context of clinicopathological parameters and expression of p185, estrogen receptor, and proliferating cell nuclear antigen in gastric carcinoma. World J Gastroenterol. 2002;8:1005-1008. [DOI] |
| 28. | Guo DL, Dong M, Wang L, Sun LP, Yuan Y. Expression of gastric cancer-associated MG7 antigen in gastric cancer, precancerous lesions and H pylori-associated gastric diseases. World J Gastroenterol. 2002;8:1009-1013. [DOI] |
| 29. | Li HL, Chen DD, Li XH, Zhang HW, Lu JH, Ren XD, Wang CC. JTE-522-induced apoptosis in human gastric adenocarcinoma [correction of adenocarinoma] cell line AGS cells by caspase activation accompanying cytochrome C release, membrane translocation of Bax and loss of mitochondrial membrane potential. World J Gastroenterol. 2002;8:217-223. [DOI] |
| 30. | Li HL, Chen DD, Li XH, Zhang HW, Lu YQ, Ye CL, Ren XD. Changes of NF-B, p53, Bcl-2 and caspase in apoptosis induced by JTE-522 in human gastric adenocarcinoma cell line AGS cells: role of reactive oxygen species. World J Gastroenterol. 2002;8:431-435. [PubMed] [DOI] |