修回日期: 2002-08-03
接受日期: 2002-08-07
在线出版日期: 2003-01-15
目的: 本实验通过测定急性胰腺炎(AP)患者血清白介素-15(IL-15)、白介素-18(IL-18)及可溶性肿瘤坏死因子受体-1(sTNF-1R)的水平, 探讨他们与AP的关系. 分析他们之间的相关性. 研究对重症急性胰腺炎(SAP)的发生可能有预测作用的指标. 探讨炎性递质在急性胰腺炎发病机制中的作用.
方法: 选择AP患者26例, 按照急性胰腺炎的临床诊断及分级标准分组, 其中SAP患者7例, 轻型急性胰腺炎(MAP)患者19例, 正常对照组10例. 用ELISA 法检测血清IL-15、IL-18及STNF-1R浓度.
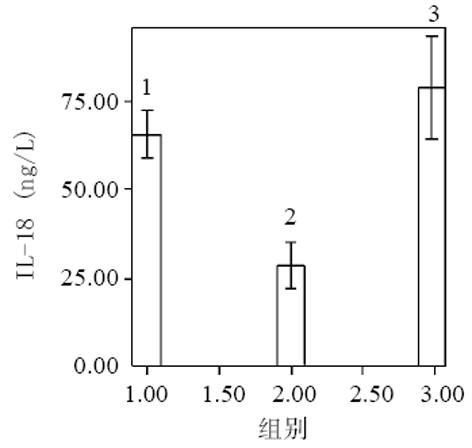
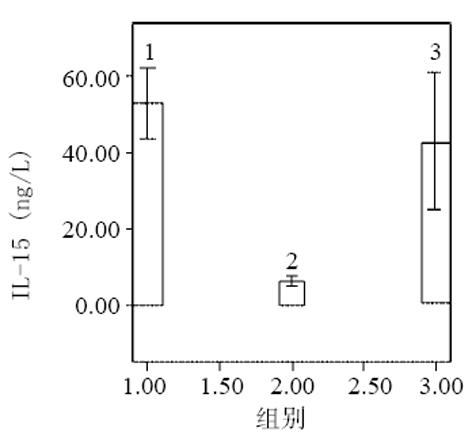
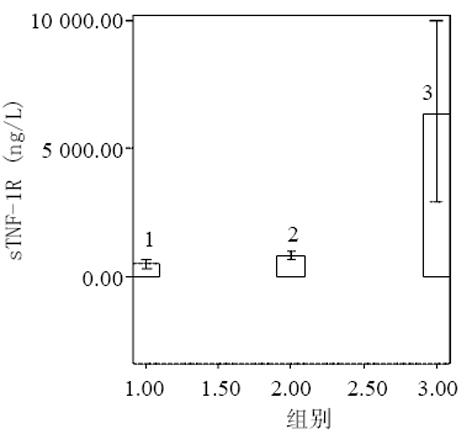
结果: 血清中 IL-15、IL-18及sTNF-1R浓度在MAP和SAP两组患者之间存在显著差异, SAP组明显高于MAP组, SAP 同MAP组比较 : IL-18: 78±15 vs 28±13 ng/L(P<0.01); IL-15: 42±19 vs 6±2 ng/L(P<0.01); sTNF-1R: 6 327±3 655 vs 832±329 ng/L(P<0.01); sTNF-1R在SAP和MAP组显著高于正常对照组, SAP同对照组比较6327±3 655 vs 545±123 ng/L(P<0.01); MAP同对照组比较 832±329 vs 545±123 ng/L(P<0.01); IL-15同IL-18有相似的结果, 即MAP组显著低于正常对照组, MAP同对照组比较 : IL-18 : 28±13 vs 66±10 ng/L(P<0.01); IL-15: 6±2 vs 53±13 ng/L(P<0.01); SAP组同正常对照组之间无显著性差异(P>0.05). 轻型急性胰腺炎患者血清sTNF-1R 同IL-18呈显著正相关(r = 0.98, P<0.01); sTNF-1R 同IL-15呈显著正相关(r = 0.823, P<0.01); IL-18同IL-15呈显著正相关(r = 0.95, P<0.01). 重型急性胰腺炎患者血清IL-18同IL-15呈显著正相关(r = 0.906, P<0.01); sTNF-1R 同IL-15呈显著正相关(r = 0.93, P<0.01); sTNF-1R同IL-18呈显著正相关(r = 0.953, P<0.01).
结论: 血清 IL-15、IL-18及sTNF-1R参与了急性胰腺炎的炎症反应过程, 可能是预测急性胰腺炎的严重程度的指标.
引文著录: 贺丽, 陈少夫, 曹晓辉, 张力达, 潘丽丽, 周卓. 急性胰腺炎患者血清IL-15 IL-18和sTNF-1R的变化意义. 世界华人消化杂志 2003; 11(1): 57-60
Revised: August 3, 2002
Accepted: August 7, 2002
Published online: January 15, 2003
AIM: To detect the serum level of IL-15, IL-18 and soluble tumor necrosis factor receptor-1 (sTNF-1R) in patients with acute pancreatitis (AP), as well as the correlation among the three factors in AP.
METHODS: According to the clinical diagnosis and criteria for acute pancreatitis, 26 patients with AP were divided into severe acute pancreatitis (SAP) group (n = 7) and mild acute pancreatitis (MAP) group (n = 19). Ten normal individuals were used as the control group (n = 10). The serum level of IL-15, IL-18 and sTNF-1R were detected by ELISA.
RESULTS: IL-15, IL-18 and STNF-1R in the SAP group was higher than that in the MAP group (IL-15, 42±19 vs 6±2 ng/L, P < 0.01; IL-18, 78±15 vs 28±13 ng/L, P < 0.01; sTNF-1R, 6 327±3 655 vs 832±329 ng/L, P < 0.01). sTNF-1R in the SAP and MAP group was higher than that in the control group (SAP vs the control group: 6 327±3 655 vs 545±123 ng/L, P < 0.01; MAP vs the control group: 832±329 vs 545±123 ng/L, P < 0.01). The results of IL-15 and IL-18 were similar to those of sTNF-1R, those in the MAP group were lower than that in the control group (MAP vs the control group, IL-18: 28±13 vs 66±10 ng/L, P < 0.01; IL-15: 6±2 vs 53±13 ng/L, P < 0.01). There was no significant difference between the SAP group and the control group (P > 0.05). In the MAP group, there was significant positive correlation between sTNF-1R and IL-18 (r = 0.98, P < 0.01), sTNF-1R and IL-15 (r = 0.823, P < 0.01), IL-18 and IL-15 (r = 0.95, P < 0.01), respectively. In the SAP group, there was significant positive correlation between IL-15 and IL-18 (r = 0.906, P < 0.01), sTNF-1R and IL-15 (r = 0.93, P < 0.01), sTNF-1R and IL-18 (r = 0.953, P < 0.01), respectively.
CONCLUSION: IL-15, IL-18 and sTNF-1R play important roles in the development of acute pancreatitis, and they may be valuable indexes to predict the severity of AP.
- Citation: He L, Chen SF, Cao XH, Zhang LD, Pan LL, Zhou Z. Changes of serum level of IL-15, IL-18 and sTNF-1R in patients with acute pancreatitis. Shijie Huaren Xiaohua Zazhi 2003; 11(1): 57-60
- URL: https://www.wjgnet.com/1009-3079/full/v11/i1/57.htm
- DOI: https://dx.doi.org/10.11569/wcjd.v11.i1.57
急性胰腺炎(acute pancreatitis, AP)是常见的消化系急症, 多数属自限性. 约15%-30%成为重症急性胰腺炎(severe acute pancreatitis, SAP).. SAP死亡率极高[1]. 目前的治疗方案不能从根本上降低SAP的死亡率及并发症的发生[2,3]. 至今, 其发病机制仍未完全阐明[4,5]. 比较公认的四大机制是胰腺胰酶自身消化[6], 胰腺血循环障碍[7-10], 白细胞过度激活[11-13]和肠道细菌移居胰腺组织学说[14-19], 炎性因子的产生及其级联瀑布效应(cascade teaction), 使得胰腺局限性炎症反应发展为威胁生命的SAP[20-22]越来越受到关注. 我们检测AP患者血清IL-15, IL-18和可溶性肿瘤坏死因子受体-1(sTNF-1R)水平间的相关性, 探讨炎性递质在AP发病机制中的作用, 用于指导治疗.
选择2001-03/2002-01中国医大二院及沈医二院消化病房住院治疗的AP患者26例. 包括男性11例, 年龄44±19(范围24-70)岁; 女性15例, 年龄54±16(范围20-74)岁, 所有患者均于症状出现后24 h内入院. 体检健康者10名作对照, 所有受试者无胃肠、肝胆胰疾病、糖尿病、肿瘤及自身免疫性疾病病史. 把AP患者分为轻型急性胰腺炎组(mild acute pancreatitis, MAP)和重症急性胰腺炎(SAP)组. 血钙浓度≤1.87mmol/L, APACHE-Ⅱ评分≥8分和Balthazar CT评分≥Ⅱ级者为SAP组, 低于以上标准者为MAP组[23,24]. IL-15、IL-18和sTNF-1R试剂盒均由上海森雄公司提供. 酶标仪型号为奥地利Tecan Sunrise.
清晨空腹取静脉血3 mL, 提取血清, 平均分装于3个试管, 均冻于-70 ℃冰箱保存, 待测血清IL-15, IL-18, sTNF-1R浓度. 采用双抗体夹心ABC-ELISA 法, 在492 nm处测定吸光度(A值). 所有A值都应减除空白值后再行计算. 绘出标准曲线. 根据样品A值求出标本中各细胞因子浓度.
统计学处理 所有测定值均用平均数±标准差(mean±SD)表示, 应用Student t检验对两个样本均数做显著性检验, 用直线回归方程做两因素间相关性分析. 统计运用SPSS统计软件完成.
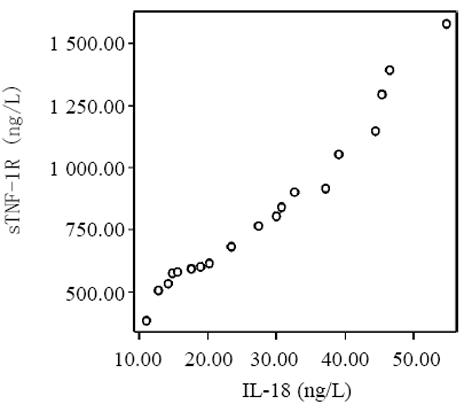
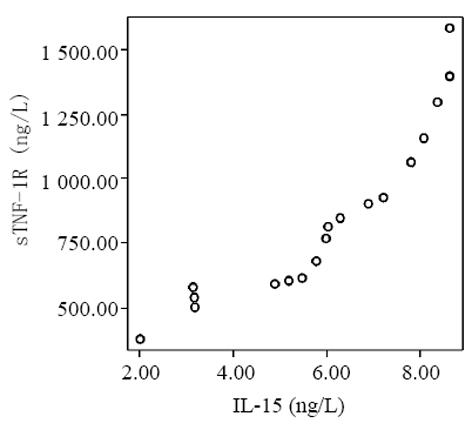
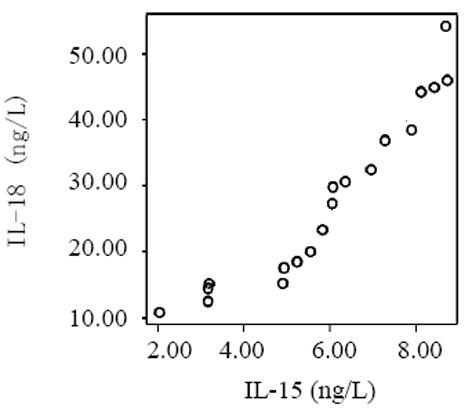
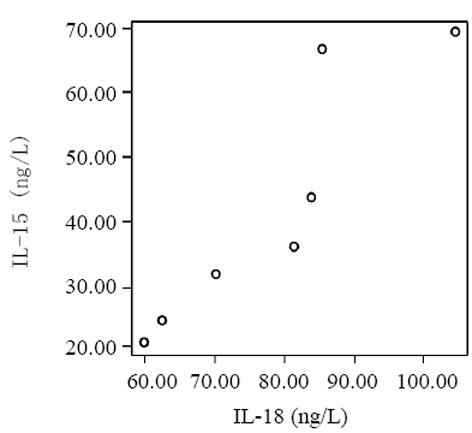
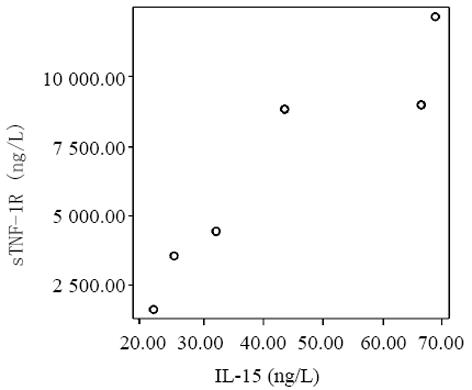
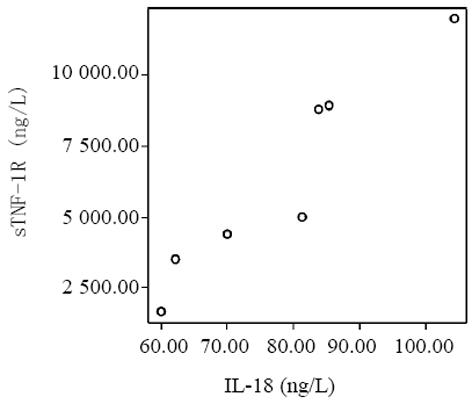
血清中IL-15, IL-18和sTNF-1R浓度在MAP和SAP两组患者之间存在显著差异, SAP组明显高于MAP组(P<0.01); sTNF-1R在MAP和SAP两组显著高于正常对照组(P<0.01); IL-15同IL-18在MAP组显著低于SAP组和正常对照组(P<0.01); IL-15在SAP略低于正常对照组, 而IL-18在SAP组略高于正常对照组, 但两组之间无显著差异(P>0.05, 表1, 图1-3). 轻型急性胰腺炎患者血清sTNF-1R 同IL-18呈显著正相关(Y = -4.428± 0.0392x, r = 0.98, n = 19, P<0.01, 图4); sTNF-1R 同IL-15呈显著正相关(Y = 1.472±0.006x, r = 0.823, n = 19, P<0.01, 图5); IL-18同IL-15呈显著正相关(Y = 1.795±0.145x, r = 0.95, n = 19, P<0.01, 图6). 重型急性胰腺炎患者血清IL-18同IL-15呈显著正相关(Y = -45.809±1.123x, r = 0.906, n = 7, P<0.01, 图7); sTNF-1R 同IL-15呈显著正相关(Y = 11.119±0.004x, r = 0.93, n = 7, P<0.01, 图8); sTNF-1R 同IL-18呈显著正相关(Y = 52.825±0.004x, r = 0.953, n = 7, P<0.01, 图9).
AP患者血清中前炎性递质如IL-1, IL-6, IL-8和TNFα升高, 提示免疫学机制参与了AP的发病机制[25]. AP时, 胰腺组织和血浆中检测到TNFa表达, 并与胰腺损伤及炎症程度密切相关, 在AP早期显著升高. 正常对照组sTNF-1R相对低水平表达, 在人体发生炎症反应时随之升高, MAP组与正常对照组比较有显著差异(P<0.01), 随着炎症反应进一步加重, SAP组升高更为显著, 与前两组比较均存在显著性差异(P<0.01). 说明随炎症反应的进一步加重, 机体的防御体系也在进一步加强, 与疾病的严重程度相关. sTNF-1R升高提示炎症反应加重. 但亦有相反的报道, 如患者sTNF-1R早期即下降, 表明受体消耗过多, 导致抗炎能力下降, 使得AP进一步恶化, 故如sTNF-1R骤减, 提示预后不良. 近年来细胞因子参与AP发病过程日益受到广泛关注, IL-15在AP中的作用尚不清楚. 在本实验中, MAP组患者血清IL-15浓度同正常对照组比较显著降低(P<0.01), 说明在疾病早期由于存在炎性因子的过度释放, IL-15呈低浓度表达, 发挥其早期保护作用, 抑制过多促炎性因子产生, 而不抑制抗炎因子的作用, 故可防止炎症反应进一步加剧. 实验结果同时显示, SAP 组患者血清IL-15浓度明显高于MAP组. 说明随炎症反应进一步加重, IL-15血清浓度随之增加, 呈高浓度表达, 增加了促炎因子的产生, 加重了高细胞因子血症的恶性循环. SAP 组同MAP组血清IL-15浓度均低于正常对照组, SAP 组同正常对照组之间无显著性差异. 说明血清IL-15在急性胰腺炎早期主要发挥其保护作用; 另一可能原因是血清IL-15在入院后24 h内未达到高峰浓度, 随着疾病发展, 处于动态上升过程. 因此, 如急性胰腺炎早期血清IL-15浓度显著下降, 提示预后良好. 如急性胰腺炎早期血清IL-15浓度下降不明显, 或轻度升高, 提示预后不良, 有恶化倾向. IL-18在重症急性胰腺炎患者中显著升高, 在并发胰腺坏死和远处脏器衰竭中IL-18升高与并发症的发生平行. AP 患者血清中一般检测不出IL-1, 可能的原因是IL-1半衰期短, 临床不易测出; 另一方面可能的原因是IL-1在检测水平之下发挥其生物学作用. 故IL-18的检测就显得尤为重要. IL-18在疾病发生后1 wk仍明显升高, 故便于跟踪监测. 本实验中, SAP组患者血清IL-18浓度显著高于MAP组(P<0.01), 说明IL-18与疾病严重程度相关. 同Rau et al[26]结果一致. MAP组血清IL-18浓度显著低于正常对照组(P<0.01), 而SAP组仅略高于正常对照组, 二者之间无显著性差异. 正常人血清中促炎细胞因子和抗炎细胞因子处于动态平衡, 在炎症发生早期, 促炎细胞因子大量产生同时, 也产生抗炎细胞因子, 这些抗炎细胞因子具有重要调节功能, 能限制炎症反应. 考虑AP早期血清IL-18浓度处于受抑制状态, 呈低水平表达, 随着炎症反应的加重, 促炎细胞因子超过抗炎细胞因子的作用, 加速炎症反应的过程, 血清IL-18浓度随之升高.
已有研究表明血清sTNF-1R 与IL-18浓度在SAP时显著升高, 我们观察到sTNF-1R 与IL-18呈显著正相关, 二者可很好预测急性胰腺炎的严重程度. IL-15同IL-18均由单核巨噬细胞系统产生, 与IFN-r产生相关, 具有相似的生物学作用, 实验结果表明, 随着病情加重, 二者同时升高, 且变化趋势相似, 二者呈显著正相关, 对AP有很好的预测作用. sTNF-1R 为TNFa的特异性受体, IL-15 能诱导TNFα的产生, sTNF-1R的产生与IL-15水平密切相关, 实验结果表明, 二者呈显著正相关, 可预测急性胰腺炎的严重程度.
编辑: N/A
| 1. | Tiscornia OM, Hamamura S, Lehmann ES, Otero G, Waisman H, Tiscornia-Wasserman P, Bank S. Biliary acute pancreatitis:a review. World J Gastroenterol. 2000;6:157-168. [PubMed] |
| 6. | Wu XN. The mechanism of actions of Octreotide, Bupleurum-Peony Cheng Qi decoction and Dan Shan in severe acute pancretitis. World J Gastroenterol. 1999;5:249-251. [PubMed] [DOI] |
| 11. | Xia Q, Jiang JM, Gong X, Chen GY, Li L, Huang ZW. Experimental study of Tong Xia purgative method in ameliorating lung injury in acute necrotizing pancreatitis. World J Gastroenterol. 2000;6:115-118. [PubMed] [DOI] |
| 13. | Brivet FG, Emilie D, Galanaud P. Pro- and anti-inflammatory cytokines during acute severe pancreatitis: an early and sustained response, although unpredictable of death. Parisian Study Group on Acute Pancreatitis. Crit Care Med. 1999;27:749-755. [PubMed] [DOI] |
| 15. | de Souza LJ, Sampietre SN, Assis RS, Knowles CH, Leite KR, Jancar S, Monteiro Cunha JE, Machado MC. Effect of platelet-activating factor antagonists (BN-52021, WEB-2170, and BB-882) on bacterial translocation in acute pancreatitis. J Gastrointest Surg. 2001;5:364-370. [PubMed] [DOI] |
| 20. | Gloor B, Schmidtmann AB, Worni M, Ahmed Z, Uhl W, Büchler MW. Pancreatic sepsis: prevention and therapy. Best Pract Res Clin Gastroenterol. 2002;16:379-390. [PubMed] [DOI] |
| 21. | Hartwig W, Maksan SM, Foitzik T, Schmidt J, Herfarth C, Klar E. Reduction in mortality with delayed surgical therapy of severe pancreatitis. J Gastrointest Surg. 2002;6:481-487. [PubMed] [DOI] |
| 24. | Osvaldt AB, Viero P, Borges da Costa MS, Wendt LR, Bersch VP, Rohde L. Evaluation of Ranson, Glasgow, APACHE-II, and APACHE-O criteria to predict severity in acute biliary pancreatitis. Int Surg. 2001;86:158-161. [PubMed] |
| 26. | Rau B, Baumgart K, Paszkowski AS, Mayer JM, Beger HG. Clinical relevance of caspase-1 activated cytokines in acute pancreatitis: high correlation of serum interleukin-18 with pancreatic necrosis and systemic complications. Crit Care Med. 2001;29:1556-1562. [PubMed] [DOI] |