Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.2083
Revised: January 30, 2003
Accepted: March 10, 2003
Published online: September 15, 2003
AIM: To study the expression of enhanced green fluorescent protein (EGFP) gene in retrovirally transduced variant HT-29 cells.
METHODS: The retroviral vector prkat EGFP/neo was constructed and transfected into the 293T cell using a standard calcium phosphate precipitation method. HT-29c cells (selected from HT-29 cells) were transduced by a retroviral vector encoding the GEFP gene. The fluorescence intensity of colorectal carcinoma HT-29c cells after transduced with the EGFP bearing retrovirus was visualized using fluorescence microscope and fluorescence activated cell sorter (FACS) analysis. Multiple biological behaviors of transduced cells such as the proliferating potential and the expression of various antigens were comparatively analyzed between untransduced and transduced cells in vitro. EGFP expression of the fresh tumor tissue was assessed in vivo.
RESULTS: After transduced, HT-29c cells displayed a stable and long-term EGFP expression under the nonselective conditions in vitro. After cells were successively cultured to passage 50 in vitro, EGFP expression was still at a high level. Their biological behaviors, such as expression of tumor antigens, proliferation rate and aggregation capability were not different compared to untransduced parental cells in vitro. In subcutaneous tumors, EGFP was stable and highly expressed.
CONCLUSION: An EGFP expressing retroviral vector was used to transduce HT-29c cells. The transduced cells show a stable and long-term EGFP expression in vitro and in vivo. These cells with EGFP are a valuable tool for in vivo research of tumor metastatic spread.
- Citation: Wang M, Boenicke L, Howard BD, Vogel I, Kalthoff H. Gene transfer and expression of enhanced green fluorescent protein in variant HT-29c cells. World J Gastroenterol 2003; 9(9): 2083-2087
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/2083.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.2083
The detection of tumor invasion and micrometastasis in fresh tissues is necessary for critical understanding of tumor progression and its control. The real-time visualization of tumor cells, micrometastasis and their progression during the course of the disease is not easy to study in current models of metastasis. The green fluorescent protein (GFP) from the jellyfish Aequorea victoria has attracted widespread interest and has become an important reporter gene since heterologous expression of the cloned gene was found to be able to generate striking green fluorescence[1,2]. GFP is a relatively small polypeptide consisting of 238 amino acid residues, and is able to produce green fluorescence when excited with a blue light. So far, it has been used as a reporter of gene expression, tracers of cell lineage, and fusion tags to monitor protein localization within living cells in a broad spectrum of model organisms[3]. No additional substrates are required to detect GFP and it can be monitored in living cells. But the sensitivity of wild type GFP is below that of standard reporter proteins, such as β-galactosidase, which utilizes enzymatic amplification. Wild type GFP exhibits lower fluorescence intensity which is hard to detect in several mammalian cells[4]. To improve the detection of GFP in transduced mammalian cells, a unique GFP variant, which contains a chromophore mutation making the protein 35 times brighter than wild type GFP, and is codon-optimized for high level expression in mammalian cells has been constructed[5,6]. These changes in the GFP coding sequence provide an enhanced GFP (EGFP) that greatly increases the sensitivity of the reporter protein[7,8].
GFP has demonstrated its potential for use as a marker for gene expression in a variety of cell types[9,10]. Numerous studies have proven the usefulness of GFP as a reporter molecule in the setting of transient gene expression[11,12]. However, it remains unclear whether colorectal carcinoma cell lines are able to stably express and maintain high level of EGFP expression over many passages in the absence of selective growth conditions. In this study, we assessed the expression of colorectal carcinoma cells after transduced with EGFP gene, and evaluated their biological behaviors in vitro. Moreover, to develop an experimental animal model of colorectal carcinoma that improves the visualization of fresh tissue, we injected EGFP-expressing human colorectal carcinoma cells subcutaneously into rats. This model involves the stable transduction of HT-29c tumor cells in vitro with the EGFP gene that could be stably and highly expressed in vivo.
Cell lines and cell culture HT-29 cell line, a gift of Dr. Dippold (Mainz, Germany), was established from a human colon adenocarcinoma with moderate differentiation, HT-29c with increased metastatic activity was a variant cell line after three cycles of selection of liver metastases from injected HT-29 cells[13]. All cell lines were grown in 75 cm2 cluture flasks in RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine and 1 mM sodium pyruvate (Life Technologies) in a humidified atmosphere of 5% CO2 and 95% air at a 37 °C incubator (Heraeus, Germany).
Plasmids For subcloning the HSV-TK gene and modifying the restriction sites on the 5’ and 3’ ends, the pSP72 cloning vector was obtained from Promega Corp., Madison, WI. The gene coding for humanized EGFP of Aequorea victoria contained in the plasmid pEGFP-C was obtained from Clontech Laboratories (Heidelberg, Germany). prkat, a retroviral vector backbone derived from the Moloney murine leukemia virus (MMLV) was provided by Cell Genesys Corp. The expression vector for the vesicular stomatitis virus G protein, pCMV VSV-G, was generously provided by Dr. Ted Friedman.
Construction of retroviral vector General molecular biological cloning techniques and the necessary solutions used to generate this plasmid vector were found in standard protocols[14]. A 0.7 kb EcoR I/BamH I fragment containing the coding region of EGFP gene was isolated and ligated into the prkat to generate prkat EGFP/neo. In this construct, the MMLV long terminal repeat (LTR) controled the expression of EGFP gene and an internal IRES sequence drived the expression of the neomycin resistance marker.
Experimental animal Three-week-old male athymic Rowett nude rats (Hsd: RH-nu/nu) were obtained from Harlan/Winkelmann (Borchen, Germany). All the rats were housed in cages with filter bonnet under special pathogen-free conditions in a laminar flow cabinet (EHRET, DIPL.-ING. W. EHRET GmbH, Germany) at constant temperature (24-26 °C), humidity (40%-50%) and 12-h light/12-h dark cycle. The rats were fed on standard rat food (Altromin, Lage/Lippe, Germany) and water ad libitum. Operative equipments, all cages and bedding were autoclaved at 121 °C for 30 minutes. All animal manipulations were done aseptically in a transverse laminar flow hood (BDK, Luft-und Reinraumtechnik GmbH, Germany).
Production of retrovirus particles and transduction of HT-29c cells with rkat EGFP/neo retroviruses 1.5 × 106 293T cells were seeded onto 10 cm2 PrimariaTM dishes. The next day, fresh medium was added 4 h prior to transduction. 10 μg of prkat EGFP/neo, 5 μg of prkat gag/pol and 5 μg pCMV-VSV were co-transfected into the 293T cells using a standard calcium phosphate precipitation method. 24 h later fresh medium (DMEM high glucose with 10% FCS plus 2 mM glutamine, 1 mM sodium pyruvate and 1X non essential amino acids) was added. 48 h after the cells were washed, the supernatant containing VSV-G pseudotyped recombinant retroviruses was harvested from the plate and filtered using a 0.45 μm low protein binding AcrodiscTM filter (Gelman Sciences, Ann Arbor, MI). 3 mL of the retroviral supernatant was then added to a 6 cm2 dish seeded with 1 × 105 HT-29c cells containing 8 μg/mL polybrene. 24 h later, the transduced HT-29c cells were placed under geneticin (G418, Life Technologies) selection (700 μg/mL). After two weeks, individual clones were generated by limited dilution. 96 well plates were seeded using cell densities of 3, 5 and 10 cells per well. Within 3-4 weeks, 12 separate clones were generated and expanded into 6 well plates. To analyze the expression of EGFP in the individual clones, 5 × 105 cells from each clone were fixed in 2% formaldehyde and the fixed cells were analyzed by FACS. The two clones with the most intense fluorescence, HT-29cEGFPclone #1 and #7 were selected and used for in vitro or in vivo studies.
Cell culture of transduced HT-29c cells HT-29cEGFP, HT-29cEGFPclone#1 and clone#7 cells were grown in supplemented RPMI-1640 medium. The cultures were incubated at 37 °C in a humidified atmosphere of 5% CO2. G418 was added to cell medium at a final concentration of 600 mg/mL from first till 15th passage for selection. After passage 15, the cells were grown in the absence of G418 and cells were passaged twice per week.
Microscopic and FACS analysis of EGFP expressing cells in vitro HT-29c EGFPclone#1 and clone#7 cells were seeded onto chamber slides. When cells grown in monolayer became confluent, the fluorescence of the cells were visualized with an Axioskop fluorescence microscope (Carl Zeiss, Germany) equipped with a FITC filter set (UV light exciter BP 546 nm, FT 580 nm, emitter LP 590 nm). Cultivated cells were harvested by trypsinization and were fixed in 0.4 mL 2% formaldehyde. The fluorescence intensity of samples was analyzed using fluorescence activated cell sorter (FACS, Epics XL, Hamburg, Germany).
Comparative analysis of biological behavior between transduced and untransduced cell lines in vitro Growth rate determination: HT-29, HT-29c, HT-29c EGFPclone#1 and clone#7 cells were seeded in six-well plastic culture plates, in triplicate at a density of 1 × 105 in supplemented medium. The cells were harvested by trypsinization and counted every 24 h using a hemocytometer. The test was repeated three times. The mean number of cells in each interval for each cell line was determined. The growth curve of each cell line was constructed. The doubling time of tumor cell growth was calculated from the cell growth curve over 5 days according to the formula: Doubling time = (T2 - T1) In 2/(In N2 - In N1), in which N1 and N2 are the number of tumor cells at time points of T1 and T2, respectively.
Three-dimensional spheroid culture of cell lines: Three-dimensional spheroid culture of HT-29c and HT-29cEGFPclone cells were performed as follows: six-well culture plates were pre-coated with 2 mL 1% (w/v) agarose gel/per well. The single-cell suspension containing 1 × 105 tumor cells in supplemented medium was seeded onto each well and incubated in a humidified 5% CO2 at a 37 °C incubator. Cell aggregation was monitored daily using a phase-contrast microscope (Carl Zeiss, Germany).
Expression of different antigens: Cells were seeded onto 10-well mask slides and incubated for 48 h as described above. Cells on the slides were fixed in cold acetone (Merck, Darmstadt, Germany) for 5 minutes. Immunohistochemical staining (IHC) was performed using the standard ABC method with VECTASTAIN ABC-kit and monoclonal antibodies (mAbs) KL-1 (Keratin), IT-ks20.10 (Cytokeratin 20), C1P83 (CEA), CA19-9 (CA19-9), MiB-1 (Ki-67) and Do7 (p53). All mAbs were commercially available except for C1P83 which was provided by Prof. Kalthoff H. The percentage of positive tumor cells was determined by calculating 1000 tumor cells in 5 random vision fields of one section under microcope.
EGFP expression of HT-29c cells in vivo All the rats were stabilized for one week in the laboratory before the experiments. 0.5 mL single-cell suspension containing 2 × 107 cells of HT-29cEGFPclone#7 was injected subcutaneously into both flanks of the rat. The rat was monitored daily. When the tumor reached 15 mm in diameter, the rat was killed. The fresh tumor tissues were sliced at 0.7-1.0 mm thickness and sliced at 60 μm cryosections, then observed directly under the fluorescence microscope.
Statistical analyses were performed using the F test.
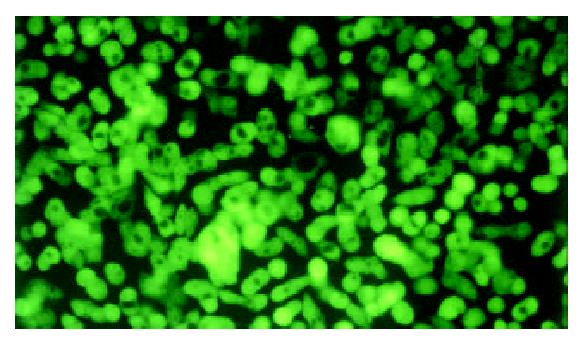
HT-29c cells could be transduced by retroviral vector with EGFP and then selected in G418. Transduced HT-29c cells with EGFP (HT-29cEGFP, HT-29c EGFPclone#1 and clone#7) were grown in vitro in G418 (600 μg/mL). Untransduced HT-29c cells did not survive in G418, suggesting all cells within the transduced pools contained at least one copy of a transcriptionally active neomycin phosphotransferase gene. Under fluorescence microscope, the selected neomycin-resistant HT-29cEGFP and HT-29cEGFPclone cells all displayed strong fluorescence (Figure 1). HT-29cEGFPclone cells exhibited stronger fluorescence than HT-29cEGFP cells, no significant difference was found between fluorescence levels of HT-29cEGFPclone#1 and clone#7 cells by FACS analysis (Table 1). After 8 weeks in culture, G418 was removed from the growth medium. The expression of EGFP fluorescence of HT-29cEGFP clone cells was still stable for over six months in vitro. No significant difference was found between passage 5 and passage 50 of HT-29cEGFPclone#1 in fluorescence intensity by FACS (Table 2).
| Cell lines | Fluorescence intensity |
| HT-29c | 2.5 ± 0.9a |
| HT-29cEGFP pool | 64.6 ± 7.4b |
| HT-29cEGFPclone#1 | 206.5 ± 39.9c |
| HT-29cEGFPclone#7 | 203.4 ± 46.4d |
| Clone #1 | Fluorescence intensity |
| Passage 5 | 217.8 |
| Passage 17 | 198.1 |
| Passage 31 | 193.4 |
| Passage 40 | 212.1 |
| Passage 50 | 208.8 |
Comparison of cell proliferation rate The results indicated that there was no significant difference in the cell proliferation rates of parental cells and selected transfectants as determined by comparing their doubling time (Table 3).
| Cell line | Doubling time (h)a |
| HT-29 | 25.3 ± 5.5 |
| HT-29c | 26.0 ± 3.3 |
| HT-29cEGFPclone #1 | 26.3 ± 4.7 |
| HT-29cEGFPclone #7 | 27.7 ± 5.3 |
Comparison of aggregation potential To compare cell aggregation potential between untransfected and transfected cells in vitro, HT-29c and HT-29cEGFPclone cells were monitored under three dimensional culture conditions. At 4 h after incubation, cell aggregation began. Most of the cell clumps were formed by 8-15 cells. The membranes of single cells in the clumps could be distinguished under phase-contrast microscope. At 24 h after incubation, the cells aggregated together to form 1-3 larger elliptic cell spheroids in each cell line. The cell spheroids consisted of more than 100 cells. There were still several cell clumps formed by 10-30 cells besides a few larger cell spheroids. Moreover, a lot of cells remained as single cells. After incubation for 1 week, most of the cell clumps remained the same size as at 24 h. No significant difference was observed in cell aggregation capability between untransduced and transduced cells in vitro.
Comparison of cell antigen expression by IHC The ratio of positive cells of antigen expression in HT-29, variant HT-29c and transfected HT-29cEGFPclone cells are shown in Table 4. No significant difference was observed in the positive ratios of HT-29, HT-29c and HT-29cEGFPclone cells.
| mAb | Percentage of positive cells (%) | ||
| HT-29 | T-29c | HT-29c EGFPclone | |
| KL-1 | 100 | 100 | 100 |
| IT-Ks20.10 | 100 | 100 | 100 |
| C183 | 33.9 | 34.6 | 36.5 |
| CA19-9 | 48.0 | 49.3 | 47.2 |
| MiB-1 | 96.7 | 97.8 | 97.2 |
| Do-7 | 96.8 | 97.1 | 98.3 |
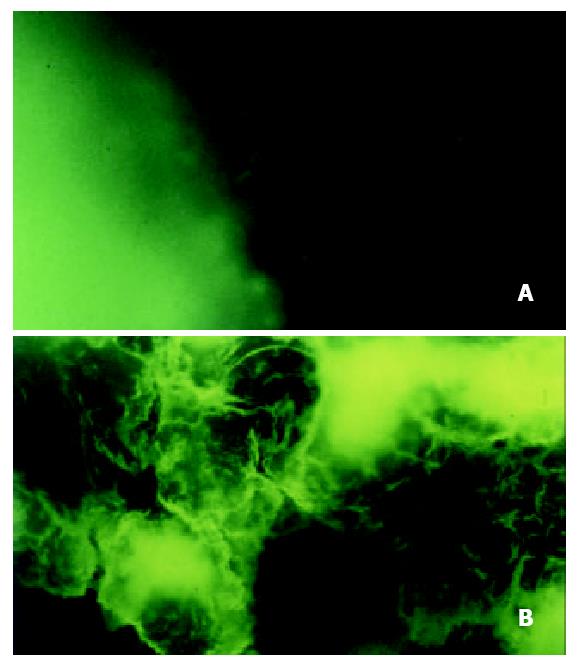
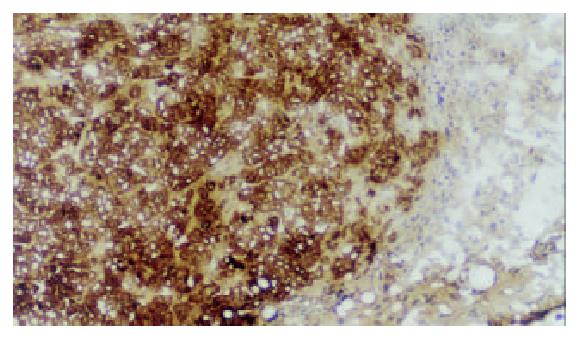
Five days after injection of HT-29cEGFPclone#7 cells, a rat s.c. tumor could be found. Two weeks after injection the rat was sacrificed. The rat had a s.c. tumor that ranged from 13.0-15.3 mm in diameter. The fresh tumor tissues were sliced at 0.7-1.0 mm and at 60 μm cryosections, then observed directly under fluorescence microscope. The tumor tissue displayed strong fluorescence (Figure 2), thereby demonstrating stable, high level EGFP expression in vivo during tumor growth. The rat s.c. tumor was also diagnosed by immunostaining with mAb KL-1 (Figure 3).
Previous studies have demonstrated the effectiveness and sensitivity of EGFP gene as a marker to visualize micrometastases in live tissue[15,16]. To use EGFP as a marker for in vivo experiments, it is necessary to establish very stable transfectants that can express EGFP constantly under nonselective conditions. In this study the retroviral vector expressing EGFP gene was transduced into HT-29c cells and the transduced cells were selected under G418. The present study showed HT-29cEGFPclone cells had stable and long-term EGFP expression under nonselective conditions in vitro. When passaged successively to passage 50 in vitro, EGFP expression was still high and stable.
The distinct metastatic potential of tumor cell is one of the most important factors in determining the outcome of metastasis. Many biological characteristics of tumor cells are associated with their metastatic ability such as proliferating potential, cell surface adhesion molecule expression, expression of oncogenes or tumor suppressor genes[17] and cell-cell junctions and active cell separation[18]. Spheroidal aggregates of malignant cells may serve as in vitro model of tumor microregions and of an early, avascular stage of tumor growth. The similarities between the original tumor and the respective spheroids include volume growth kinetics, cellular heterogeneity, e.g. induction of proliferation gradients and quiescence, differentiation characteristics, development of specific histological structures or expression of antigens[19]. Some research using cell aggregates has focused on mechanisms involved in the control of distribution, spread, invasion and metastasis of tumors[20]. Cellular heterogeneity, which is a general property of solid tumors may occur in multicellular spheroids rather than in conventional monolayer cultures. In the present study some biological behaviors were compared between transduced and parental cells in vitro. No differences were found in the expression of antigens. There was no difference in the cell proliferation rate determined by comparing their doubling times. And there was no difference in the cell aggregation capability either, which correlated with the metastatic potential.
In the present study, EGFP gene-transduced HT-29c cells were successfully used to visualize s.c. tumors in rat. The fresh tumor tissues could be analyzed directly under fluorescence microscope. The tumor tissue showed strong fluorescence, demonstrating stable, high level of EGFP expression in vivo during tumor growth. Other studies also demonstrated that EGFP gene transduced tumor cells were successfully used to visualize extensive peritoneal seeding[21], lung metastasis[22], skeletal metastasis[23] and bone metastasis[24,25], brain tumor[26,27] and liver metastasis[28]. Using EGFP fluorescence, diagnosis of tumor metastasis can be detected down to the single-cell level. This method has a higher resolution and is much more feasible than the traditional cumbersome pathological examination procedures, such as histology and immunohistochemistry. It is possible that when EGFP-expressing cells undergo apoptosis, they could be engulfed by macrophages. However, when EGFP-expressing cells die, they lose their fluorescence, such as in necrotic areas of tumors, suggesting that these macrophages will not interfere with the detection of metastases[29]. Studies have shown that EGFP transfectants should also be useful with new techniques such as intravital videomicroscopy, which previously involved labeling of tumor cells with dyes[30]. Flotte et al[31] reported gene transfer and expression could be detected by a fluorescence video-endoscopy technique. This method could be used to reliably track transfer in living animals or patients. Other results also showed all intravital imaging, that is, imaging of an intact primary tumor in a living animal was carried out on the laser scanning confocal microscope using the whole-animal platform in animal models with EGFP-expressing tumor cells[32]. Recent studies showed whole-body optical imaging, in real time, of genetically EGFP-expressing tumor growth and metastases. The whole-body optical imaging system is external and noninvasive. It affords unprecedented continuous visual monitoring of malignant growth and spread within intact animals[33,34]. A major advantage of EGFP-expressing tumor cells is that they do not need any preparation and can be seen in fresh living tissues at the microscopic level, and it allows direct observations of metastasis in an intact orthotopically growing primary tumor in a living animal.
We sincerely thank Dr. Zhu Kejian in the Department of Dermatology of the Second Affiliated Hospital of Medical College, Zhejiang University, Hangzhou, Zhejiang Province for performing the FACS analysis.
Edited by Zhu LH
| 1. | Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1490] [Cited by in RCA: 1335] [Article Influence: 40.5] [Reference Citation Analysis (0)] |
| 2. | Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4830] [Cited by in RCA: 4352] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 3. | Cubitt AB, Heim R, Adams SR, Boyd AE, Gross LA, Tsien RY. Understanding, improving and using green fluorescent proteins. Trends Biochem Sci. 1995;20:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 979] [Cited by in RCA: 928] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 4. | Stearns T. Green fluorescent protein. The green revolution. Curr Biol. 1995;5:262-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 83] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373:663-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1268] [Cited by in RCA: 1244] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 6. | Zhang G, Gurtu V, Kain SR. An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun. 1996;227:707-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 268] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Kimata Y, Iwaki M, Lim CR, Kohno K. A novel mutation which enhances the fluorescence of green fluorescent protein at high temperatures. Biochem Biophys Res Commun. 1997;232:69-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Cheng L, Fu J, Tsukamoto A, Hawley RG. Use of green fluorescent protein variants to monitor gene transfer and expression in mammalian cells. Nat Biotechnol. 1996;14:606-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 119] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Wysocka A, Krawczyk Z. Green fluorescent protein as a marker for monitoring activity of stress-inducible hsp70 rat gene promoter. Mol Cell Biochem. 2000;215:153-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | D'Assoro AB, Stivala F, Barrett S, Ferrigno G, Salisbury JL. GFP-centrin as a marker for centriole dynamics in the human breast cancer cell line MCF-7. Ital J Anat Embryol. 2001;106:103-110. [PubMed] |
| 11. | Ahmed F, Wyckoff J, Lin EY, Wang W, Wang Y, Hennighausen L, Miyazaki J, Jones J, Pollard JW, Condeelis JS. GFP expression in the mammary gland for imaging of mammary tumor cells in transgenic mice. Cancer Res. 2002;62:7166-7169. [PubMed] |
| 12. | Zhao H, Hart LL, Keller U, Holth LT, Davie JR. Characterization of stably transfected fusion protein GFP-estrogen receptor-alpha in MCF-7 human breast cancer cells. J Cell Biochem. 2002;86:365-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Vogel I, Shen Y, Soeth E, Juhl H, Kremer B, Kalthoff H, Henne-Bruns D. A human carcinoma model in athymic rats reflecting solid and disseminated colorectal metastases. Langenbecks Arch Surg. 1998;383:466-473. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Sambrook J, Gething MJ. Protein structure. Chaperones, paperones. Nature. 1989;342:224-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 48] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Chishima T, Miyagi Y, Wang X, Yamaoka H, Shimada H, Moossa AR, Hoffman RM. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997;57:2042-2047. [PubMed] |
| 16. | Shintani S, Mihara M, Nakahara Y, Aida T, Tachikawa T, Hamakawa H. Lymph node metastasis of oral cancer visualized in live tissue by green fluorescent protein expression. Oral Oncol. 2002;38:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Takahashi Y, Ellis LM, Wilson MR, Bucana CD, Kitadai Y, Fidler IJ. Progressive upregulation of metastasis-related genes in human colon cancer cells implanted into the cecum of nude mice. Oncol Res. 1996;8:163-169. [PubMed] |
| 18. | Guvakova MA, Adams JC, Boettiger D. Functional role of alpha-actinin, PI 3-kinase and MEK1/2 in insulin-like growth factor I receptor kinase regulated motility of human breast carcinoma cells. J Cell Sci. 2002;115:4149-4165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Mueller-Klieser W. Multicellular spheroids. A review on cellular aggregates in cancer research. J Cancer Res Clin Oncol. 1987;113:101-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 287] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 20. | Grill J, Lamfers ML, van Beusechem VW, Dirven CM, Pherai DS, Kater M, Van der Valk P, Vogels R, Vandertop WP, Pinedo HM. The organotypic multicellular spheroid is a relevant three-dimensional model to study adenovirus replication and penetration in human tumors in vitro. Mol Ther. 2002;6:609-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Fujiwara H, Kubota T, Amaike H, Inada S, Takashima K, Atsuji K, Yoshimura M, Ueda Y, Hagiwara A, Yamagishi H. [Functional analysis of peritoneal lymphoid tissues by GFP expression in mice--possible application for targeting gene therapy against peritoneal dissemination]. Gan To Kagaku Ryoho. 2002;29:2322-2324. [PubMed] |
| 22. | Huang MS, Wang TJ, Liang CL, Huang HM, Yang IC, Yi-Jan H, Hsiao M. Establishment of fluorescent lung carcinoma metastasis model and its real-time microscopic detection in SCID mice. Clin Exp Metastasis. 2002;19:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Yang M, Hasegawa S, Jiang P, Wang X, Tan Y, Chishima T, Shimada H, Moossa AR, Hoffman RM. Widespread skeletal metastatic potential of human lung cancer revealed by green fluorescent protein expression. Cancer Res. 1998;58:4217-4221. [PubMed] |
| 24. | Yang M, Jiang P, Sun FX, Hasegawa S, Baranov E, Chishima T, Shimada H, Moossa AR, Hoffman RM. A fluorescent orthotopic bone metastasis model of human prostate cancer. Cancer Res. 1999;59:781-786. [PubMed] |
| 25. | Peyruchaud O, Winding B, Pécheur I, Serre CM, Delmas P, Clézardin P. Early detection of bone metastases in a murine model using fluorescent human breast cancer cells: application to the use of the bisphosphonate zoledronic acid in the treatment of osteolytic lesions. J Bone Miner Res. 2001;16:2027-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Jung S, Kim HW, Lee JH, Kang SS, Rhu HH, Jeong YI, Yang SY, Chung HY, Bae CS, Choi C. Brain tumor invasion model system using organotypic brain-slice culture as an alternative to in vivo model. J Cancer Res Clin Oncol. 2002;128:469-476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 27. | MacDonald TJ, Tabrizi P, Shimada H, Zlokovic BV, Laug WE. Detection of brain tumor invasion and micrometastasis in vivo by expression of enhanced green fluorescent protein. Neurosurgery. 1998;43:1437-1442; discussion 1437-1442;. [PubMed] |
| 28. | Li X, Wang J, An Z, Yang M, Baranov E, Jiang P, Sun F, Moossa AR, Hoffman RM. Optically imageable metastatic model of human breast cancer. Clin Exp Metastasis. 2002;19:347-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Steff AM, Fortin M, Arguin C, Hugo P. Detection of a decrease in green fluorescent protein fluorescence for the monitoring of cell death: an assay amenable to high-throughput screening technologies. Cytometry. 2001;45:237-243. [PubMed] [DOI] [Full Text] |
| 30. | Chambers AF, MacDonald IC, Schmidt EE, Koop S, Morris VL, Khokha R, Groom AC. Steps in tumor metastasis: new concepts from intravital videomicroscopy. Cancer Metastasis Rev. 1995;14:279-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 166] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 31. | Flotte TR, Beck SE, Chesnut K, Potter M, Poirier A, Zolotukhin S. A fluorescence video-endoscopy technique for detection of gene transfer and expression. Gene Ther. 1998;5:166-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 32. | Farina KL, Wyckoff JB, Rivera J, Lee H, Segall JE, Condeelis JS, Jones JG. Cell motility of tumor cells visualized in living intact primary tumors using green fluorescent protein. Cancer Res. 1998;58:2528-2532. [PubMed] |
| 33. | Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li L, Hasegawa S, Bouvet M, Al-Tuwaijri M, Chishima T. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA. 2000;97:1206-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 338] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 34. | Bouvet M, Wang J, Nardin SR, Nassirpour R, Yang M, Baranov E, Jiang P, Moossa AR, Hoffman RM. Real-time optical imaging of primary tumor growth and multiple metastatic events in a pancreatic cancer orthotopic model. Cancer Res. 2002;62:1534-1540. [PubMed] |