Published online Sep 15, 2003. doi: 10.3748/wjg.v9.i9.1930
Revised: February 23, 2003
Accepted: March 21, 2003
Published online: September 15, 2003
AIM: To investigate the effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells.
METHODS: The gastric cancer SGC-7901 adenocarcinoma cells were treated with allicin and the cell cycle, inhibitory rate, apoptosis, telomerase activity and morphologic changes were studied by MTT assay, flow cytometry (FCM), TRAP-PCR-ELISA assay, light microscope, electron microscope respectively. Results were compared with that of AZT (3’-Azido-3’-deoxythymidine).
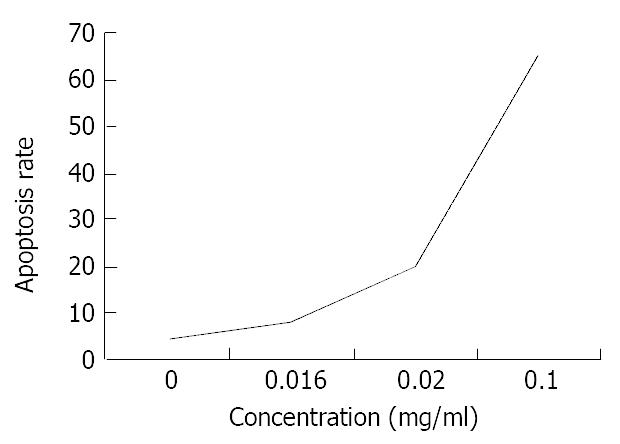
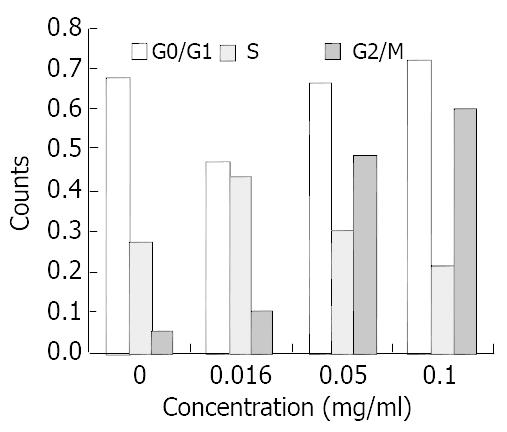
RESULTS: SGC-7901 cells were suppressed after exposure to allicin of 0.016 mg/mL, 0.05 mg/mL, and 0.1 mg/mL for 48 h. Compared with the control, the difference was significant (P < 0.05). Allicin could induce apoptosis of the cells in a dose-dependent and non-linear manner and increase the proportion of cells in the G2/M phase. Compared with the control, the difference was significant in terms of the percentage of cells in the G2/M phase (P < 0.05). Allicin could inhibit telomerase activity in a time-dependent and dose-dependent pattern. After exposure to allicin at 0.016 mg/mL for 24 hours, SGC-7901 cells showed typical morphologic change.
CONCLUSION: Allicin can inhibit telomerase activity and induce apoptosis of gastric cancer SGC-7901 cells. Allicin may be more effective than AZT.
- Citation: Sun L, Wang X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J Gastroenterol 2003; 9(9): 1930-1934
- URL: https://www.wjgnet.com/1007-9327/full/v9/i9/1930.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i9.1930
The relationship between telomere, telomerase and cancer has been the hotspot of study since Kim found telomerase activity in cancer in 1994. It was reported that telomerase activity and malignancy had a close association. Telomerase activity was detected in approximately 80%-90% of immortal cells. In contrast, telomerase activity was not detected in most mature somatic cells[1-3]. The observed differences in telomerase activity in normal versus tumor derived cells led to the hypothesis that the activation of telomerase might be essential to tumor progression and the proliferation of tumor cells, and that telomerase might represent a suitable target for highly specific anti-cancer therapies[4,5].
Gastric cancer is the most common alimentary tract cancer in China in terms of incidence. It is one of the malignancies that do serious harm to people’s health with a high mortality and are short of effective therapeutic methods. Researchers are not only trying to enchance the therapeutic effects of the current methods but also working hard to find new ways and medicines to treat gastric cancer. We have studied the relationship between telomere, telomerase and malignancies, and tried to find new medicines to treat gastric cancer since 1997 in our laboratory. The results suggest that the presence of telomerase activity itself can be used as an excellent tool for the early diagnosis of cancer[6-8]. We carried out further studies to try to find out medicines from traditional Chinese herbs, which can inhibit telomerase activity and provide a new therapeutic approach on gastric cancer. Allicin is the bulb of Allium. Epidemiological studies and animal experiments have suggested that several garlic-derived compounds have potential anticarcinogens[8-13]. Allicin is one of them, but the mechanism of anticancer is not clearly demonstrated. In this paper, we first studied the effect of 3’-Azido-3’-deoxythymidine (AZT) on telomerase activity and apoptosis. Then the test was continued by using cheap allicin, instead of the expensive AZT. The results were compared between allicin and AZT.
Allicin was obtained from HeFeng Pharmaceutical Company (15 mg/mL, Batch Number: 010101). AZT was purchased from Sigma Company. Human gastric adenocarcinoma SGC-7901 cell line was obtained from the Cell Biology Institute of Chinese Academy of Sciences. RPMI-1640 was the product of GBICO. Fetal bovine serum (FBS) was purchased from Tianjin Hematological Diseases Research Institute. Trypsin, tetrazolium bromide (MTT), ribonuclease A, DMSO and propidium iodide (PI) were purchased from the Sino-American Hua Mei Biotechnology Company of Beijing. The telomerase detection kits were obtained from the Sino-American Hua Mei Biotechnology Company of Shanghai.
Cell culture Cells were maintained in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), streptomycin (100 μg/mL) and penicillin (100 IU/mL) at 37 °C in a humidified atmosphere containing 5% CO2.
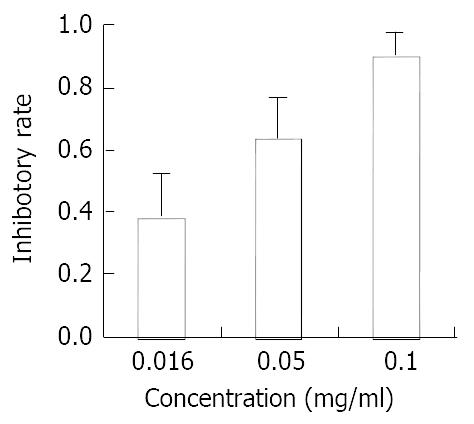
Effect of allicin on cell proliferation of SGC-7901 cells SGC-7901 cells were suspended at a concentration of 5 × 104/mL. Then 200 μL of the cell suspension was placed in each well of a replicate 96-well microtiter plate. The cells were allowed to adhere overnight. Then different concentrations (0.016 mg/mL, 0.05 mg/mL, 0.1 mg/mL) of allicin were added to the cells. MTT assay was performed after 48 h growth. 40 μL of 5 mg/mL of MTT was added to each well followed by incubation for 4 h at 37 °C. The formazan crystals were dissolved in 200 μL DMSO and the absorbance measured by enzyme-linked immunosorbent assay (ELISA). Optical density value (OD) was measured at a wavelength of 570 nm. Each assay was performed three times and the average results were calculated.
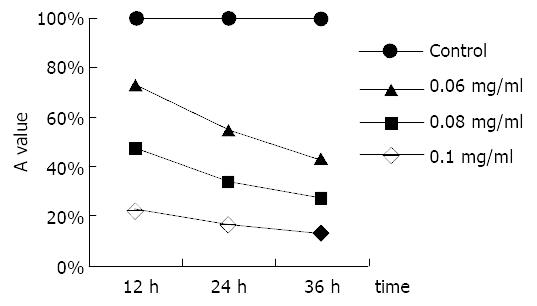
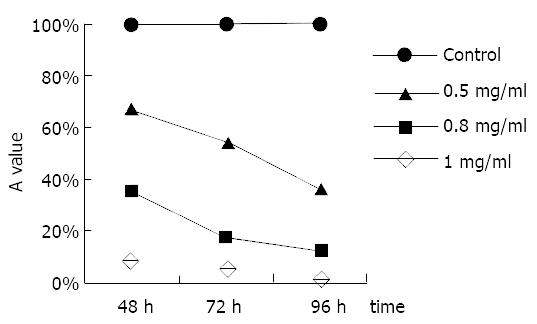
Effect of allicin on telomerase activity of SGC-7901 cell Cultured cells in logarithmic growth were digested by 0.25% trypsin and suspended at a concentration of 2 × 104/mL, then 5 mL was placed into a cell culture flask of 25 mL and allowed to adhere overnight. Cells were harvested after 12 h, 24 h, and 36 h. Cells were washed once with PBS and scraped into a wash buffer, The cells were washed in the buffer, homogenized in 150 μL cell lysis buffer, and incubated on ice for 30 min. Cell homogenates were then centrifuged at 12000 × g for 20 min at 4 °C. The supernatants were recovered and snap-frozen in liquid nitrogen and stored at -80 °C. The TRAP-PCR-ELISA assay was performed using a telomerase detection kit. In brief, 2 μL of tissue extract and 48 μL TRAP reaction mixture were placed into tubes, PCR was then performed at 94 °C for 120 s and at 94 °C for 30 s, at 48 °C for 30 s, at 72 °C for 90 s for 35 cycles. The PCR products (25 μL) were hybridized to a digoxigenin (DIG)-labelled telomeric repeat specific detection probe. The PCR products were immobilized via the biotin-labelled primer to a streptavidin-coated microtiter plate. The immobilized PCR products were detected with a peroxidase-conjugated anti-DIG antibody and visualized following addition of the stop reagent. The microtitre plate was assessed on an enzyme-linked immunosorbent assay (ELISA) plate reader at a wavelength of 490 nm.
Effect of allicin on the cell cycle of SGC-7901 cells Cell culture was the same as before, and treated with allicin. Cultured cells were harvested after 24 h and fixed with 70% cold ethanol for 4 h. The percentage of the cells in different cell cycle was determined by a flow cytometer. Analysis of the DNA content was done by using FACSalibur. Briefly, 1 × 105 cells were suspended in 0.2% Triton-X-100/PBS solution containing 0.5% ribonuclease A, After incubation for 20 min, DNA was stained with 50 ug/mL of propidum iodide (PI), then applied to flow cytometer analysis at the inspiring wavelength of 488 nm.
Effect of allicin on morphological changes of SGC-7901 cells Observations under light microscope The cells were treated with 0.016 mg/mL allicin for 24 h, 48 h, then morphological changes were observed under the inverted light microscope and photographed.
Observations under transmission electron microscope (TEM) For TEM, SGC-7901 cells were incubated in culture dishes with allicin for 24 h. A total of 5 × 106 cells were pelleted at 12000 × g for 5 min and washed twice with PBS. Cells were fixed in 2.5% cold glutaraldehyde, 0.1 M of sodium cacodylate/1% sucrose buffer for 24 h. The cells were washed three times with PBS, then postfixed in 1% osmium tetroxide (60 min), encapsulated in 1% agar, stained with uranyl acetate and phosphotungstic acid, and dehydrated in a series of graded ethanolic solutions, finished with propylene oxide before finally embedded in Epon 812-Araldite mixture. Ultrathin sections (50 nm) were cut on a LKL-208 ultramicrotome and placed under 200 mesh standard copper grids, examined with an HA-600 transmission electron microscope.
The oneway test was used to evaluate the significance of cell proliferation, cell cycle and apoptosis. The ANOVA was used to evaluate the significance of telomerase activity. P < 0.05 was considered statistically significant.
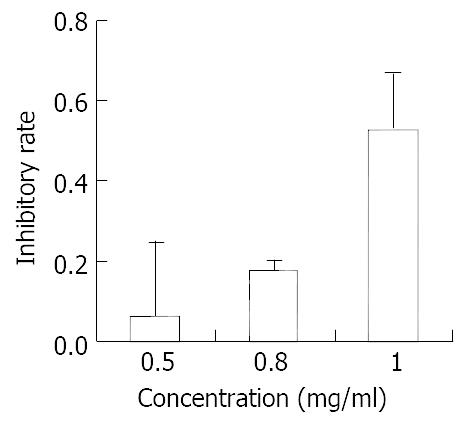
SGC-7901 cells were treated with different concentrations of allicin for 48 h. MTT assay was used to measure cell proliferation. The results are shown in Figure 1. Allicin could inhibit gastric cancer cell proliferation in A dose-dependent pattern. Allicin’s action was notably stronger than AZT (Figure 2). At the highest concentration, the inhibitory rate of allicin on SGC-7901 was 89%, while that of AZT was only 52.6%.
The cells were harvested after treated at different concentrations of allicin for 12 h, 24 h, and 36 h, respectively. SGC-7901 cells were treated by AZT for 48 h, 72 h, 96 h. Then the cells were harvested. The telomerase activity was measured by TRAP-PCR-ELISA assay. The results suggested that both allicin and AZT could inhibit telomerase activity by different degrees (Figure 3 and Figure 4).
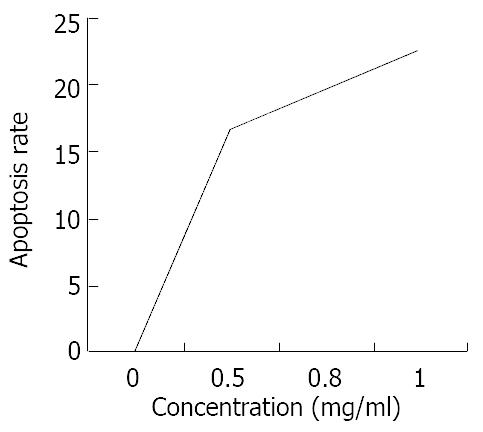
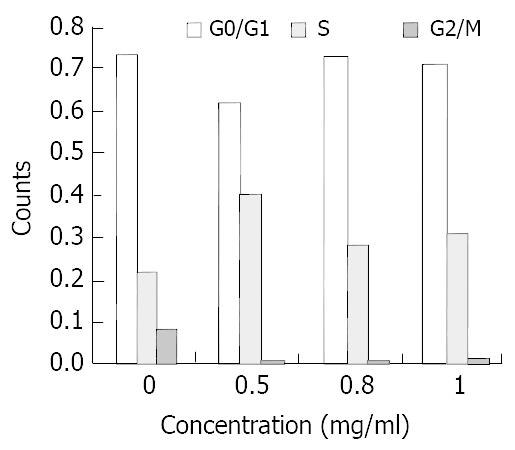
Three different concentrations of allicin acted on SGC-7901 cells for 24 h, the flow cytometry results showed the sub-G1 wave which was the apoptosis wave. Allicin could induce apoptosis in a dose-dependent and non-linear manner (Figure 5). At the same time allicin could change the cell cycle of SGC-7901 cell. In a certain range of concentrations, when the concentration increased, the cells of G2/M phase increased (Figure 6). As shown in Figure 7 and Figure 8, AZT could also change the cells cycle. It could increase the cells of S phase and induce apoptosis in a dose-dependent and non-linear manner.
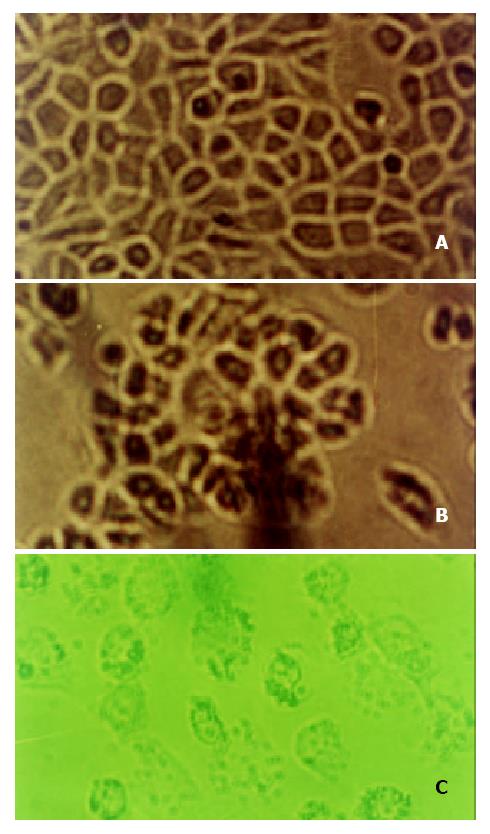
The results of light microscopy Cells became round after treated with allicin for 24 h and the intercellular gaps were loose. After 48 h, the cells were crimpled and floated. There were a lot of fragments around the cells (Figure 9).
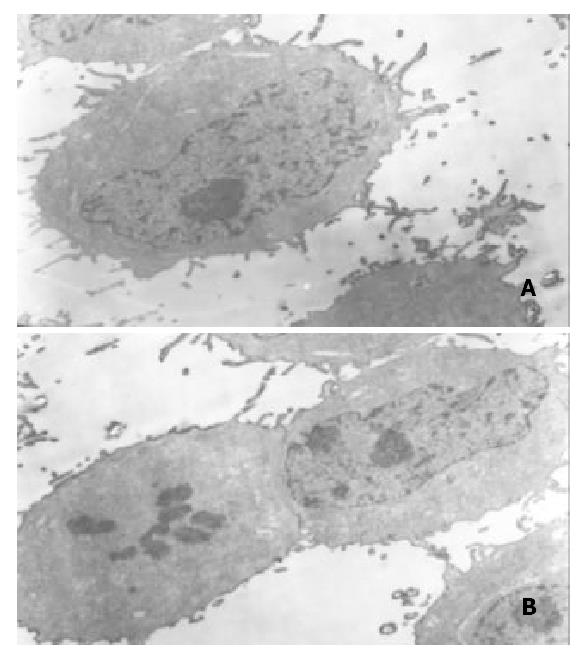
Results of transmission electron microscopy SGC-7901 cells had big nucleoli and aberrant nuclei. There were a lot of prominences of microvilli on the surface of cell membranes. After treated with allicin for 24 h, the prominence was disappeared and nuclei deflated, but the cell membranes were intact (Figure 10).
Telomeres, the ends of eukaryotic chromosomes, are composed of tandemly repeated guanine-rich sequences 5’TTAGGG3’[14]. However, due to the nature of DNA synthesis, the 5’ ends of telomeres are shortened by 50-100 bp with each round of cell division. When the telomere reaches a certain critical length, the cell cannot undergo division. This has been described as the “end-replication” problem of linear chromosomes[15-17]. Telomerase is a ribonucleoprotein and its internal RNA component serves as a template for directing the appropriate telomeric sequences onto the 3’ end of a telomeric primer, and then the cells can continue to divide[18]. To date, three major components of telomerase, namely, human telomerase RNA component (hTR), human telomerase-associated protein (TP) and human telomerase catalytic subunit (hTERT) have been identified[19]. Recent studies have demonstrated a close correlation between telomerase activity and hTERT expression[19,20]. In a number of laboratories, experiments have shown that down-regulation of telomerase activity can be used to treat cancer[21-28]. AZT, which has been among the most extensively studied reverse transcriptase inhibitors, is a telomerase inhibitor that works on hTERT. AZT can effectively cause telomere shortening and inhibit telomerase activity in cancer cells. The results have shown that tumor incidence is reduced and survival is prolonged, at the same time the number and size of spontaneous metastases are also decreased. But AZT has toxical effects and is so expensive that it cannot be used widely[27-29]. Allicin, which has been used to decrease blood pressure, cholesterol and as an antioxidant, antimicrobial, etc[30-33], has little toxicity and is easily available. In the current study, the SGC-7901 cells were treated at different concentrations of allicin and AZT. The results strongly suggest that allicin can effectively inhibit telomerase activity in a time- and dose-dependent manner. This study also indicates that allicin can induce cell apoptosis (arrest in G2/M phase). The cause of apoptosis is related to decreased telomerase activity. When telomerase activity degrades, telomere shortens, mitoses of cells are arrested, which leads to cell apoptosis[34]. It was reported that apoptosis inhibitor Bcl-2 could modulate telomerase activity. Overexpression of Bcl-2 leads to a significant enchancement in the level of telomerase activity. On the other hand, with down-regulation of Bcl-2 expression, telomerase activity also decreases[35-37]. Some researches found that the mechanism of degraded Bcl-2 expression by allicin was through the secondary messengers, namely cAMP, PKC of the second signal system, which led to overexpression of Fas and Bax, and at the same time the Bcl-2 expression decreased[38]. But the telomerase activity modulation is a complex system[39]. We were unable to rigorously determine if decrease of telomerase activity after treatment by allicin was associated with the down-regulation of Bcl-2.
Furthermore, this work also provides a direct comparison between two classes of medicine, both of which can inhibit telomerase activity and induce apoptosis. Allicin is more effective than AZT. At the same time, both Allicin and AZT can arrest the cells in different cell cycle phases. Allicin arrests cells at the G2/M phase while AZT does at the S phase, indicating that the mechanism of allicin on telomerase activity inhibition is different from that of AZT and telomerase activity is lack of cell cycle regulation[40-42]. In conclusion, our results may provide important insights into using allicin as a therapeutic approach against neoplasm.
Edited by Zhu LH and Wang XL
| 1. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5234] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 2. | Harley CB. Telomere loss: mitotic clock or genetic time bomb. Mutat Res. 1991;256:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 782] [Cited by in RCA: 773] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 3. | Rhyu MS. Telomeres, telomerase, and immortality. J Natl Cancer Inst. 1995;87:884-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 269] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 4. | Neidle S, Kelland LR. Telomerase as an anti-cancer target: current status and future prospects. Anticancer Drug Des. 1999;14:341-347. [PubMed] |
| 5. | Zhou HP, Wang X, Zhang NZ. Early apoptosis in intestinal and diffuse gastric carcinomas. World J Gastroenterol. 2000;6:898-901. [PubMed] |
| 6. | Gu T, Wang X, Wang X, Wang W, Liu Y, Zhang B, Shi Y, Zhang Z, Sun Q, Xue T. [The value of detecting telomerase activity on early diagnosis of lung cancer]. Zhongguo Fei Ai Za Zhi. 2001;4:37-40. [PubMed] |
| 7. | Peng MQ, Wang X, Zhu SY, Luo T, Gu T, Yan YL, Liu H. The value of CT scan and detection of telomerase activity in biopsy specimens for early diagnosis of lung carcinoma. Linchuang Fangshe Xue Zazhi. 2002;21:1-4. |
| 8. | Wang XB, Wang X, Zhang NZ. Inhibition of somatostatin ana-log Octreotide on human gastric cancer cell MKN-45 growth in vitro. Shijie Huaren Xiaohua Zazhi. 2002;10:40-42. |
| 9. | Li Y, Lu YY. Applying a highly specific and reproducible cDNA RDA method to clone garlic up-regulated genes in human gastric cancer cells. World J Gastroenterol. 2002;8:213-216. [PubMed] |
| 10. | Li Y, Yang L, Cui JT, Li WM, Guo RF, Lu YY. Construction of cDNA representational difference analysis based on two cDNA libraries and identification of garlic inducible expression genes in human gastric cancer cells. World J Gastroenterol. 2002;8:208-212. [PubMed] |
| 11. | Li X, Xie J, Li W. [Garlic oil induces differentiation and apoptosis of human gastric cancer cell line]. Zhonghua Zhong Liu Za Zhi. 1998;20:325-327. [PubMed] |
| 12. | Arivazhagan S, Nagini S, Santhiya ST, Ramesh A. Protection of N-methyl-N'-nitro-N-nitrosoguanidine-induced in vivo clastogenicity by aqueous garlic extract. Asia Pac J Clin Nutr. 2001;10:238-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Takezaki T, Gao CM, Wu JZ, Ding JH, Liu YT, Zhang Y, Li SP, Su P, Liu TK, Tajima K. Dietary protective and risk factors for esophageal and stomach cancers in a low-epidemic area for stomach cancer in Jiangsu Province, China: comparison with those in a high-epidemic area. Jpn J Cancer Res. 2001;92:1157-1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 75] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Tang F, Zhou J, Gu L. [In vivo and in vitro effects of selenium-enriched garlic on growth of human gastric carcinoma cells]. Zhonghua Zhong Liu Za Zhi. 2001;23:461-464. [PubMed] |
| 15. | Kim Sh SH, Kaminker P, Campisi J. Telomeres, aging and cancer: in search of a happy ending. Oncogene. 2002;21:503-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 145] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345:458-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4007] [Cited by in RCA: 4042] [Article Influence: 115.5] [Reference Citation Analysis (0)] |
| 17. | Huffman KE, Levene SD, Tesmer VM, Shay JW, Wright WE. Telomere shortening is proportional to the size of the G-rich telomeric 3'-overhang. J Biol Chem. 2000;275:19719-19722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 183] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | de Lange T. Activation of telomerase in a human tumor. Proc Natl Acad Sci USA. 1994;91:2882-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 190] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Harley CB. Telomerase is not an oncogene. Oncogene. 2002;21:494-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 111] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564-579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 440] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 21. | Jong HS, Park YI, Kim S, Sohn JH, Kang SH, Song SH, Bang YJ, Kim NK. Up-regulation of human telomerase catalytic subunit during gastric carcinogenesis. Cancer. 1999;86:559-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 22. | Corey DR. Telomerase inhibition, oligonucleotides, and clinical trials. Oncogene. 2002;21:631-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 71] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Elayadi AN, Demieville A, Wancewicz EV, Monia BP, Corey DR. Inhibition of telomerase by 2'-O-(2-methoxyethyl) RNA oligomers: effect of length, phosphorothioate substitution and time inside cells. Nucleic Acids Res. 2001;29:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Tamura Y, Tao M, Miyano-Kurosaki N, Takai K, Takaku H. Inhibition of huma n telo merase a ctivity by a ntisense phosphorothioate oligonucleotides encapsulated with the trans-fection reagent, FuGENE6, in HeLa cells. Antisense Nucleic Acid Drug Dev. 2000;10:87-96. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Yegorov YE, Akimov SS, Akhmalisheva AK, Semenova IV, Smirnova YB, Kraevsky AA, Zelenin AV. Blockade of telomerase function in various cells. Anticancer Drug Des. 1999;14:305-316. [PubMed] |
| 26. | Yokoyama Y, Takahashi Y, Shinohara A, Wan X, Takahashi S, Niwa K, Tamaya T. The 5'-end of hTERT mRNA is a good target for hammerhead ribozyme to suppress telomerase activity. Biochem Biophys Res Commun. 2000;273:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Herbert BS, Pongracz K, Shay JW, Gryaznov SM. Oligonucleotide N3'--& gt; P5' phosphoramidates as efficient telomerase inhibitors. Oncogene. 2002;21:638-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 28. | Melana SM, Holland JF, Pogo BG. Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by 3'-azido-3'-deoxythymidine. Clin Cancer Res. 1998;4:693-696. [PubMed] |
| 29. | Tejera AM, Alonso DF, Gomez DE, Olivero OA. Chronic in vitro exposure to 3'-azido-2', 3'-dideoxythymidine induces senescence and apoptosis and reduces tumorigenicity of metastatic mouse mammary tumor cells. Breast Cancer Res Treat. 2001;65:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Komata T, Kanzawa T, Kondo Y, Kondo S. Telomerase as a therapeutic target for malignant gliomas. Oncogene. 2002;21:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 31. | Mohamadi A, Jarrell ST, Shi SJ, Andrawis NS, Myers A, Clouatre D, Preuss HG. Effects of wild versus cultivated garlic on blood pressure and other parameters in hypertensive rats. Heart Dis. 2000;2:3-9. [PubMed] |
| 32. | Neil HA, Silagy CA, Lancaster T, Hodgeman J, Vos K, Moore JW, Jones L, Cahill J, Fowler GH. Garlic powder in the treatment of moderate hyperlipidaemia: a controlled trial and meta-analysis. J R Coll Physicians Lond. 1996;30:329-334. [PubMed] |
| 33. | Liao F, Jiao L. Ligustrazine, allicin and shear-induced platelet aggregation. Clin Hemorheol Microcirc. 2000;22:167-168. [PubMed] |
| 34. | Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 533] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 35. | Hackett JA, Greider CW. Balancing instability: dual roles for telomerase and telomere dysfunction in tumorigenesis. Oncogene. 2002;21:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Mandal M, Kumar R. Bcl-2 modulates telomerase activity. J Biol Chem. 1997;272:14183-14187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 113] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Fan Y, Lin GJ, Qian LP, Xu ZD, Li H. Effects of β-elemene on both telomerase activity and expression of Bcl-2 gene of gastric cancer SGC-7901 cell. Shanghai Yixue. 2001;24:490-492. |
| 38. | Johnson VL, Cooper IR, Jenkins JR, Chow SC. Effects of differential overexpression of Bcl-2 on apoptosis, proliferation, and telomerase activity in Jurkat T cells. Exp Cell Res. 1999;251:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 39. | Li Y, Liu JH, Zhao Q, Fan LF, Yu YM, Wang LL, Zhao XF, Zhang TD, Zhang TP, Ma TX. The study of allicin inducing BGC-823 human gastric adenocarcinoma cells apoptosis. Zhongguo Zhongxi Yi Jiehe Waike Zazhi. 2001;7:307-310. |
| 40. | Kyo S, Inoue M. Complex regulatory mechanisms of telomerase activity in normal and cancer cells: how can we apply them for cancer therapy. Oncogene. 2002;21:688-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Holt SE, Wright WE, Shay JW. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16:2932-2939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 270] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Holt SE, Aisner DL, Shay JW, Wright WE. Lack of cell cycle regulation of telomerase activity in human cells. Proc Natl Acad Sci USA. 1997;94:10687-10692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 122] [Article Influence: 4.4] [Reference Citation Analysis (0)] |