INTRODUCTION
Retinoid receptors are nuclear receptors, and belong to members of the steroid and thyroid hormone receptor superfamily[1-4]. As retinoid receptor ligands, retinoids exert their effects on proliferation and differentiation through regulating the transcriptional and post-transcriptional activities of retinoid receptors[5-8]. Besides retinoids, other agents such as tetradecanoylphorbol-1, 3-acetate (TPA), and nerve growth factor (NGF), have an effect on retinoid receptor regulation[9-11]. Although up-regulation of retinoid receptor mRNA by retinoids has been demonstrated among a number of different types of cells[12-15], it is also indicated that retinoid receptors are degraded through a distinct pathway[16]. However, little is known how and where retinoid receptors are degraded, especially when other agents, rather than their ligands, are used to stimulate carcinoma cells.
Eukaryotic cells contain multiple proteolysis systems, which are mainly responsible for degradation of proteins in the extracellular milieu[17]. The multicomplex 26S proteasome contains two 19S regulatory complexes and a 20S catalytic core complex that may be responsible for 80%-90% of protein degradation in the cells[18,19]. It has been reported that proteasomes play an important role in thymocyte apoptosis and inflammatory response[20-23]. Proteasome inhibitors can induce cell apoptosis, accompanied by activation of several Caspases, such as Caspase-3 and Caspase-7[24,25]. In contrast, proteasome inhibitors may prevent cells from apoptosis through promoting resistance of cells at higher temperature and toxicity[26-28].
Retinoid receptors can be divided into two types, retinoic acid receptor (RAR) and retinoid X receptor (RXR)[29-32]. Usually, to regulate retinoid receptor by retinoid is preferential to measure its mRNA level, little has been done on retinoid receptor protein itself. Recently, Tanaka et al[16] pointed out that although retinoic acid caused up-regulation of RARα and RARγ mRNA, it down-regulated the expression of RARα and RARγ protein in breast cancer cell line MCF-7. This evidence suggests that the mechanism of retinoic acid on transcriptional or post-transcriptional activity of retinoid receptor is quite different. In the course of investigating the effect of TPA on RXR protein expression, we found that TPA could down-regulate the expression of RXRα protein effectively in gastric cancer cells. In present study, we focused on the mechanism of TPA in RXRα degradation. The results demonstrated that degradation of RXRα by TPA was proteasome-dependent in gastric cancer cells. More importantly, both degradation and proteasome-dependency of RXRα were associated with translocation of RXRα protein. These findings may provide a novel insight into the cross-talk between degradation and translocation of retinoid receptor and TPA signaling pathway.
MATERIALS AND METHODS
Cancer cell line
Human gastric cancer cell line, BGC-823, was obtained from the Institute of Cell Biology in Shanghai. The cells were maintained in RPMI-1640 medium, supplemented with 10% FCS, 1 mM glutamine, and 100 u/mL penicillin.
Detection of western blot[
9,
33]
Cells treated by different agents were harvested, and suspended in RIPA buffer (10 mmol/L Tris (pH7.4), 150 mmol/L NaCl, 1% Triton X-100, 1% deoxycholic acid, 0.1% SDS, 5 mmol/L EDTA (pH8.0), 1 mmol/L PMSF). Protein concentration was determined using the Bio-Rad protein assay system according to the manufacturer’s instructions (Bio-Rad Hereules, CA). Total protein (50 μg) was subjected to SDS-PAGE and transferred to nitrocellulose membrane for Western blot analysis. The membrane was subsequently blocked with 5% dry milk in TBS-T and then immunnoblotted with the corresponding antibody. Protein was detected using the ECL kit (Peirce) according to the manufacturer’s directions. Before incubation with antibody, the same membrane was stained by ponceau S (Sigma) to show the amount of protein used in each well. The intensities of bands indicated by Western blot were quantified by a densitometer. To separate the cytoplasmic and nuclear fractions, cells were suspended in 500 μl of 10 mM Tris-Cl (pH7.8), 1% Nonidet P-40, 10 mM mercaptoethanol, 0.5 mM phenylmethylsulfonyl fluoride, 1 mg/L leupeptin and 1 mg/L aprotinin for 2 min at 0 °C, then 500 μl of DDW was added, and the cells were allowed to swell for 2 min. Cells were sheared by 10 passages through a 22 gauge needle. The nuclear and cytoplasmic fraction was obtained by centrifugation at 4000 × g for 5 min. The supernatant was cytoplasmic fraction and pellet was nuclear fraction.
Immunofluorescent labeling and confocal microscopy[
9,
34]
Cells were cultured on a cover glass overnight, then treated with various agents. After washed with PBS, cells were fixed in 4% paraformaldehyde. To identify RXRα protein, cells were firstly incubated with anti-RXRα IgG antibody (Santa Cruz), and then reacted with corresponding FITC-conjugated anti-IgG (Pharmingen) as secondary antibody. To visualize nuclei, cells were stained with propidium iodine (50 mg/L) containing 100 mg of DNase-free RNase A per liter. Fluorescent images were observed and analyzed under laser-scanning confocal microscope (Bio-Rad MRC-1024ES).
RESULTS
TPA induces degradation of RXRα
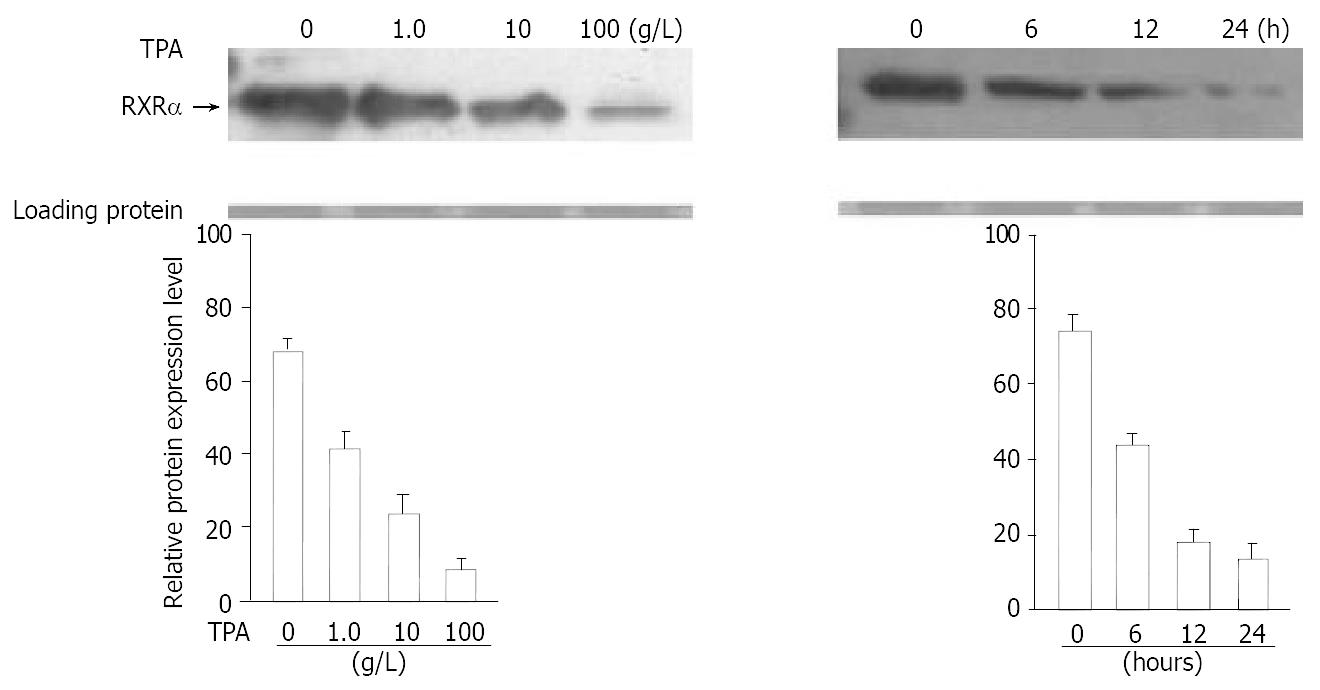
Western blot analysis was carried out to investigate the effect of TPA on the expression level of RXRα protein. When BGC-823 cells were treated at different concentrations of TPA (ranging from 100-1.0 g/L) for 24 hrs, the expression of RXRα protein showed a concentration-dependent decrease (Figure 1A), 87% reduction of RXRα protein expression was observed at the concentration of 100 g/L TPA determined by a densitometer (Figure 1B). Similar repressing tendency was also seen in time-course of TPA treatment. As shown in Figure 1A-B, treatment of cells with TPA for different time periods caused a marked down-regulation of RXRα protein in BGC-823 cells. After 24 hr of TPA treatment, the expression of RXRα protein was almost inhibited. Thus, TPA could induce the degradation of RXRα protein in BGC-823 cells. Moreover, this degradation was time- and TPA concentration-dependent.
Figure 1 Effect of TPA on expression of RXRα protein.
Cells were treated at different concentrations of TPA or different time periods. (A) Expression of RXRα was detected by Western blot. The amount of protein used in each lane was indicated by staining with ponceau S. (B) The intensity of each band shown in Figure 1A was quantified by a densitometer.
TPA induces RXRα translocation
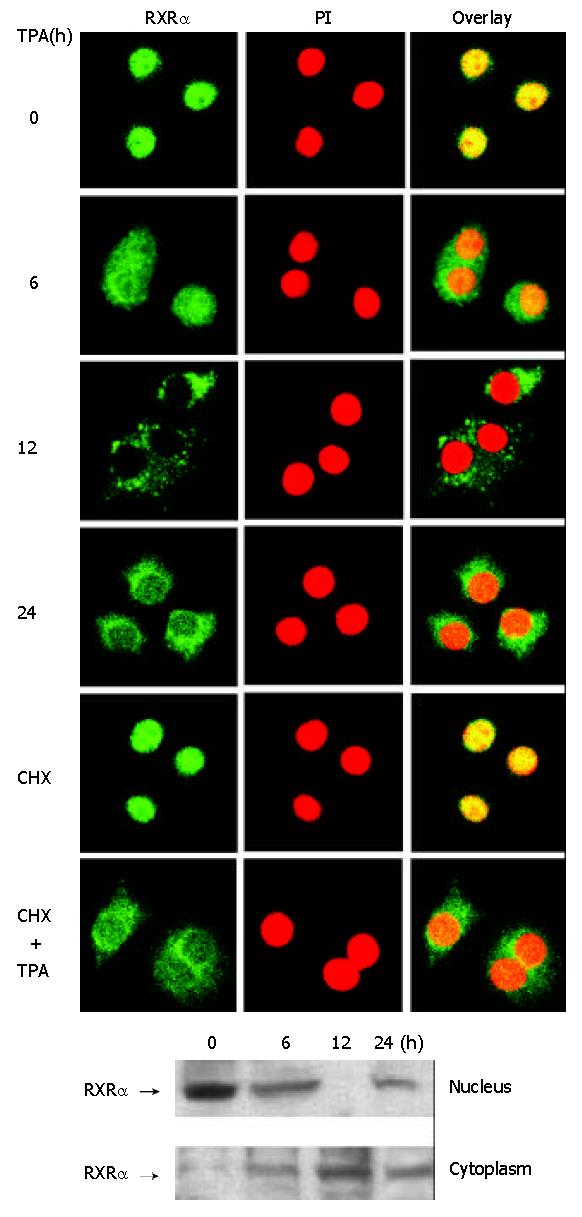
To determine the effect of TPA on the subcellular localization of RXRα, immunofluorescent labeling of RXRα protein was performed and observed under laser-scanning confocal microscope. The result indicated that RXRα was accumulated in the nuclei in intact BGC-823 cells (Figure 2A). Treatment of TPA resulted in redistribution of RXRα protein in a time-dependent manner. When cells were treated with TPA for 6 hrs, RXRα protein started to be translocated from the nucleus to the cytoplasm, and after 12 h of treatment, RXRα protein was completely accumulated in the cytoplasm, and after 24 h of treatment, most of the RXRα protein became localized in the cytoplasm, but little in the nucleus (Figure 2A). To further confirm this translocation, nuclear and cytoplasmic fractions were prepared from BGC-823 cells, and the protein extracts were analyzed by Western blot. It clearly showed that in the intact BGC-823 cells, RXRα was expressed mainly in the nucleus (Figure 2B). TPA treatment resulted in a time-dependent shift of RXRα protein from nuclear portion to cytoplasmic portion. The maximum cytoplasmic RXRα accumulation occurred in the 12 h TPA treatment, and after 24 h TPA treatment, trace RXRα was detected in the nucleus, mostly in the cytoplasm (Figure 2B). Accordingly, this result was in agreement with that of Figure 2A, strongly suggesting that the translocation of RXRα did occur in the course of TPA treatment.
Figure 2 Translocation of RXRα from the nucleus to the cyto-plasm induced by TPA.
Cells were treated with TPA for differ-ent time periods or CHX (10 g/L) for 3 hrs as required. (A) Cells were immunostained with anti-RXRα antibody followed by corresponding FITC-conjugated anti-IgG secondary anti-body to show RXRα protein. Simultaneously, cells were stained with PI to display the nucleus. The fluorescent image was ob-served under laser-scanning confocal microscope. (B) Nuclear and cytoplasmic fractions were prepared as described in the Materials and Methods. RXRα was revealed by Western blot.
To verify that cytoplasmic RXRα protein was originated from nuclear RXRα, rather than being synthesized in the cytoplasm, cells were pre-incubated with cycloheximide (CHX) for 3 hrs to specifically prevent new cytoplasmic protein synthesis[35]. Confocal microscopy observation showed that RXRα protein was seen only in the nucleus when cells were treated with CHX alone (Figure 2A), indicating that cytoplasmic protein synthesis was inhibited by CHX effectively. However, when cells were continuously incubated with TPA for another 24 hrs, RXRα protein was still translocated into the cytoplasm (Figure 2A). Therefore, it convincingly demonstrated that cytoplasmic RXRα protein was from the nucleus.
Effect of proteasome inhibitor on RXRα degradation
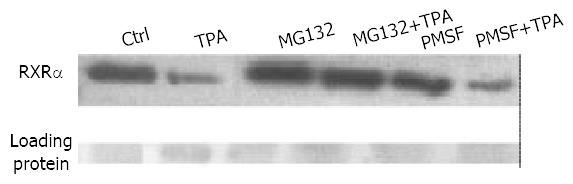
To determine whether the degradation of RXRα by TPA was 26S proteasome-dependent, BGC-823 cells were incubated with proteasome-specific inhibitor MG132[16,18] in the absence or presence of TPA. Western blot analysis showed that MG132 was able to inhibit TPA-induced RXRα degradation (Figure 3). By contrast, when PMSF, a potent inhibitor of protease but not specific-inhibitor of proteasome[36], was used as a positive control, it could not inhibit TPA-induced RXRα degradation, TPA still down-regulated expression of RXRα (Figure 3). Thus, this result suggested that TPA-induced degradation of RXRα occurred through the proteasome pathway.
Figure 3 Effect of different agents, including TPA, MG132 (10 μmol/L) and PMSF (100 mg/L), on expression of RXRα.
Cells were pre-treated with MG132 or PMSF for 3 hrs, followed by TPA for another 24 hrs. RXRα expression was analyzed by Western blot.
RXRα translocation by TPA is proteasome-dependent
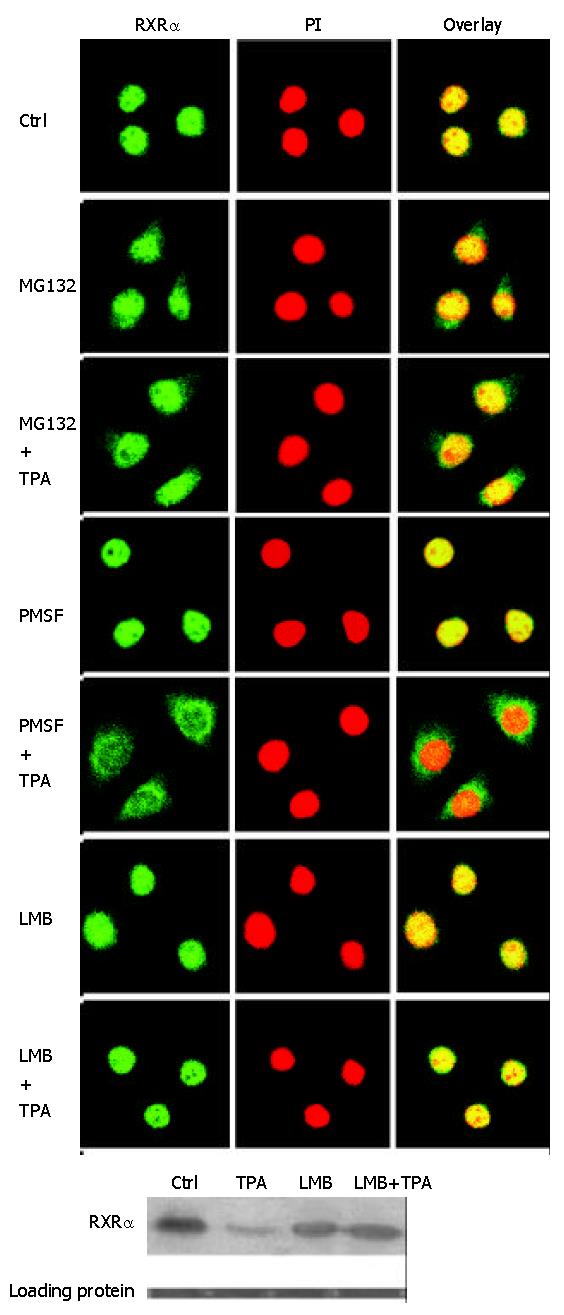
To determine the relationship between RXRα translocation and proteasome pathway, the behavior of RXRα translocation in the presence of MG132 was investigated. Confocal microscopy observation showed that cells treated with MG132 alone for 3 hrs could hardly induce RXRα protein translocation into the cytoplasm, majority of RXRα protein was localized in the nucleus, but very little in the cytoplasm. After TPA treatment for another 12 hrs, most of RXRα protein remained in the nucleus, similar to MG132 treatment alone (Figure 4A). As a positive control, when PMSF was used to treat cells alone, RXRa was not translocated, still in the nucleus. However, when cells were continuously incubated with TPA, translocation of RXRα was detected (Figure 4A). Thus, it clearly revealed that MG132 and PMSF had different effects on inducing RXRα translocation, MG132 could inhibit TPA-induced translocation of RXRa protein, and RXRα translocation induced by TPA was proteasome-dependent.
Figure 4 Effect of different agents, including TPA, MG132, PMSF and LMB (1 ng/mL), on translocation and expression of RXRα.
Cells were pre-treated with different inhibitors for 3 hrs as required, followed by TPA treatment for another 12 hrs. (A) Translocation of RXRα in response to different agents, observed under laser-confocal microscope. (B) Expression of RXRα in-duced by different agents, revealed by Western blot.
In addition, we used another inhibitor LMB, an inhibitor of protein export from the nucleus[37,38], to block RXRα protein nucleocytoplasmic translocation, and then determine whether this blockage could affect TPA-induced RXRα degradation. As shown in Figure 4A, when LMB was incubated with cells alone for 3 hrs, or followed by TPA treatment for another 12 hrs, RXRα was not translocated into the cytoplasm, indicating that LMB did block RXRα translocation no matter TPA existed or not. Under such circumstances, TPA could not repress the expression level of RXRα protein (Figure 4B). These results clearly indicated that TPA-induced RXRα degradation was not only proteasome-dependent, but also translocation-dependent.
DISCUSSION
The redistribution of proteins between nucleus and cytoplasm is an important event for the regulation of their activities and the execution of their functions[39-41]. For example, phosphorylation is needed for redistribution of some proteins. In some cases, phosphorylation of a protein promotes its entry from the cytoplasm to the nucleus[41], in other cases, it causes the protein translocation from the nucleus to the cytoplasm[41,42]. Our study showed that degradation of RXRα protein by TPA was associated with its translocation from the nucleus to the cytoplasm. In gastric cancer BGC-823 cells, TPA could down-regulate the expression of RXRα protein significantly, and this repression was time-dependent and TPA-concentration-dependent (Figure 1A). In the treatment of cells with TPA for 24 hrs, 87% of RXRα protein expression was inhibited (Figure 1B). Moreover, we found that RXRα was translocated from the nucleus to the cytoplasm as degraded by TPA, also in a time-dependent manner (Figure 2A). However, when this translocation was blocked by LMB (Figure 4A), degradation of RXRα by TPA was not detected by Western blot (Figure 4B). Therefore, it clearly demonstrated that TPA-induced RXRα degradation was time-dependent and translocation-dependent.
Although we have detected the degradation of RXRα by TPA during the translocation of RXRα, its molecular mechanism is still largely unknown. More evidences revealed that the proteasome played not only a proteolytic role in protein degradation, but also a non-proteolytic role in transcriptional elongation, nuclear excision repair, and protein trafficking[43-48]. To determine whether degradation of RXRα was responsible for proteasome pathway, we used MG132, a specific proteasome inhibitor, to study the potential linkage among RXRα degradation, translocation and proteasome pathway. The results showed that inhibition of proteasome function by MG132 could repress TPA-induced RXRα degradation (Figure 3), and further block TPA-induced RXRα translocation (Figure 4A), which strongly supported the non-proteolytic role of the proteasome in protein translocation. Accordingly, it is likely that regulation of post-transcriptional activity of RXRα through the proteasome pathway may be involved in multiple mechanisms.
Recently, Tanaka et al[16] found that incubation of breast cancer cells MCF-7 with 10-6 mol/L retinoic acid induced a rapid breakdown of both RARα and RARγ in spite of the accumulation of their mRNA. Eliezer et al[49] also pointed out that retinoic acid-induced degradation of RARs and RXRs could play an important role in the control of the activity of RAR/RXR heterodimer. These reports, at least, provide a crucial clue: retinoid receptor (including RARs and RXRs) activity may be regulated through a distinct mechanism at transcriptional or post-transcriptional level, and formation of heterodimer/or homodimer between these receptors is essential. In our previous studies, we found that RXR could form heterodimer with RAR in the presence of retinoic acid[50]. Moreover, we have indicated that besides RAR, RXR also formed heterodimer with Nur77, an orphan receptor, bound to the RARβ (retinoic acid receptor β) promoter, and then induced RARβ expression, which is critical for inducing apoptosis and inhibiting cell growth in carcinoma cells[15,50]. Therefore, it is likely that degradation and translocation of RXRα by TPA are not a simple event. RXRα might form heterodimer with some proteins, such as Nur77 or RAR, then export from the nucleus to exert its function in the cytoplasm. But, at least, it has been confirmed that the RXRα is degraded through a proteasome pathway in the present study.