Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1878
Revised: March 14, 2003
Accepted: April 3, 2003
Published online: August 15, 2003
AIM: To study the intestinal permeability (IP) following stress of abdominal operation and the different effects on IP of enteral nutrition (EN) and parenteral nutrition (PN).
METHODS: Forty patients undergoing abdominal surgery were randomized into EN group and PN group. Each group received nutritional support of the same nitrogen and calorie from postoperative day (POD) 3 to POD 11. On the day before operation (POD-1), POD 7 and POD 12, 10 g of lactulose and 5 g of mannitol were given orally, and urine was collected for 6 hours. Urine excretion ratios of lactulose and mannitol (L/M) were measured.
RESULTS: L/M ratios of EN group on POD-1, POD 7 and POD 12 were 0.026 ± 0.017, 0.059 ± 0.026, 0.027 ± 0.017, respectively, and those of PN group were 0.025 ± 0.013, 0.080 ± 0.032, 0.047 ± 0.021, respectively. Patients of both groups had elevated L/M ratios on POD 7 vs. POD-1. However the ratio returned toward control level in EN group by POD 12. In contrast, PN group still had elevated L/M ratios on POD 12.
CONCLUSION: L/M ratio increases for a period of time after surgical trauma and the loss of gut mucosal integrity can be reversed by substitution of enteral nutrition.
- Citation: Jiang XH, Li N, Li JS. Intestinal permeability in patients after surgical trauma and effect of enteral nutrition versus parenteral nutrition. World J Gastroenterol 2003; 9(8): 1878-1880
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1878.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1878
Apart from the major function for digestion and absorption of nutrients, intestine also acts as "a central organ of stress". In many pathological conditions such as severe trauma, operation, chemotherapy and acute severe pancreatitis, intestine is a barrier to prevent microorganisms and toxins in the lumen from spreading to distant tissues and organs.
Nutrtional support has been used in clinical care for more than forty years. Total parenteral nutrition (TPN) is the form of nutritional support most suitable to patients with gut failure, in which it is lifesaving[1]. However, studies have found that TNP has many disadvantages, such as gut barrier dysfunction and bacterial translocation. Enteral nutrition may not have these disadvantages[2-4].
Many studies have demonstrated that intestinal permeability (IP) can reflect gut barrier function[5,6]. When the integrity of gut mucosal barrier is damaged, increased intestinal permeability may occur. The excretion ratio of L/M of urine has been used to measure intestinal permeability[7]. However, few studies have directly comparied the effect of EN versus PN on intestinal permeability after surgical trauma. Therefore, the present study was to observe intestinal permeability following operation and to investigate the different effects of enteral nutrition and parenteral nutrition on IP.
A prospective and randomized study was designed. Forty patients with digestive tract tumor were enrolled. All the patients had normal liver and kidney functions but no metabolic diseases. Informed consent was obtained from all the patients preoperatively.
All the patients were randomized intraoperatively after complete resection of tumor. The groups were defined as follows. EN group: Patients received enteral nutrition via jejunostomy tube or nasojejunal tube starting from POD 3. All the tubes were placed approximately 20 cm distal to the ligament of Treitz. PN group: Patients received total parenteral nutrition via central venous catheter starting from POD 3. Control group: It consisted of twenty healthy volunteers. All controls underwent an overnight fast and took the test solution orally.
All the patients of the two groups received isonitrogenous (0.1728 g/Kg/d) and isocaloric (30 Kcal/Kg/d) nutritional support starting from POD 3. The aim of enteral or parenteral nutrition was to meet 50% of nutritional requirements according to the protocol and GI tolerance of the patient on POD3, 75% on the next day and 100% on POD5.
EN group: Patients received Isosource (Novartis Corp, Switzerland) fluid polymeric formulation containing 14% protein, 29% fat, 57% carbohydrate calories. Nutrient solution was given at a steady speed.
PN group: Patients received total parenteral nutrition. Amino acids, fat, glucose and minerals were mixed and infused steadily.
Intestinal permeability was performed on POD-1, POD7 and POD12. All the patients fasted for at least 6 hours and their bladders were emptied before the test. The test solution consisted of 10 g of lactulose and 5 g of mannitol in a total volume of 50 mL with osmotic pressure 1 200 mOsm/L. The solution was given via the jejunostomy tubes or by nasojejunal or oral routes. The urine volume was collected for the subsequent 6 hours. One hour after the test started, the patients were encouraged to drink. After 2 h, liberal intake of food was allowed. The urine volume was recorded, and 10-mL portion was frozen and stored at -80 °C.
Urinary lactulose and mannitol were assayed using high- pressure liquid chromatography (HPLC) as described by Willems D and colleagues[8]. Calibration was performed on a daily basis with authentic standards at multiple concentrations, and the experimental standards were diluted so that the areas of all peaks fell within the calibration range. Fractional excretions (lactulose and mannitol) and L/M ratios were calculated. Fractional excretion was defined as the fraction of the gavaged dose recovered in the urine sample, and L/M ratio was a ratio of fractional excretions (lactulose-mannitol).
Statistical analysis was performed using analysis of variance (ANOVA) when comparing mean L/M ratios within groups and by an independent t test for differences between groups and vs control. Enumeration data were analyzed by χ2 square test. Differences were considered significant when P < 0.05, and obviously significant when P < 0.01. All values were expressed as means ± SD.
From April 2000 to July 2001, forty patients with digestive tract tumors were randomized to receive enteral nutrition or total parenteral nutrition. Preoperative and procedure related data for the two groups are listed in Table 1.
| EN group | PN group | |
| Age (y) | 50.8 ± 14.9 | 53.1 ± 15.6 |
| Sex (M/F) | 13/7 | 11/9 |
| Weight (Kg) | 60.0 ± 6.8 | 61.3 ± 12.3 |
| Cancer of stomach | 15 | 11 |
| Cancer of colon | 5 | 9 |
| Complete gastrectomy | 7 | 5 |
| Partial gastrectomy | 8 | 6 |
| Left hemicolectomy | 4 | 5 |
| Right hemicolectomy | 1 | 4 |
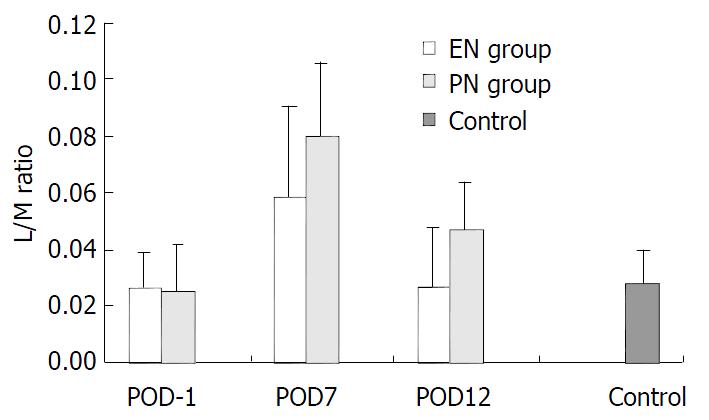
Figure 1 depicts the mean L/M ratios for all groups. On POD-1, there were no significant differences in EN group (0.026 ± 0.017) and PN group (0.025 ± 0.013) vs control (0.028 ± 0.012) (P > 0.05). Also there was no significant difference in EN group vs PN group (P > 0.05). On POD7, there was one-fold to two-fold increase in the L/M ratios in both EN group (0.059 ± 0.026) and PN group (0.080 ± 0.032) vs that on POD-1 (P < 0.01). However, there was a significant difference between PN group and EN group (P < 0.05). On POD12, there was a significant difference in L/M ratios in both EN group and PN group vs. that on POD7 (P < 0.01). However, there was a decreasing trend in L/M ratio in EN group (0.027 ± 0.017) vs. that on POD-1 (P > 0.05), while there was a significant difference in PN group (0.047 ± 0.021) vs. that on POD-1 (P < 0.01). There was a significant difference between PN group and EN group on POD12 (P < 0.01).
Small intestinal permeability has been used to quantify the damage of gut mucosal barrier[9,10]. Intestinal permeability changes have been detected by oral administration of probes such as 51Cr-EDTA, sucrose, lactulose, cellobiose, and polyethylene glycol[11-14]. The measurement of urinary excretion of nonmetabolized sugars has been widely used as a noninvasive method to assess mucosal integrity of the small bowel[15-17]. Monosaccharides such as mannitol and L-rhamnose pass through the transcellular routes of aqueous pores, reflecting the degree of absorption of small molecules (0.65 nm). Disaccharides, including lactulose and cellobiose, pass through the intercellular junctional complexes and extrusion zones at the villous tips, reflecting the permeability of large molecules (0.93 nm). The permeabilities of mono- and disaccharides are usually compared and expressed as an excretion ratio such as lactulose/mannitol or lactulose/L-rhamnose in urine samples. Lactulose and mannitol represent ideal compounds for measuring differential sugar absorption because they have a negligible affinity for the monosaccharide transport system and are passively absorbed and not metabolized before urine excretion. Intraindividual differences in gastric emptying, small intestinal transit, and urinary excretion are therefore eliminated[5-7,16].
Several enzymatic, colorimetric, and thin- layer chromatographic methods have been developed for the determination of lactulose and mannitol[5,6]. However, most of them are time-consuming and do not allow a simultaneous assay of both sugars. More recently, gas chromatographic and HPLC procedures have been proposed to overcome these problems[8,18]. Data from our study suggested that HPLC was a good method of measuring lactulose and mannitol. Our data also showed that L/M ratios could reflect intestinal permeability.
Sepsis, systemic inflammatory response and trauma in both animal and human are associated with gut mucosal damage and dysfunction[19-21]. Gut dysfunction is a common problem, resulting in loss of gut mucosal barrier selectivity, increased permeability to various hydrophilic solutes and translocation of bacterial products into the circulation, which may then further increase the inflammatory response in distant organs, leading to multiple organ dysfunction and death[22]. As a result, many authors considered the gut as an “engine” that drived sepsis. One possible contributory mechanism to endotoxin-induced gut mucosal damage was the increased apoptosis[11]. Inflammatory mediators enhanced apoptosis in a large number of cell lines. In intact animals, increased cardiac and hepatic apoptosis during sepsis might contribute to sepsis-related dysfunction of those organs. Thus paracellular tight junction may be injured and intestinal permeability increases. Our study showed an increased permeability after the stress of surgical trauma.
Nowadays nutritional support has become a routine therapy method. In the stress condition, proper nutritional support may provide necessary nutrients, reduce clinical complications and promote patients’ recovery from illness[3]. Total parenteral nutrition (TPN) provides significant benefits to surgical patients. However, there are still many complications. The effects of total parenteral nutrition on the gastrointestinal tract include decreasing brush-border hydrolase and nutrient-transporter activity, increasing mucosal permeability, and decreasing microvillus height[23-25]. Thus TPN is complicated by bacterial translocation (BT). Enteral nutrition after stress can maintain immunocompetence, and promote wound healing. Furthermore, it is considered that enteral nutrition can maintain gut barrier integrity, reduce septic complications[26] and the risk of death of critical care patients[3,27]. In our study, we used L/M ratio as a marker to reflect gut mucosal barrier. On POD7, L/M ratios in both EN group and PN group were elevated, but L/M ratio of EN group was significantly lower than that of PN group. And on POD12, L/M ratio of EN group returned to the level of POD-1, while L/M ratio of PN group was still higher than that of POD-1 and EN group.
It is concluded that L/M ratio increases in a period of time after surgical trauma and institution of enteral nutrition can reverse the loss of gut mucosal integrity.
Edited by Zhao P and Wang XL
| 1. | Jeejeebhoy KN. Total parenteral nutrition: potion or poison. Am J Clin Nutr. 2001;74:160-163. [PubMed] |
| 2. | Windsor AC, Kanwar S, Li AG, Barnes E, Guthrie JA, Spark JI, Welsh F, Guillou PJ, Reynolds JV. Compared with parenteral nutrition, enteral feeding attenuates the acute phase response and improves disease severity in acute pancreatitis. Gut. 1998;42:431-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 395] [Article Influence: 14.6] [Reference Citation Analysis (1)] |
| 3. | Minard G, Kudsk KA. Nutritional support and infection: does the route matter. World J Surg. 1998;22:213-219. [PubMed] |
| 4. | Zhu L, Yang ZC, Li A, Cheng DC. Protective effect of early enteral feeding on postburn impairment of liver function and its mechanism in rats. World J Gastroenterol. 2000;6:79-83. [PubMed] |
| 5. | Travis S, Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond). 1992;82:471-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 223] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 6. | Bjarnason I, MacPherson A, Hollander D. Intestinal permeability: an overview. Gastroenterology. 1995;108:1566-1581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 630] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 7. | Barboza Junior MS, Silva TM, Guerrant RL, Lima AA. Measurement of intestinal permeability using mannitol and lactulose in children with diarrheal diseases. Braz J Med Biol Res. 1999;32:1499-1504. [PubMed] |
| 8. | Willems D, Cadranel S, Jacobs W. Measurement of urinary sugars by HPLC in the estimation of intestinal permeability: evaluation in pediatric clinical practice. Clin Chem. 1993;39:888-890. [PubMed] |
| 9. | Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 10. | Wu CT, Huang XC, Li ZL. Intestinal mucosal permeability in-crease and intestinal bacterial translocation. Shijie Huaren Xiaohua Zazhi. 1999;7:605-606. |
| 11. | Uil JJ, van Elburg RM, van Overbeek FM, Mulder CJ, VanBerge-Henegouwen GP, Heymans HS. Clinical implications of the sugar absorption test: intestinal permeability test to assess mucosal barrier function. Scand J Gastroenterol Suppl. 1997;223:70-78. [PubMed] |
| 12. | Fink MP. Gastrointestinal mucosal injury in experimental models of shock, trauma, and sepsis. Crit Care Med. 1991;19:627-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 192] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S, Mauriño E, Meddings J. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001;49:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 14. | Blomquist L, Bark T, Hedenborg G, Norman A. Evaluation of the lactulose/mannitol and 51Cr-ethylenediaminetetraacetic acid/14C-mannitol methods for intestinal permeability. Scand J Gastroenterol. 1997;32:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Smecuol E, Bai JC, Vazquez H, Kogan Z, Cabanne A, Niveloni S, Pedreira S, Boerr L, Mauriño E, Meddings JB. Gastrointestinal permeability in celiac disease. Gastroenterology. 1997;112:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Dong HL. Intestinal permeability test and its clinical significance. Shijie Huaren Xiaohua Zazhi. 2000;8:562-563. |
| 17. | Tibble JA, Bjarnason I. Non-invasive investigation of inflammatory bowel disease. World J Gastroenterol. 2001;7:460-465. [PubMed] |
| 18. | Smecuol E, Bai JC, Sugai E, Vazquez H, Niveloni S, Pedreira S, Mauriño E, Meddings J. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001;49:650-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 19. | Deitch EA. Intestinal permeability is increased in burn patients shortly after injury. Surgery. 1990;107:411-416. [PubMed] |
| 20. | Wang W, Smail N, Wang P, Chaudry IH. Increased gut permeability after hemorrhage is associated with upregulation of local and systemic IL-6. J Surg Res. 1998;79:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 21. | Sun XQ, Fu XB, Zhang R, Lu Y, Deng Q, Jiang XG, Sheng ZY. Relationship between plasma D(-)-lactate and intestinal damage after severe injuries in rats. World J Gastroenterol. 2001;7:555-558. [PubMed] |
| 22. | Peng YZ, Yuan ZQ, Xiao GX. Effects of early enteral feeding on the prevention of enterogenic infection in severely burned patients. Burns. 2001;27:145-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 66] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 23. | Li J, Langkamp-Henken B, Suzuki K, Stahlgren LH. Glutamine prevents parenteral nutrition-induced increases in intestinal permeability. JPEN J Parenter Enteral Nutr. 1994;18:303-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Helton WS. The pathophysiologic significance of alterations in intestinal permeability induced by total parenteral nutrition and glutamine. JPEN J Parenter Enteral Nutr. 1994;18:289-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Illig KA, Ryan CK, Hardy DJ, Rhodes J, Locke W, Sax HC. Total parenteral nutrition-induced changes in gut mucosal function: atrophy alone is not the issue. Surgery. 1992;112:631-637. [PubMed] |
| 26. | Braunschweig CL, Levy P, Sheean PM, Wang X. Enteral compared with parenteral nutrition: a meta-analysis. Am J Clin Nutr. 2001;74:534-542. [PubMed] |
| 27. | Omura K, Hirano K, Kanehira E, Kaito K, Tamura M, Nishida S, Kawakami K, Watanabe Y. Small amount of low-residue diet with parenteral nutrition can prevent decreases in intestinal mucosal integrity. Ann Surg. 2000;231:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |