Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1808
Revised: November 5, 2002
Accepted: February 9, 2003
Published online: August 15, 2003
AIM: To directly investigate the relationship between telomerase activity and its subunit expression and the inhibitory effect of antisense hTR on pancreatic carcinogenesis.
METHODS: We examined the telomerase activity and its subunit expression by cell culture, polymerase chain reaction (PCR), PCR-silver staining, PCR-ELISA, DNA sequencing, MTT and flow cytometry methods.
RESULTS: PCR-silver staining and PCR-ELISA methods had the same specificity and sensitivity as the TRAP method. Telomerase activity was detected in the extract of the 10th, 20thand 30th passages of P3 cells,while it was absent in fibroblasts. Furthermore, after the 30th generation, the proliferation period of fibroblast cells was significantly prolonged. Telomerase activity and hTERTmRNA were detected in two pancreatic carcinoma cell lines, but were found to be negative in human fibroblast cells. Telomerase activity and hTERTmRNA were tested in pancreatic carcinoma specimens of 24 cases. The telomerase activity was positive in 21 of the 24 cases (87.5%), and the hTERTmRNA in 20 cases (83.3%). In adjacent normal tissues positive rates were both 12.5%. There was a significant difference between the two groups. This indicated a significant correlation between the expression level of telomerase activity and histologic differentiation, metastasis and advanced clinical stage of pancreatic carcinoma. Our findings showed that the expressions of hTR and TP1mRNA were not correlated with the activity of telomerase but the expression of hTERTmRNA was. After treatment with PS-ODNs, telomerase activity in P3 cells weakened and the inhibiting effect became stronger with an increase in PS-ODNs concentration. There was a significant difference between different PS-ODN groups (P < 0.05). Inhibition of telomerase activity occurred most significant with PS-ODN1.The results of the FCM test of pancreatic cancer P3 cells showed an increase in the apoptotic rate with increasing PS-ODN1 and PS-ODN2 concentrations.
CONCLUSION: The expression of telomerase activity has a significant relationship to carcinogenesis. A strong correlation exists between telomerase activity and hTERTmRNA expression. The up-regulation of hTERTmRNA expression may play a critical role in human carcinogenesis. The expression of telomerase activity and its subunit level in pancreatic carcinoma significantly correlate with the clinical stage of pancreatic carcinoma and hence, may be helpful in its diagnosis and prognosis. The anti-hTR complementary to the template region of hTR is sufficient to inhibit P3 cell telomerase activity and cell proliferation in vitro, and can lead to a profound induction of programmed cell death.
- Citation: Zhou JH, Zhang HM, Chen Q, Han DD, Pei F, Zhang LS, Yang DT. Relationship between telomerase activity and its subunit expression and inhibitory effect of antisense hTR on pancreatic carcinoma. World J Gastroenterol 2003; 9(8): 1808-1814
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1808.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1808
Telomerase is a DNA-dependent RNA polymerase[1] carrying template features. It is different from reverse transcriptase, DNA polymerase of commonly pure proteins. The activated telomerase takes the 3’ distal end of telomeres as the primer and its RNA component acts as the template. The protein component of telomerase regulates the catalytic activity in the synthesis of telomere repetitive sequence to maintain the telomere length. Human telomerase mainly consists of three subunits-human telomerase RNA (hTR), human telomerase-associated protein 1(TPl) and human telomerase reverse transcriptase (hTERT). Telomerase activity has been found in most human tumor cells[2], while there is no evident expression in normal human tissues other than in germ cells, hemopoietic stem cells and cuticle basal cells[3,4]. This suggests that telomerase is a broad spectrum tumor marker[5-9]. It is recognized that activation of telomerase and stability of telomere length are necessary for tumor immortalization. However, it has been found that expression of hTERTmRNA is obviously related to telomerase activity. It is suggested that up-regulation of the expression level of hTERTmRNA is a key factor in the formation of tumor cells.
Human pancreatic cancer is one of the most frequent tumors. The present clinical treatment however has a low curative effect. The mortality rate of human pancreatic cancer is very high. Post-operative metastases are common and less than 3% of patients have a survival rate of 5-years. In recent years, a close relationship has been found between telomerase and pancreatic cancer. By cell culture, PCR-silver staining, PCR-ELISA, RT-PCR, DNA sequencing technology, MTT and Flow cytometry methods, we have investigated the expression of telomerase activity and its subunits in pancreatic cancer cell line, fibroblasts, and in pancreatic cancer samples of 24 cases and their adjacent normal tissues. The purpose of this research was to explore the relationship between the biological behavior and clinico-pathological characteristics of human pancreatic cancer, as well as the inhibitory effects of hTR antisense oligonucleotide on pancreatic cancer cells.
In this study, the pancreatic cancer P3 cells were provided by Peking Union Medical College Hospital, PaTu-8801 cells were provided by Shanghai Changhai Hospital, and the human skin fibroblasts were developed in the Zhongda Hospital. Cancer tissues and adjacent normal tissues from 24 patients with pancreatic cancer were provided and assayed by the Surgical Department of Zhongda Hospital Affiliated to Southeast University and Jiangsu Provincial People,s Hospital. These samples were frozen at -80 °C within 15 min after surgical removal and stored until use. Of the 24 patients, 13 were male and 12 female.Their age ranged between 55-74 years with an average of 63 years.
Culture of the pancreatic cancer P3 cells and PaTu-8801 cells: P3 cells and human skin fibroblasts were cultured in 1640 culture medium and PaTu-8801 cells in DMEM culture medium (high glucose content) with 10% inactivated calf serum and all were put in 5% CO2 at 37 °C for generational culture at 100% RH.
Telomerase extracts and assays of activity were done as TRAP method. Briefly frozen pancreatic tissue samples of approximately 200 mg were homogenized in 200 μl of lysis reagent containing 5% CHPAS (3-(3-cholamidopropyl dimethyammonio)-1-propanesulfonate lysis buffer, Roche Co.). The cells obtained by cell culture were also treated with lysis reagent containing 5% CHPAS (Roche Co.). After 30 min of incubation on ice,the lysates were centrifuged at 16000×g (tissue) or 34000×g (cell) for 20 min at 4 °C, and the supernatants were rapidly frozen in liquid nitrogen and stored at -80 °C until use. In the extracts from frozen tissues, the concentration of protein was measured using the Coomassie brilliant blue method. BCA. Extracts of 293 cell line with telomerase activity were used as the standard, while extracts obtained by inactivation for 10 min at 94 °C or treated with RNase for 10 min at 37 °C were used as the negative control. For cell samples, aliquots corresponding to extracts derived from approximately 102, 103, 104, 105 cells were used for TRAP assay. Extracts of tissues containing 0.06, 0.6 and 6 μg of protein were used for TRAP assay. Each extract specimen was assayed in 25 μl reaction mixture (Roche Co assay kit containing dNTP, Taq enzyme, biotin tagged TS primer and CX primer) which was diluted to 50 μl with DEPC water solution. After 30 min incubation at 25 °C for telomerase-mediated extension of the TS primer, the reaction mixture was heated at 94 °C for 5 min and then subjected to 30 PCR cycles at 94 °C for 30 s, at 50 °C for 30 s and at 72 °C for 50 s and then extended for 10 min at 72 °C. Firstly, the PCR product went through enzyme-linked immunosorbent assay (PCR-ELISA) containing DIG-labeled probes (Roche Co assay kit). After spectrophotometric determination, the absorbance value A( = A450 - A690) was calculated. The result was positive if A > 0.2. Secondly, the PCR product was electrophoresed on a 10% polyacrylamide gel. The telomerase activity was positive if the specific band appeared. For each sample of pancreatic carcinoma tissue, the intensity of telomerase was graded according to the different contents of protein in the extract specimen. A sample intensity of telomerase was represented as (-) if the specimen containing 6 μg extracted protein was tested to be negative, or (+) if the specimen containing 6 μg extracted protein was tested positive. If both sample specimens containing 6 μg and 0.6 μg extracted protein were tested positive, while the specimen containing 0.06 μg extracted protein was tested to be negative, it was represented by (++), and (+++) if all three specimens containing 6 μg, 0.6 μg, and 0.06 μg extracted protein were tested positive[10].
Analysis of the expression of each telomerase subunit was performed by RT-PCR amplification[11-13]. hTERT mRAN was amplified by using the primer pair (145 bp): LT5 5’-CGGAAGAGTATCTGGAGCAA-3’, LT6 5’-GGATGAAGCGGAGTCTGGA-3’. TP1 mRAN was amplified by using the primer pair (340 bp), TP1.1 5’-TCAAGCCAAACCTGAATCTGAG-3’, TP1.2 5’-CCCCGAGTGAATCTTTCTACGC-3’. hTR was amplified by using the primer pair (134 bp): F3b 5’-TCTAACCCTAACTGAGAAGGGCGTAG-3’, R3c 5’-GTTTGCTCTAGAATGAACGGTGGAAG-3’. The efficiency of cDNA synthesis from each sample was estimated by PCR with glyceraldehyde-3-phosphate-dehydrogenase (GAPDH)-specific primers of (450 bp): K136 5’-CTCAGACACCATGGGGAAGGTGA-3’,K137 5’-ATGATCTTGAGGCTGTTGTCATA-3’. cDNA was synthesized in 20 μl of reaction mixture containing 5×RT Buffer 4 μl, RNasin 0.5 μl, total RNA 1 μl, and MLV 0.8 μl with 1 μl random primers. The reaction mixture was incubated at 94 °C for 5 min before it was heated at 95 °C for 5 min to inactivate MLV. To amplify the cDNA 2 μl aliquots of the reversely-transcribed cDNA was subjected to 35 cycles of PCR in 50 μl containing 10×buffer (10 mM Tris-HCl (pH8.3), 25 mM MgCl2, 500 mM KCl) 10 mM dNTP 1 μl, 25 mM MgCl2 2-3 μl, Taq enzyme 4 unit and 10 pM of specific primers 22 μ1. After heated at 94 °C for 5 min, each cycle consisted of de-naturation at 94 °C for 45 s, annealing at 56 °C for 45 s (hTERT) or at 61 °C for 45 s (hTR) or at 60 °C for 45 s (TP1) and extension at 72 °C for 90 s and then extended at 72 °C for 10 min. PCR products were electrophoresed on 3% agarose gel with ladder marker to determine the concentrations and purity of PCR amplified products. hTERTmRNA PCR products were sequenced (sent to Shanghai Biolottering Co, Ltd.).
Four nucleotides were synthesized as hTR template sequence (5’-CUAACCCUAAC-3’) and modified by thiophosphoric acid. They were PS-ODN1: 5’-GTTAGG-3’ (antisense), PS-ODN2: 5’-GTTAGGGTTAG-3’ (antisense), PS-ODN3: 5’-CCTAAC-3’ (pro-sense), and a PS-ODN4 with random sequence: 5’-AACTCGTAGTC-3’. After 5 × 10 P3 cells and human fibroblasts were inoculated into culture bottles and replaced the old culture medium with a fresh one after 24-h-lasting cultivation, the cells stuck to the wall of bottle. The test group was arranged PS-ODN1, PS-ODN2, PS-ODN3, PS-ODN4 so that each had four concentrations, 3.16 μmol/L, 10 μmol/L, 31.6 μmol/L, and 100 μmo1/L. They were added into the culture bottles, respectively. Additional sets were the control groups which had no PS-ODN. The following tests were conducted when obvious change in cytomorphology was observed under the optical microscope. Firstly, P3 and human fiber forming monolayer anchorage-dependent cells were digested. The suspended cells were cultured in RPMI 1640. After the suspended cell liquor was inoculated into a 96-cave culture board (100 μ1 per cave), 20 μ1 of freshly prepared 5 mg/ml MTT solution was added into each cave. This was incubated for 4 h at 37 °C, the culture medium in the caves was discarded, and then dimethyl sulfoxide (DMSO) 150 μ1 was added into each cave to be shaken for 10 min to solve the crystal. The activity of succinic dehydrogenase (SDH) was determined according to the optical absorbance of the content in each cave at 540 μm in an enzyme-linked immunoassay analyzer. Secondly, after 2 × 106 P3 cells were treated with each of the different PS-ODNs, the activity of telomerase extracts was assayed according to the TRAP, PCR-ELISA and polyacrylamidedel gel (10%) electrophoresis (PAGE). The telomerase extract of 293 cell line was taken as the positive control. The extract that was subjected to inactivation for 10 min at 94 °C or RNase treatment for 10 min at 37 °C was taken as the negative control. Finally, the single cell suspension was prepared by digesting anchorage-dependent cells after treatment with PS-ODN1 and PS-ODN2, respectively. It was cool-washed three times, and then centrifuged for 10 min at 2000 rpm. After the supernatant was discarded and dried, precooled 95% alcohol was added and the sample was placed into a refrigerator at 4 °C for 1 h, and then incubated with 10 μg/ml RNase at 4 °C for 3 h. The apoptotic rate and the cell cycle distribution of pancreatic cancer cells were analyzed by a flow cytometer (DB Corp. United States) after 1 ml propidium iodide comprehensive staining solution was added in an ice-bath for 15 min. Data were collected by Cellquest software and analyzed by Mmodift Lt software.
The data were processed by t-test and variance analysis. The P value was determined according to the t value, F value and FI value.
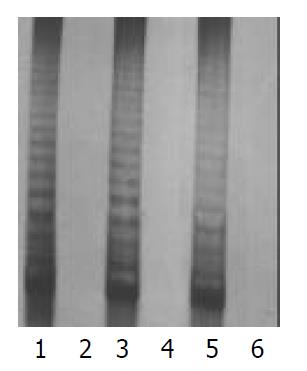
There were morphological changes in the fibroblasts (HE stain) of the 32nd generation, such as karyopyknosis, cytoplasmic concentration, and cell body shrinkage. No changes were found in P3 cells. P3 cells, and fibroblasts of the 10th, 20th, and 30th generations were used to detect the activity of telomerase in this test. All results were positive for P3 cells, and negative for fibroblasts (Figure 1).
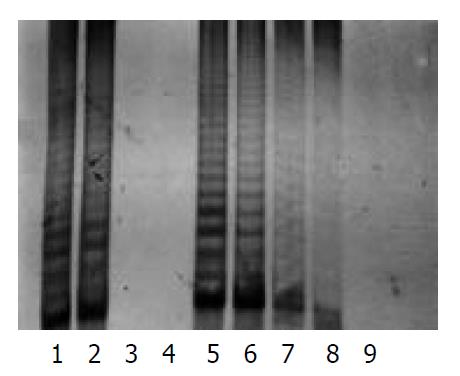
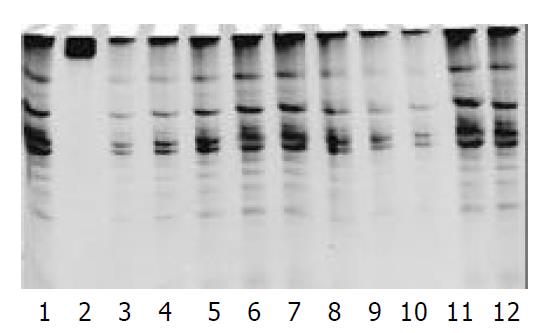
The protein content was calculated from the sample extract, and standard curves were prepared by using the data. The protein contents in the sample extracts were determined by Coomassie brilliant blue method. PCR-silver staining method was used to detect the activities of telomerase in 10 cells or 1 μg protein extracted from a sample. PCR-ELISA method had a same sensitivity as PCR-silver staining (Figure 2).
The results were positive for the activity of telomerase in pancreatic cancer cells, and negative for fibroblasts. There were 21 cases whose telomerase activity was detected in the 24 pancreatic cancer tissues by PCR-ELISA or PCR silver staining. Four cases of them were (+++), 11 cases (++), and 6 cases (+). The rate of positive findings was 87.5%. However, there were only 3 cases with telomerase activity (+) was found in adjacent normal tissues, with a positive rate of 12.5% (Figure 3).
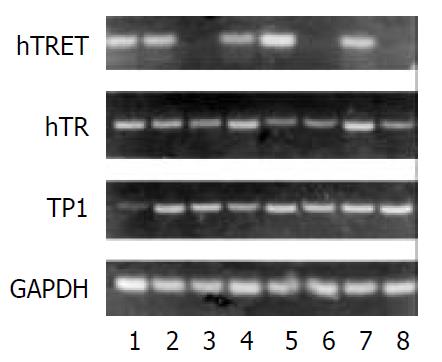
Pancreatic cancer cell P3, and PaTu-8801 were taken as the positive control, while the fibroblast was taken as the negative control. There were 20 cases which expressed hTERTmRNA in cancer tissues of 24 cases, and 3 cases expressed hTERTmRNA in the adjacent normal tissues. 4 of 24 cases of cancer tissue did not express hTR and 3 cases of the adjacent normal tissues did not express hTR. Two cases tested were negative for TP1 (Figure 4).
The cDNA sequencing of hTERTmRNA PCR products showed that there were 143 base groups that were significant sequences between the 51st and the 193rd base groups.The homology with hTERTcDNA reached 98.6%, proving that the amplification products were the hTERTcDNA sequence amplified from hTERT.
Table 1 shows the relationship between telomerase activity and tumor and adjacent normal tissue of pancreas.
There were significant differences in the expressions of telomerase activity and hTERTmRNA between pancreatic cancer tissues and adjacent normal tissues
Table 2 shows the relationship between telomerase activity and biological behavior of human pancreatic carcinoma.
| Biological behavior | Telomerase activity | ||
| – | + | AverageA (+) | |
| Age | 63.15 ± 0.45 | 62.50 ± 0.31 | 1.21 ± 0.11 |
| Sex | |||
| Male | 1 | 12 | 1.22 ± 0.13 |
| Female | 2 | 9 | 1.15 ± 0.44 |
| Pathologic type | |||
| Cystadenocarcinoma | 1 | 1 | 1.19 |
| Duct cell adenocystoma | 2 | 19 | 1.13 ± 0.39 |
| Mucinous adenocystoma | 1 | 1.22 | |
| Histologic differentiation | |||
| Well-diff. | 3 | 3 | 0.66 ± 0.33a |
| Mod-diff. | 10 | 1.10 ± 0.16 | |
| Poorly-diff. | 8 | 1.46 ± 0.12 | |
| Invasion stage | |||
| Non-invasive | 1 | 3 | 0.86 ± 0.31 |
| Invasive | 2 | 18 | 1.20 ± 0.35 |
| Lymphnode metastasis | |||
| Absent | 1 | 6 | 0.88 ± 0.31b |
| Present | 2 | 15 | 1.31 ± 0.24 |
| TNM stage | |||
| I | 1 | 1 | 1.19 |
| II | 5 | 0.81 ± 0.35c | |
| III | 2 | 10 | 1.28 ± 0.24 |
| IV | 5 | 1.34 ± 0.12 | |
A correlation existed between the expression level of telomerase activity in pancreatic cancer tissues with histologic differentiation, presence of lymph node metastasis, and clinical TNM stage of the tumor (no significant difference between stages III and IV). However, in pancreatic cancer patients no correlation was found between the expression level of telomerase activities and age or sex of patients, pathologic category or tumor infiltration.
Table 3 shows the relationship between telomerase activity and telomerase subunit.
There was also an obvious correlation between expression of hTERT and telomerase activities in pancreatic cancer tissues, but no correlation was found between expressions of hTR and TPlmRNA and telomerase activities.
In 24 pancreatic cancer tissues and adjacent normal tissues, their hTR/GAPDH ratios were 0.592 ± 0.056 and 0.510 ± 0.059, respectively. No difference was found in the expression level of hTR.
In the control groups, pancreatic cancer cells extended in polygon, showed large differences in their size and shape. The cells were more transparent, strong refractive, overlapped to grow after fully covering the bottom of the bottle and mitotic figures increased. After treatment with PS-ODN1 and PS-ODN2, morphologic changes of cells were obvious-refractiv, intercellular space became larger and the cells gradually became round, crenated, and fell off[14]. Occasionally, ballooning could be seen. When P3 cells and human fibroblasts were treated with four PS-ODNs for reflecting the survival rate of cells, the results of SDH activity are shown in Table 4 and Table 5. There was a significant difference in the effects between the different PS-ODN groups (P < 0.05). Along with an increase in concentrations of PS-ODN1 and PS-ODN2, the survival rate of cells significantly decreased. There was a significant difference between different concentrations in the same groups (P < 0.05). No significant difference was found between PS-ODN1 and PS-ODN2. It was found that PS-ODN3 also might cause a decrease in SDH activity,but the decrease was less obvious than that of the former two. Inhibition of SDH activity occurred earliest with PS-ODN1. Comparison between different PS-ODNs and different concentrations of any PS-ODN (P < 0.05) showed that the four PS-ODNs of any concentration had no significant effect on the survival rate of normal human fibroblasts.
| PS-ODN | C1(3.16) V(%,-x±s) | C2(10) V(%,-x±s) | C3(31.6) V(%,-x±s) | C4(100) V(%,-x±s) |
| PS-ODN1 | 92.16 ± 4.2 | 80.39 ± 5.9 | 58.11 ± 3.4 | 33.34 ± 4.6 |
| PS-ODN2 | 93.40 ± 2.6 | 84.22 ± 3.6 | 74.10 ± 3.8 | 27.35 ± 2.4 |
| PS-ODN3 | 91.74 ± 4.1 | 88.75 ± 7.1 | 86.38 ± 6.7 | 81.37 ± 5.3 |
| PS-ODN4 | 94.23 ± 3.3 | 92.36 ± 5.2 | 93.72 ± 4.9 | 91.54 ± 4.4 |
| PS-ODN | C1(3.16) V(%,-x±s) | C2(10) V(%,-x±s) | C3(31.6) V(%,-x±s) | C4(100) V(%,-x±s) |
| PS-ODN1 | 104.16 ± 6.4 | 100.48 ± 3.9 | 107.20 ± 8.4 | 103.04 ± 5.7 |
| PS-ODN2 | 100.80 ± 5.4 | 97.12 ± 6.7 | 99.52 ± 7.0 | 106.72 ± 6.4 |
| PS-ODN3 | 94.72 ± 4.7 | 90.56 ± 6.1 | 92.80 ± 6.8 | 95.36 ± 3.5 |
| PS-ODN4 | 100.16 ± 5.6 | 104.48 ± 5.3 | 99.04 ± 7.6 | 106.40 ± 9.2 |
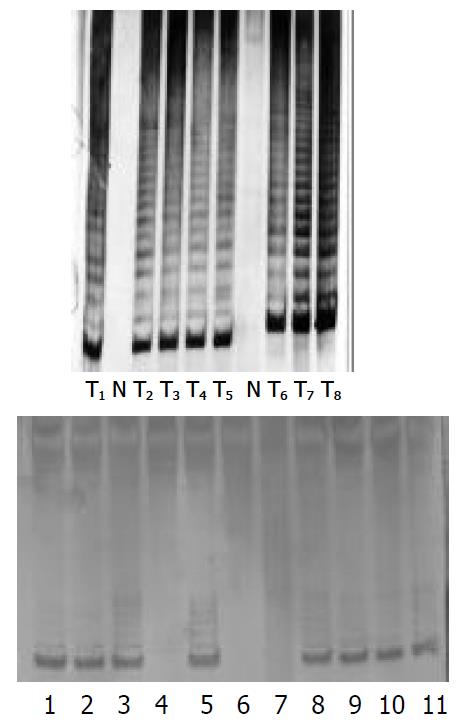
After P3 cells were treated by four PS-ODNs, their telomerase activity was detected using the PCR-ELISA method. The results showed that there was a significant difference between different PS-ODN groups (P < 0.05). PS-ODN1 and PS-ODN2 showed similar results when different concentration groups were compared (P < 0.05), suggesting that telomerase activity in P3 cells weakened and the inhibiting effect became stronger with increasing concentration. There was no significant difference of telomerase activity between the different concentration groups of PS-ODN3 and PS-ODN4 (P > 0.05) (Table 6). The results of quantitative tests of telomerase activity in PS-ODNs-treated P3 cells by TRAP-PAGE silver staining method are shown in Figure 5. After treatment by PS-ODN1 and PS-ODN2, the telomerase activity in P3 cells was positive. The brightness of the bands darkened with increasing PS-ODN1 and PS-ODN2 concentrations, and expression of the telomerase activity had a tendency to be depressed.
| PS-ODN | C0(0) B(-x±s) | C1(3.16) B(-x±s) | C2(10) B(-x±s) | C3(31.6) B(-x±s) | C4(100) B(-x±s) |
| PS-ODN1 | 2.25 ± 0.56 | 1.53 ± 0.23 | 1.20 ± 0.15 | 0.97 ± 0.12 | 0.65 ± 0.47 |
| PS-ODN2 | 2.25 ± 0.56 | 1.66 ± 0.46 | 1.40 ± 0.44 | 0.69 ± 0.29 | 0.49 ± 0.32 |
| PS-ODN3 | 2.25 ± 0.56 | 2.28 ± 0.32 | 2.32 ± 0.37 | 2.26 ± 0.31 | 2.19 ± 0.26 |
| PS-ODN4 | 2.25 ± 0.56 | 2.16 ± 0.35 | 2.24 ± 0.50 | 2.27 ± 0.43 | 2.13 ± 0.38 |
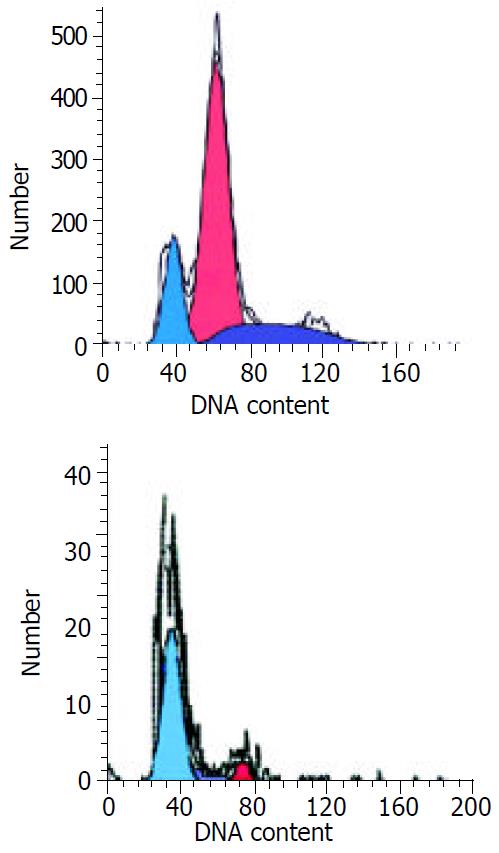
The FCM results of pancreatic cancer P3 cells showed the apoptotic rate of cells increased along with increasing PS-ODN1 and PS-ODN2 concentrations. Fifty days or so after continuous treatment of the cells at 100 μM concentration of PS-ODN1 and PS-ODN2, the apoptotic rate of cells was 81.03% and 70.75%, respectively. The cycle distribution increased from 86.51% and 83.89% to 94.53% and 95.39% for cells in stage Go/G1, and reduced from 13.49% and 16.11% to 5.47% and 2.11% for cells in stage S, respectively (Figure 6).
The activation of telomerase and maintenance of telomere length are essential for tumor cell immortalization. The positive rate of telomerase activity was 85%-95% in patients with pancreatic cancer, much higher than that in patients with benign pancreatic diseases[15-18]. Therefore, it can be deduced that there is a close link between telomerase activity and metastatic potential of pancreatic cancer cells[19].
The 10th, 20th, and 30th generations of cultured tumor cells and fibroblasts showed a significant difference in their telomerase activities. The results showed a positive telomerase activity for tumor cells, whereas it was absent in all generations of fibroblasts. After the 30th generation, the fibroblast cell proliferation period was obviously elongated, and part of the cells were found to have morphologic changes such as karyopyknosis, cytoplasmic concentration and cell-body shrinkage on HE stain. These findings indicate that telomerase is involved in immortalization of pancreatic cancer cells.
Hiyama et al[15]. reported positive telomerase activity was found in 43 cases. Among them, five cases were found to have a low level of telomerase activity expression in the adjacent normal tissues. In 24 pancreatic cancer samples, the positive rate of telomerase activity was 87.5%, while there were 3 cases whose telomerase activity appeared in the adjacent normal tissue, a positive rate of 12.5%. Of the three cases, one was proven to be infiltrated by tumor cells. The positive rate of hTERTmRNA in pancreatic cancer samples was 83.3%, and 12.5% in the adjacent normal tissue.
The results of this study support the theory that telomerase is involved in the formation of pancreatic cancer. It is a consensus that the prognosis is poor in patients with low differentiation and wide infiltration of human pancreatic cancer, i.e., those falling into the TNM stages III-IV of the pancreatic cancer[20-22]. By statistic analysis, our data showed that there was a definite correlation between the expression level of telomerase activity in pancreatic cancer and histologic differentiation, presence of lymph node metastasis and TNM staging of the tumor. However, no significant correlation was found between it and age, sex of the subjects, pathologic category and degree of infiltration. Along with increasing expression level of telomerase, the tumor showed a higher lymph node metastasis. Therefore, the prognosis became much worse. We did not perform any post-operative follow-up studies.Tang et al[22] reported that patients with pancreatic endocrine neoplasm who were positive in telomerase expression usually had a shorter life expectancy than those who were negative in telomearse expression.
In the tumor cell line with positive telomerase expression, hTERTmRNA was found to have a higher expression level, whereas it was absent in normal tissues. In 21 cases of pancreatic cancer tissues with positive telomerase activity, hTERTmRNA was detected in 18 cases and only one case was found to be positive in the adjacent normal tissue which showed no telomerase activity.
Statistic analysis showed a significant correlation between hTERTmRNA and telomerase activity. Therefore, it may be thought that expression of hTERT is an important factor in governing telomerase activity[23,24] and that up-regulation of hTERTmRNA expression may play an important role in the formation of pancreatic cancer. However hTERTmRNA was detected in two of the pancreatic cancer samples in which no telomerase activity was found. This may be explained by the following factors: (1) The expression level of each subunit or an equilibrium state between their expression levels determines the activity of telomerase; (2) Modification of the telomerase subunit after its transcription may have a regulating effect on the enzyme activity; (3) telomerase inhibitor existing in the cell extract reduces the activity of telomerase. Furthermore, there were 3 cases whose telomerase activity was detected, while the expression of hTERTmRNA showed negative results. There may be other factors to determine the activity of telomerase in this latter situation.
This study found that though the expression level of hTR in pancreatic cancer tissue was higher than that in adjacent normal tissue, it had no statistical significance. hTR itself cannot reflect the activity of telomerase. However, its function is essential for the activity of telomerase. This point is consistent with other studies[25-28].
It was found in our study that inhibition of the growth of P3 cells by PS-ODN1 occurred the earliest. This was probably not only due to the smaller PS-ODN1 molecules entering cells, but also due to two important basic UC groups at the junction of the closed hTR templates. No significant effect was found on the survival rate of the P3 cells in the random sequence group, while slight inhibition of P3 cells growth was found in the pro-sense group. This could be explained as the pro-sense oligonucleotide competed against telomerase RNA in quantity and space, thus impeding the attachment of telomere with RNA template zone to a certain degree[29]. The results showed that inhibition of PS-ODN1 and PS-ODN2 on the growth of P3 cells was dosage-dependent. This implies that after hTR template is closed, telomerase can no longer bring its activity into full play. Therefore it can be concluded that hTR is involved in the regulation of telomerase activity[30].
FCM results showed that the apoptotic rate of P3 cells would increase along with increase of the concentrations of antisense oligonucleotide, at the same time, cells in G0/G1 stage increased in quantity while those in S stage decreased. This suggests that treatment with PS-ODN1 and PS-ODN2 may block the cells at G0/G1 stage and induce apoptosis.
In regard to the relationship between telomerase activity and cell cycle, it was thought[31] that the tolemerase activity was regulated in the cell cycle-leading mode. It was also thought that tolemerase should be activated at the DNA replication stage as tolemerase activity was essential to maintain tolemere. This activity is not required at the non- replication stage of the cell cycle. Telomerase has different activity at different stages of the cell cycle, its highest rate is at S stage, lowest at G2/M stage, and almost no activity at G0/G1 stage. Our results support this point of view.
We simultaneously observed the influence of PS-ODNs on SDH activity and proliferation of normal human fibroblasts. The results showed that it had a significantly inhibitory effect on the metabolism and proliferation of cells. Therefore, it can be assumed that the effect of antisense hTR is cell-specific, and has no harmful action on normal cells.
Our study suggested that there was no correlation between the expression of TPlmRNA and telomerase activity in pancreatic cancer. However, the function of TP1 is still not clearly known, and it may be related to interaction with protein. TP1 may play a role in the interface between telomerase and telomere conjugated proteins. Modification after transcription of TPlmRNA may regulate the telomerase activity.
In conclusion, telomerase may be taken as a subsidiary parameter for the diagnosis and outcome of pancreatic cancer. For preoperative patients, detection of the telomerase activity may be conducted on blood, or pancreatic juice and duct cells taken from ERCP on fine needle aspiration specimens for early diagnosis[32-35]. Antisense oligonucleotide can reduce the activity of telomerase in pancreatic cancer P3 cells, inhibit the growth of P3 cells, promote changes in cell cycle and induce their apoptosis[36,37].
Edited by Lu HM and Wang XL
| 1. | Greider CW, Blackburn EH. A telomeric sequence in the RNA of Tetrahymena telomerase required for telomere repeat synthesis. Nature. 1989;337:331-337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1147] [Cited by in RCA: 1210] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 2. | Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD, Ho PL, Coviello GM, Wright WE, Weinrich SL, Shay JW. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266:2011-2015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5156] [Cited by in RCA: 5234] [Article Influence: 168.8] [Reference Citation Analysis (0)] |
| 3. | Wan M, Li WZ, Duggan BD, Felix JC, Zhao Y, Dubeau L. Telomerase activity in benign and malignant epithelial ovarian tumors. J Natl Cancer Inst. 1997;89:437-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Härle-Bachor C, Boukamp P. Telomerase activity in the regenerative basal layer of the epidermis inhuman skin and in immortal and carcinoma-derived skin keratinocytes. Proc Natl Acad Sci USA. 1996;93:6476-6481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 358] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Wang J, Liu X, Fang J. [Expression and clinical significance of telomerase catalytic subunit gene in lung cancer and its correlations with genes related to drug resistance and apoptosis]. Zhonghua Zhongliu Zazhi. 1999;21:350-353. [PubMed] |
| 6. | Zhang YL, Zhang ZS, Wu BP, Zhou DY. Early diagnosis for colorectal cancer in China. World J Gastroenterol. 2002;8:21-25. [PubMed] |
| 7. | Shen ZY, Xu LY, Li EM, Cai WJ, Chen MH, Shen J, Zeng Y. Telomere and telomerase in the initial stage of immortalization of esophageal epithelial cell. World J Gastroenterol. 2002;8:357-362. [PubMed] |
| 8. | Qin LX, Tang ZY. The prognostic molecular markers in hepatocellular carcinoma. World J Gastroenterol. 2002;8:385-392. [PubMed] |
| 9. | Yao XX, Yin L, Sun ZC. The expression of hTERT mRNA and cellular immunity in gastric cancer and precancerosis. World J Gastroenterol. 2002;8:586-590. [PubMed] |
| 10. | Kojima H, Yokosuka O, Imazeki F, Saisho H, Omata M. Telomerase activity and telomere length in hepatocellular carcinoma and chronic liver disease. Gastroenterology. 1997;112:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 11. | Suzuki T, Suzuki Y, Fujioka T. Expression of the catalytic subunit associated with telomerase gene in human urinary bladder cancer. J Urol. 1999;162:2217-2220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Takakura M, Kyo S, Kanaya T, Tanaka M, Inoue M. Expression of human telomerase subunits and correlation with telomerase activity in cervical cancer. Cancer Res. 1998;58:1558-1561. [PubMed] |
| 13. | Lanham S, Herbert A, Watt P. HPV detection and measurement of HPV-16, telomerase, and survivin transcripts in colposcopy clinic patients. J Clin Pathol. 2001;54:304-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Zhang FX, Zhang XY, Fan DM, Deng ZY, Yan Y, Wu HP, Fan JJ. Antisense telomerase RNA induced human gastric cancer cell apoptosis. World J Gastroenterol. 2000;6:430-432. [PubMed] |
| 15. | Hiyama E, Kodama T, Shinbara K, Iwao T, Itoh M, Hiyama K, Shay JW, Matsuura Y, Yokoyama T. Telomerase activity is detected in pancreatic cancer but not in benign tumors. Cancer Res. 1997;57:326-331. [PubMed] |
| 16. | Tsutsumi M, Tsujiuchi T, Ishikawa O, Majima T, Yoshimoto M, Sasaki Y, Fukuda T, Oohigashi H, Konishi Y. Increased telomerase activities in human pancreatic duct adenocarcinomas. Jpn J Cancer Res. 1997;88:971-976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Büchler P, Conejo-Garcia JR, Lehmann G, Müller M, Emrich T, Reber HA, Büchler MW, Friess H. Real-time quantitative PCR of telomerase mRNA is useful for the differentiation of benign and malignant pancreatic disorders. Pancreas. 2001;22:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Iwao T, Hiyama E, Yokoyama T, Tsuchida A, Hiyama K, Murakami Y, Shimamoto F, Shay JW, Kajiyama G. Telomerase activity for the preoperative diagnosis of pancreatic cancer. J Natl Cancer Inst. 1997;89:1621-1623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Sato N, Maehara N, Mizumoto K, Nagai E, Yasoshima T, Hirata K, Tanaka M. Telomerase activity of cultured human pancreatic carcinoma cell lines correlates with their potential for migration and invasion. Cancer. 2001;91:496-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 20. | Ohta K, Kanamaru T, Morita Y, Hayashi Y, Ito H, Yamamoto M. Telomerase activity in hepatocellular carcinoma as a predictor of postoperative recurrence. J Gastroenterol. 1997;32:791-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Suda T, Isokawa O, Aoyagi Y, Nomoto M, Tsukada K, Shimizu T, Suzuki Y, Naito A, Igarashi H, Yanagi M. Quantitation of telomerase activity in hepatocellular carcinoma: a possible aid for a prediction of recurrent diseases in the remnant liver. Hepatology. 1998;27:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Tang SJ, Dumot JA, Wang L, Memmesheimer C, Conwell DL, Zuccaro G, Goormastic M, Ormsby AH, Cowell J. Telomerase activity in pancreatic endocrine tumors. Am J Gastroenterol. 2002;97:1022-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, Ziaugra L, Beijersbergen RL, Davidoff MJ, Liu Q. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1302] [Cited by in RCA: 1318] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 24. | Nakamura TM, Morin GB, Chapman KB, Weinrich SL, Andrews WH, Lingner J, Harley CB, Cech TR. Telomerase catalytic subunit homologs from fission yeast and human. Science. 1997;277:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1680] [Cited by in RCA: 1660] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 25. | Müller M, Krause H, Heicappell R, Tischendorf J, Shay JW, Miller K. Comparison of human telomerase RNA and telomerase activity in urine for diagnosis of bladder cancer. Clin Cancer Res. 1998;4:1949-1954. [PubMed] |
| 26. | Kanaya T, Kyo S, Takakura M, Ito H, Namiki M, Inoue M. hTERT is a critical determinant of telomerase activity in renal-cell carcinoma. Int J Cancer. 1998;78:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 27. | Kyo S, Kanaya T, Takakura M, Tanaka M, Yamashita A, Inoue H, Inoue M. Expression of human telomerase subunits in ovarian malignant, borderline and benign tumors. Int J Cancer. 1999;80:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Harrington L, McPhail T, Mar V, Zhou W, Oulton R, Bass MB, Arruda I, Robinson MO. A mammalian telomerase-associated protein. Science. 1997;275:973-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 456] [Cited by in RCA: 476] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 29. | Yuan X, Zhang B, Ying J, Wang H, Zhang H, Hou L, Wu B. [Expression of antisense telomerase genes suppressing human cancer malignant phenotypes]. Zhonghua Binglixue Zazhi. 1999;28:356-360. [PubMed] |
| 30. | Teng L, Chen S, Thomas J F. Stable expression of antisense hTR inhibits in vitro pancreatic cancer cell growth. Chin Med J (Engl). 2002;115:1196-1200. [PubMed] |
| 31. | Zhu X, Kumar R, Mandal M, Sharma N, Sharma HW, Dhingra U, Sokoloski JA, Hsiao R, Narayanan R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc Natl Acad Sci USA. 1996;93:6091-6095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 32. | Inoue H, Tsuchida A, Kawasaki Y, Fujimoto Y, Yamasaki S, Kajiyama G. Preoperative diagnosis of intraductal papillary-mucinous tumors of the pancreas with attention to telomerase activity. Cancer. 2001;91:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Myung SJ, Kim MH, Kim YS, Kim HJ, Park ET, Yoo KS, Lim BC, Wan Seo D, Lee SK, Min YI. Telomerase activity in pure pancreatic juice for the diagnosis of pancreatic cancer may be complementary to K-ras mutation. Gastrointest Endosc. 2000;51:708-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Seki K, Suda T, Aoyagi Y, Sugawara S, Natsui M, Motoyama H, Shirai Y, Sekine T, Kawai H, Mita Y. Diagnosis of pancreatic adenocarcinoma by detection of human telomerase reverse transcriptase messenger RNA in pancreatic juice with sample qualification. Clin Cancer Res. 2001;7:1976-1981. [PubMed] |
| 35. | Pearson AS, Chiao P, Zhang L, Zhang W, Larry L, Katz RL, Evans DB, Abbruzzese JL. The detection of telomerase activity in patients with adenocarcinoma of the pancreas by fine needle aspiration. Int J Oncol. 2000;17:381-385. [PubMed] |
| 36. | Sato N, Mizumoto K, Nagai E, Tanaka M. Telomerase as a new target for pancreatic cancer treatment. J Hepatobiliary Pancreat Surg. 2002;9:322-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 37. | Pirocanac EC, Nassirpour R, Yang M, Wang J, Nardin SR, Gu J, Fang B, Moossa AR, Hoffman RM, Bouvet M. Bax-induction gene therapy of pancreatic cancer. J Surg Res. 2002;106:346-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |