INTRODUCTION
For a malignant tumor cell to metastasize, it must break away from its neighbors, force its way through the surrounding stroma, and penetrate basement membranes to enter the stroma and the circulation. When it arrives at its destination, these steps must be repeated in reverse order[1]. A critical event in the process of cancer invasion and metastasis is therefore degradation of various constituents of the extracellular matrix (ECM) including collagen, laminin, fibronectin, and heparan sulfate proteoglycans (HSPGs). The malignant cell is able to accomplish this task through the concerted sequential action of enzymes such as metalloproteinases, serine proteases, and endoglycosidases[2,3]. Among these enzymes, an endo-β-glucuronidase (heparanase) selectively degrades the heparan sulfate chains of HSPGs which are essential and ubiquitous macromolecules associated with the cell surface and ECM of a wide range of cells and tissues[4,5]. Heparanase cleaves heparan sulfate (HS) and has been implicated in many important pathological processes, including tumor metastasis and angiogenesis[6,7]. Therefore, heparanase plays an essential role in these pathological processes which makes it a potentially important target for cancer therapy and be helpful to investigate the mechanism, by which the expression of heparanase is regulated.
Eukaryotic initiation factor 4E (eIF-4E) is a 25 kDa mRNA cap-binding phosphoprotein that is rate-limiting for the initiation of cap-dependent mRNA translation by the eIF-4F translation initiation complex[8,9]. Overexpression of eIF-4E has been found in human carcinoma tissues and tumor cell lines. The factor (eIF-4E)[10] dramatically impacts upon the quantitative expression of key malignancy-related genes and can be considered as a critical determinant of malignancy. It seems that involvement of eIF-4E in tumor progression is more closely associated with the impact of enhanced eIF-4E activity on specific, malignancy-related molecules such as ODC, c-myc, cyclin D1, VEGF or MMP-9. Cooperative overexpression of these potent molecules leads to occurrence of tumorigenic phenotype that conspires to drive metastatic progression. The aim of this study was to determine whether eIF-4E was involved in the regulation of heparanase expression and to postulate the probable mechanism.
MATERIALS AND METHODS
Materials
Cell lines Human colon adenocarcinoma cell line LS-174T was an ATCC cell line and was maintained in RPMI 1640 supplemented with 2 mM L-glutamine and 10% FCS at 37 °C in a humidified atmosphere containing 5% CO2.
Antisense oligonucleotides Oligonucleotides containing phosphorothioate were customarily-made and purified with high-performance liquid chromatography. The eIF-4E antisense oligonucleotide comprised the following sequence[11]: 5’-AGTCGCCATCTTAGATCGAT-3’ (20 mer), complementary to nucleotides (nt) -11 to + 9 of human eIF-4E mRNA. The complementary sense sequence used was 5’-ATCGATCTAAGATGGCGACT-3’. Sense oligonucleotide was used as controls in each of the antisense oligonucleotide experiments.
Methods
Antisense oligonucleotide treatments The day before transfection, the cells were trysinized, counted and plated in a 5 × 106 cells/60-mm dish so that 90%-95% confluency was reached on the day of transfection. As it was a unique cationic lipid formulation, LIPOFECTAMlNE 2000 was more convenient in that it could be used in the presence of serum containing media, by adding it directly to the culture without washing the cells. For transfecting oligonucleotides to cells, the LIPOFECTAMlNE 2000 reagent (Invitrogen) was used according to the manufacturer’s instructions. Briefly, LIPOFECTAMlNE 2000 reagent and oligonucleotides (ODNs) were diluted separately into RPMI 1640 medium and ODNs were mixed with liposome in a charge ratio of 1:2. The mixtures were incubated at room temperature for 20 min to form complexes and then ODN-LIPOFECTAMlNE 2000 reagent complexes was added directly to each well and mixed gently by rocking the plate back and forth. The ODNs were delivered to tumor cells at the final concentration of 2 μmol/L. Tumor cells were incubated in the presence of oligonucleotides/ LIPOFECTAMlNE 2000 reagent mixture for 24 h and 48 h for RT-PCR assays or Western blot.
Semi-quantitative RT-PCR analysis of eIF-4E mRNA Total RNA was extracted by the guanidine salts and phenol-chloroform method. cDNA was prepared from 2 μg of total RNA, using poly-T as primer and MuLV reverse transcriptases (Promega). For PCR amplification, one-fourth of the reverse transcription product and 0.3 μmol/L of each oligonucleotide as primers were used. After one denaturation step at 96 °C for 3 minutes, 30 cycles of amplification were performed: denaturation at 94 °C for 45 s, annealing at 55 °C for 45 s, synthesis at 72 °C for 1 minute, and extension at 72 °C for 5 minutes. Oligonucleotides for eIF-4E expression analysis were sense primer 5‘AGATGGCGACTGTCGAACC3’, antisense primer 5’CAGCGCCACATACATCAT3’[12]. To check cDNA quality, GAPDH was amplified using sense primer 5’CTGGCGCTGAGTACGTCGTG3’ and antisense primer 5’CAGTCTTCTGGGTGGCAGTG3’. One-tenth of the amplified products was run on 2% agarose gels in 1×Tris borate buffer and visualized with ethidium bromide.
Western blot analysis Total proteins were extracted at 48 h after transfection. Cells were lysed in RIPA buffer [10 mM Tris-HCl (pH 7.4), 1% deoxycholate, 1% NP40, 150 mM NaCl, 0.1% SDS, 0.2 mM phenylmethylsulfonyl fluoride, 1 μg/ml aprotinin and 1 μg/ml leupeptin. The lysates were then centrifuged at 10000×g for 15 min to remove debris. Protein content of the lysate was determined by using Bradford assay (Bio-Rad). For each sample, equal amounts (50 μg) of protein lysates were analyzed on a sodium dodecyl sulfate-10% or 12% polyacrylamide gel. Proteins were electroblotted onto Immobilon PVDF membranes (Millipore, Bedford, MA), blocked for 1 h in 5% dry skim milk, and then incubated with the indicated antibodies. The antibodies used and their dilutions were as follows: 1:500 mouse monoclonal anti-eIF-4E (BD Biosciences), 1:700 rabbit polyclonal anti-heparanase (a kind gift from Mark D. Hulett). The secondary antibodies used were those in an enhanced chemiluminescence detection kit (Amersham) and were chosen according to the species used for the primary antibodies. Exposure times varied with the antibodies used and ranged from 5 to 60 s.
Assays for heparanase activity Heparanase activity was defined as the ability to degrade high molecular weight (40-100 kDa) radio-labeled HS substrate into low molecular weight (5-15 kDa) HS fragments[13-15], which could be differentiated by gel filtration chromatography[16,17]. Radiolabeled HS substrate was prepared by metabolically labeling the extracellular matrix with [35S] as described[16,17]. Soluble substrate was made by releasing 35S-labeled HS proteoglycans from the culture plate with trypsin, and incubated with cell homogenates which were prepared by suspending 106 cells in 0.2 ml of aq. 0.1% (v/v) Triton X-100 and disrupted by freezing and thawing three times in a solid CO2/ethanol mixture. Samples were taken from the above supernate fractions. Protein concentrations were estimated by a Bio-Rad Coomassie protein assay kit (Bio-Rad Laboratories) using BSA as a standard. Cell lysates containing 50 μg of protein were incubated at 37 °C for 24 h in 10 mM sodium phosphate-citrate buffer, pH 6.0, with 20-25000 cpm of 35S-labeled HS substrate. The incubation medium was centrifuged and the supernatant was analyzed by gel filtration on a Sepharose CL-6B column (0.7 × 25 cm). Fractions (0.2 ml) were eluted with PBS and their radioactivity was measured. Degradation fragments of HS side chains were eluted from Sepharose CL-6B at 0.5 < Kav < 0.8 (peak II). A nearly intact HSPG was eluted just after the Vo (Kav < 0.2, peak I). Each experiment was repeated at least three times and the variation of elution positions (Kav values) did not exceed ± 15%. Blue dextran and phenol red were added to the sample to mark the excluded (V0) and included (Vt) volumes, respectively.
In vitro invasion assaysIn vitro invasion assays were performed using modified Boyden chambers, 24-well plates. Polycarbonate filters (8 μm porosity), used to separate the upper compartment, were coated with 50 μg Matrigel. After the lower wells were filled with 600 μl of culture media, supplemented with 0.1% BSA and 50 μg solubilized Matrigel as a chemo-attractant. The cells in media containing 2 μM oligonucleotides, 1% FBS at a density of 106 cells/ml were resuspended. Gelled Matrigel was gently washed with warmed serum free-culture media. 100 μl of the cell suspension was put onto the Matrigel. Chambers were placed in a 37 °C incubator with 5% CO2/95% air for 48 h. Then, the cells remaining on the upper surface of the membrane were removed with a cotton swab and the filters were fixed and stained with a solution of methanol/Coomassie blue 0.1% in acetic acid (1:3). Cells that had invaded the lower surface of the filter were counted under an inverted microscope. 10 fields per well were counted. The invasion score of untreated control cells was taken as 100% and that of treated cells was expressed as a percentage of control. All experiments were performed in duplicate and the results from 3 separate sets of experiments were averaged.
RESULTS
Down-regulation effects of antisense oligonucleotides on eIF-4E expression
LS-174T cells were treated with either antisense oligonucleotides, sense control oligonucleotides or saline for 24 h or 48 h in the presence of liposome. By using quantitative RT-PCR analysis, a high level of eIF-4E mRNA was shown in LS-174T cell lines, and treatment with antisense oligonucleotides at concentrations up to 2 μM, induced a significant decrease in the eIF-4E mRNA expression levels compared to control groups. There was no down-regulation of eIF-4E expression in the sense oligonucleotide-treated cells (Figure 1). In order to verify that the decrease in mRNA expression levels corresponded also to decreases in protein levels, Western blot analysis was performed (Figure 2). We used eIF-4E standard antigen (BD Transduction Laboratories) as a positive control. A significant reduction of eIF-4E protein level was observed in LS-174T cell line, treated with antisense oligonucleotides. In contrast, there was no apparent down-regulation of eIF-4E protein levels in the cells exposed to sense oligonucleotides in comparison with untreated ones.
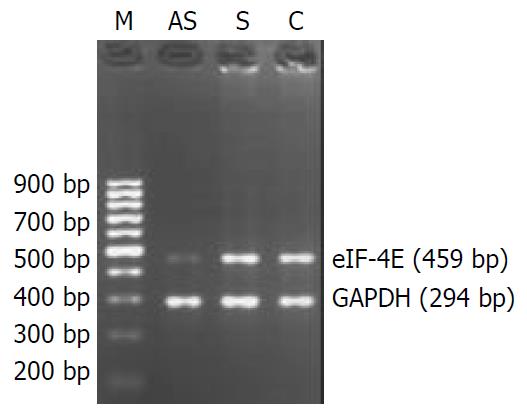
Figure 1 eIF-4E and GAPDH mRNA expression in oligonucle-otide-treated human colon adenocarcinoma cells.
AS. anti-sense oligonucleotide-transfected cells; S. sense oligonucleotide-transfected cells; C. cells treated with liposome only as control. LS-174T cells were treated with 2 μM oligonucleotides, accord-ing to the optimal condition of this cell line in the preliminary experimental results. The numbers under each band showed the relative amount of eIF-4E fragments normalized to that of GAPDH by densitometry. The results suggest that antisense oligonucleotides against eIF-4E inhibit eIF-4E mRNA expres-sion in LS-174T cells, and sense oligonucleotides have no in-hibitory effect on eIF-4E mRNA expression.
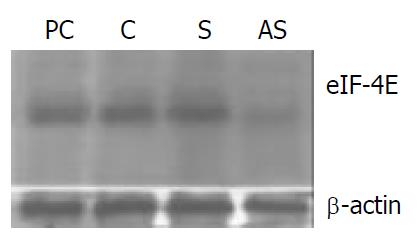
Figure 2 eIF-4E protein expression in oligonucleotide-treated colon adenocarcinoma cells.
Protein extracts were prepared from oligonucleotide-treated cells at 48 h after transfection. Cell lysates were size-fractionated by 12% SDS-PAGE, and immunodetection on the blotted membrane was performed using the eIF-4E MAb. AS, antisense oligonucle-otide-treated cells; S. sense oligonucleotide-treated cells; C, cells treated with liposome only; PC, eIF-4E standard anti-gen (derived from a human neuroblastoma cell line) as a positive control marker.
eIF-4E down-regulation is correlated with alterations in expression of heparanase protein
It has shown that eIF-4E may be involved in regulating a variety of tumor invasion-associated molecules[10]. We investigated the relationship between eIF-4E overexpression and the effects of this expression on heparanase levels in metastatic colon adenocarcinoma cell line. Substantial alterations in the levels of both 50 kDa and 65 kDa heparanase protein expression were observed in the transfectants. In LS-174 cell lines treated with asODN, eIF-4E down-regulation appeared to be associated with the decrease in heparanase protein expression. A 50 kDa protein, corresponding to the expected active mature heparanase according to published data[18], was detected at lower levels in LS-174T cells transfected with antisense oligonucleotides. And so did a 65 kDa protein, which corresponded to the expected size for unprocessed heparanase. In the meantime, heparanase protein levels did not change apparently in the cells treated with sense oligonucleotides or liposome only. Our data demonstrated that eIF-4E did affect the expression of heparanase protein of LS-174T cells under these experimental conditions (Figure 3).
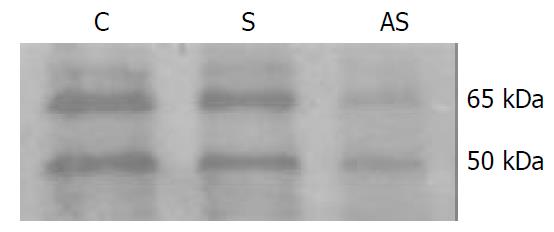
Figure 3 Western blot analysis of heparanase expression in LS-174T cells.
The colon adenocarcinoma cells were lysed, and Western blot analysis was performed with an anti-hu-man heparanase polyclonal antibody. AS, antisense oligo-nucleotide-treated cells; S. sense oligonucleotide-treated cells; C, cells treated with liposome only. Cells treated with anti-sense oligonucleotides showed markedly reduced levels of heparanase protein compared to cells treated with sense oli-gonucleotides or saline in the presence of liposome. The ap-proximately 65-kDa protein represents a heparanase precursor, whereas the 50-kDa protein is a processed form, and data showed that both of them were less expressed while eIF-4E was inhibited.
Alteration in heparanase activity
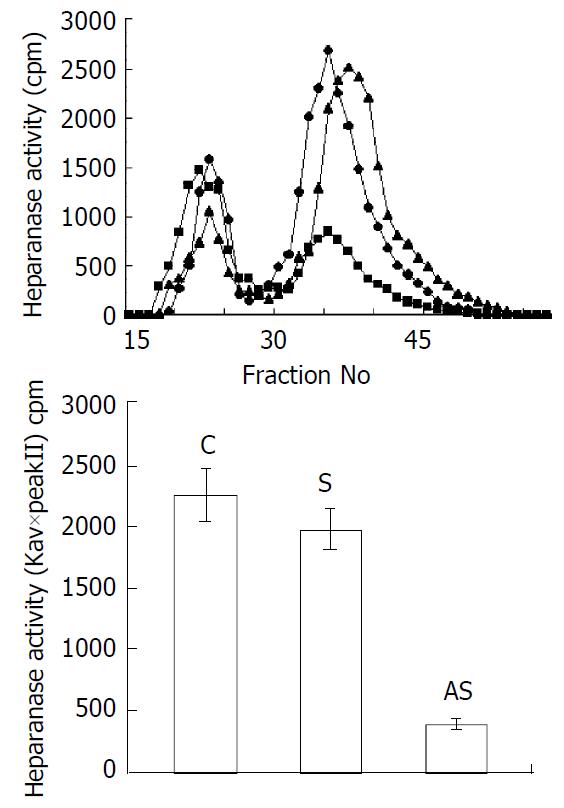
Heparanase activity was determined by degradation of purified ECM [35S] HS using gel filtration chromatography analysis. Degradation fragments eluted in peak II were shown to be degradation products of HS. In this experiment, incubation of an equal amount of protein with sulfate-labeled ECM resulted in release of high level HS degradation fragments (Peak II) in the samples derived from the cells treated with sense oligonucleotides or liposome alone, whereas degradation was less pronounced during incubation of ECM with extracts derived from the cells treated with antisense oligonucleotides (Figure 4). Heparanase activity was also expressed as the total amount of radioactivity eluted in peak II multiplied by the Kav of peak II, thereby representing both the total amount and the size of HS-degradation fragments. The result was consistent with heparanase expression at the protein level. Thus, the alteration of heparanase protein seen by immunostaining was also reflected by a decreased heparanase activity found in the extracts derived from the tumor cells treated with antisense oligonucleotides. These results suggested that the high levels of heparanase were parallel to over-expressed eIF-4E in LS-174T cells and the expression of heparanase might be regulated by eIF-4E in these tumor cells.
Figure 4 Effects of eIF-4E down-regulation upon enzyme activity of heparanase.
Cells were treated for 48 h with antisense oligonucleotide (■), sense oligonucleotide (▲) or liposome only(◆). The soluble fraction from transfected, freezed and thawed LS-174T cells was incubated with 35S-la-beled HS. The incubation medium was then subjected to gel filtration over Sepharose CL-6B. Low-molecular-weight HS degradation fragments (peak II) were mainly produced dur-ing incubation with medium conditioned by sense oligonucle-otide infected cells. The cells treated with antisense oligonucle-otide exhibited low levels of heparanase activity as compared to untreated ones. Heparanase activity was also expressed as Kav×total cpm eluted in peak II.
Inhibition of heparanase suppresses in vitro invasiveness of LS-174T cells
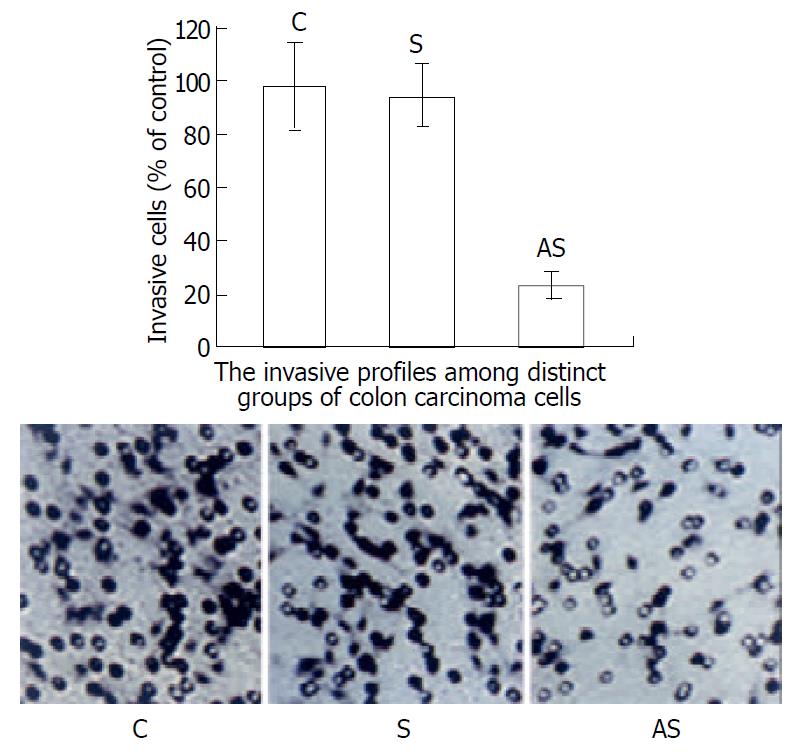
We first examined whether the variation of eIF-4E expression was associated with heparanase activity in LS-174T cells and its consequent influence on the invasive potential of these tumor cells (Figure 5). The effect of this antisense sequence on invasive capacity was studied in vitro by measuring the ability of tumor cells to cross a barrier composed of Matrigel. In these conditions, the invasiveness of LS-174T cells was reduced to 23% by this antisense sequence. In a control experiment, cells treated at the same concentration of sense oligonucleotides did not show any significantly inhibitory effect on tumor cells invasion as compared to the untreated ones. These results argued that eIF-4E was involved in the regulation of heparanase expression and might further influence the invasive potential of tumor cells.
Figure 5 Invasive profiles of LS-174T cells measured in vitro using Matrigel as ECM barrier in modified Boyden chambers.
The invasiveness of the cells on Matrigel was normalized to 100% (control, untreated cells). Compared with the control, antisense oligonucleotide reduced invasion to 23%. Sense sequence didn’t have a significant influence on invasive potential of LS-174T cells. These results suggest that alteration of heparanase activity via inhibition of the expression of eIF-4E results in decreased inva-siveness in LS-174T cells. The small rings are membrane pores.
DISCUSSION
Inhibition of tumor invasion is an attractive approach for the treatment of highly malignant tumors. Tumor cell invasion is characterized by secretion of enzymes that facilitate tumor cell spread by degrading ECM surrounding the tumors and solubilizing the vascular basement[19]. Thus, heparanase, which degrades HSPG in ECM, is an attractive target for the development of new antimetastatic drugs because of evidences implicating the enzyme in tumor cell invasion. Heparanase facilitates tumor cell invasion and metastasis by at least two different mechanisms. First, it breaks down physical barriers to invasion through degradation of HS chains of HSPGs which are the chief components in BM and ECM, not cleaved by MMPs. Second, it may regulate angiogenesis, tissue repair, inflammation and lipid metabolism by releasing HS-bound growth factor (bFGF) and lipoprotein lipase, which may be a pertinent molecular mechanism for tumor metastasis. Prior reports have mentioned that heparanase is involved in the metastatic potential of various tumor cells, however heparanase as a key regulator of tumor metastasis has not been established with regard to the lack of purified enzymes and cDNA of the gene[20-24]. Two studies concerned with cloning and sequencing of mammalian heparanase and new findings not only confirmed previous studies but revealed that heparanase played an important role in invasion and metastasis of tumor cells[25,26].
As judged by semi-quantitative RT-PCR, heparanase mRNA was increased in human malignancies and xenografts of human breast, colon, lung, prostate, ovary, and pancreas tumors, compared with the corresponding normal tissues[27]. Further support for the role of heparanase in tumour metastasis came from transfection studies where the metastatic ability of lymphoma and melanoma cell lines was substantially enhanced when they were stably transfected with the heparanase gene[25,28]. Additional evidences for heparanase involvement in tumour metastasis came from the in vivo administration of heparanase inhibitors, with a dramatic reduction (90%) in the incidence of tumour metastases reported in a number of tumour models when animals were treated with heparanase inhibitors[29,30]. As to human colon carcinoma[31], heparanase mRNA and protein accumulated even at early stages in the progression of neoplastic disorders, and their levels increased gradually as cells progressed from severe dysplasia through well-differentiated to poorly differentiated colon carcinoma. Adjacent, morphologically normal colonic tissue showed no expression of the enzyme. Deeply invading colon carcinoma cells and the adjacent desmoplastic stromal fibroblasts showed high levels of heparanase mRNA and protein. These results suggest that increased expression of heparanase as in tumor cells indicates less differentiation and more invasive ness. Considering the key role of heparanase in the metastasis of tumor cells, it seems very important to investigate the mechanism by which the expression of heparanase in tumor cells is regulated. Virtually nothing is known about the factors that upregulate heparanase expression and activity in malignant cells, and the molecular basis of this upregulation at the gene level has not been delineated yet. Our research dealed with the translational regulation of heparanase via modulation of the mRNA cap-binding phosphoprotein, eIF-4E and probed the subsequent alterations of invasive potential in LS-174T cells.
To date, Overexpression of eIF-4E has been found in human carcinoma tissues and tumor cell lines. A recent publication[32] also suggested that increased expression of eIF-4E might be an early event in the development human colon cancer. Investigators have found that eIF-4E is increased in both colon adenomas and carcinomas. A compelling body of evidences now indicates that eIF-4E is centrally involved not only in cellular transformation, but also in tumor formation, invasion and metastasis. The major intracellular signaling pathways involved in tumor growth and malignancy induce eIF-4E activity. MAP kinase activation stimulates MNK-mediated phosphorylation and activation of eIF-4E directly, while activation of the pI3 kinase/PTEN/AKT pathway ultimately yields phosphorylation and inactivation of 4E-BP1, and subsequent release of eIF-4E. Zimmer et al[10] indicated that increased eIF-4E activity might provide a central regulatory step facilitating tumor invasion and metastasis via upregulation of selected, potent gene products (such as bFGF, VEGF, MMP-9 and CD44v6). In this report, the colon adenocarcinoma cell line also showed expression of eIF-4E. By transfecting LS-174T cells with a 20-mer antisense s-oligodeoxynucleotide targeted against eIF-4E at well tolerated doses, eIF-4E expression was specifically and significantly inhibited at transcriptional and translational levels. Furthermore, as eIF-4E protein expression decreased, the expression and activity of heparanase were effectively retarded as well and the tumor cells also displayed a reduced invasive potential. It seems that heparanase down-regulation is closely correlated with alterations in the expression of eIF-4E. This study firstly demonstrated that eIF-4E might be involved in the regulatory processes of heparanase in colon adenocarcinoma cell line LS-174T. However, the mechanisms underlying these regulatory events remain unclear, since eIF-4E has the potential to alter gene expression at levels including translational initiation, mRNA splicing, mRNA 3’-end processing, mRNA nucleocytoplasmic transport, and protection against 5’-exonucleolytic degradation[33]. Possible roles of eIF-4E in regulatory processes of heparanase expression remain unidentified and the mechanism deserves further investigation.
Our results, together with the previous reports, indicate that constitutively high-level expression of eIF-4E in tumor cells is involved in the regulation of heparanase expression, although the exact mechanism is not quite certain. Since matrix metalloprotease 9 (MMP-9) and CD44 expression are directly related to metastatic capacity, they are certainly to be governed by enhanced eIF-4E activity. Therefore, eIF-4E antisense strategy should be an effective treatment against tumour invasion and metastasis. This targeting strategy in antisense chemistry may have practical applications in experimental or clinical anti-metastatic gene therapy of human colorectal carcinoma.