Published online Aug 15, 2003. doi: 10.3748/wjg.v9.i8.1667
Revised: April 19, 2003
Accepted: May 19, 2003
Published online: August 15, 2003
AIM: To characterize enhancement patterns of focal hepatic lesions using C-cube gray scale sonography with a microbubble contrast agent and to evaluate its usefulness in differential diagnosis of hepatic lesions.
METHODS: Fifty-four patients with 58 focal hepatic lesions were examined with Levovist-enhanced C-cube gray scale sonography. The final diagnosis of hepatic lesions was 29 primary liver cancers, 4 metastases, 8 hemangiomas, 12 focal nodular hyperplasias, 2 inflammatory pseudotumors of the liver and 3 angiomyolipomas. The initiation time of enhancement in various lesions and enhancement duration after administration of contrast agent were compared. Vascular findings in lesions were classified as peripheral enhancement, homogenous enhancement, mosaic enhancement and no enhancement depending on microbubble signals in the lesion relative to the liver parenchyma.
RESULTS: The initiation time of enhancement in hemangioma (48 ± 12 s) was significantly later compared to other lesions (P < 0.05). The enhancement duration of malignancies (69 ± 33 s in primary liver cancer, 61 ± 23 s in metastasis) was significantly shorter compared to benign lesions (P < 0.05). Intranodular enhancement appearing at arterial phase and decreasing at portal venous phase was considered characteristic for malignancy. Intranodular enhancement did not appear earlier than the liver parenchyma, and peripheral enhancement pattern was regarded as positive findings for hemangioma. Intranodular enhancement appeared in the arterial phase, and homogenous enhancement pattern sustained in the whole portal venous phase were regarded as positive findings for focal nodular hyperplasia. No microbubble signals appeared in two inflammatory pseudotumors of the liver.
CONCLUSION: C-cube gray scale sonography can demonstrate dynamic intranodular enhancement in various focal hepatic lesions. The information provided by this methodology may be useful in the differential diagnosis of hepatic lesions.
- Citation: Wang WP, Ding H, Qi Q, Mao F, Xu ZZ, Kudo M. Characterization of focal hepatic lesions with contrast-enhanced C-cube gray scale ultrasonography. World J Gastroenterol 2003; 9(8): 1667-1674
- URL: https://www.wjgnet.com/1007-9327/full/v9/i8/1667.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i8.1667
Color Doppler ultrasonography (US) is the most widely used imaging modality in screening detection of hepatic tumors and differential diagnosis of malignancies based on tumor vascularity. Unfortunately, conventional Doppler US does not provide satisfactory results in the evaluation of tumor vascularity because of limitations such as a lack of sensitivity to slow flow and deeply located flow, inevitable motion artifacts from either respiratory or cardiac activity, and poor showing of tumor stain.
Over the past decade, great efforts have been made to improve both ultrasound instruments and echo-enhancing agents to demonstrate tumor flow more sensitively with non-invasive modalities. Harmonic imaging is a newly developed technique used with microbubble contrast agents that interact with the imaging process. The microbubbles reflect ultrasonic echoes with a low acoustic power, additionally, they resonant and emit multiple frequencies when acoustic power is sufficiently elevated[1]. Second harmonic imaging, which transmits sonographic pulses at one frequency and then selectively receives echoes at twice that frequency, has been shown to be excellent in eliminating clutter noises and displaying the slower blood flow in smaller vessels when compared to conventional Doppler US[2-5].
However, the intensity of second harmonic frequency of some microbubble contrast agents (e.g., Levovist) is lower than that of fundamental ones[1,6]. In addition to second harmonics, the microbubbles emit multiple frequencies such as subharmonic, ultraharmonic, etc. Recently, a commercially available C-cube gray scale US (C3-ModeTM, Esaote Biomedica, Genoa, Italy) technique uses comparative digital decorrelation and a digital adaptive band pass filtering process, providing a combination of gray scale, Doppler and contrast signals. It utilizes the signal coming from the microbubbles with not only the second harmonics, but also the fundamental, sub- and ultra-harmonics from the contrast agent. Therefore, this new technique might be able to provide increased sensitivity in demonstrating slow blood flow in focal liver lesions with good spatial resolution in comparison to contrast-enhanced conventional US or second harmonic imaging.
The purpose of this study was to characterize enhancement patterns in focal hepatic lesions with contrast-enhanced C-cube gray scale US.
Between October 2001 and March 2002, 54 patients with clinically and histopathologically diagnosed hepatic tumors who were referred for hepatic color Doppler US were examined with C-cube gray scale US in combination with a microbubble contrast agent. All the patients gave fully informed consent for the study that had received approval from our institutional review board.
The patients consisted of 31 men and 23 women aged between 20-68 years (mean, 47 years). Contrast-enhanced C-cube gray scale US was performed 2-3 days before treatment. In patients with multiple lesions, the largest one was selected for contrast study except 4 patients with different types of hepatic lesions. Therefore, a total of 58 hepatic lesions were studied with contrast agents. They were 29 cases of primary liver cancer (PLC) (hepatocellular carcinoma, HCC, 27; cholangiocarcinoma, 2), 8 of hemangiomas, 12 of focal nodular hyperplasias (FNH), 2 of inflammatory pseudotumors of the liver (IPT), 3 of angiomyolipomas, and 4 of metastases (gastric cancer, 1; breast cancer, 2; nasopharyngeal carcinoma, 1). Histopathological diagnosis was obtained by surgical resection or US-guided percutaneous biopsy in all the 33 malignancies, 3 hemangiomas, 7 FNHs, 2 IPTs and 3 angiomyolipomas. Other 5 hemangiomas and 5 FNHs were diagnosed according to the typical imaging findings on contrast-enhanced computed tomography (CT), magnetic resonance imaging, as well as clinical follow-up which showed no change of lesion size for more than one year.
The maximal diameters of the 58 hepatic lesions measured on conventional US were as follows: PLC, 1.1-8.2 cm (mean, 3.3 cm); metastasis, 2.2-3.5 cm (mean, 2.8 cm); hemangioma, 1.0-8.0 cm (mean, 5.5 cm); FNH, 1.1-7.3 cm (mean, 4.5 cm); angiomyolipoma, 2.4-7.3 cm (mean, 6.3 cm) and IPT, 1.7-4.1 cm (mean, 2.9 cm).
The contrast agent was Levovist (Schering AG, Berlin, Germany), which is a suspension of monosaccharide microparticles (galactose) in sterile water. Microbubbles were stabilized in the microparticle suspension with an average diameter of 1.3 μm, which could traverse the pulmonary capillary bed and enhance the signal of blood. Before US examination, the agent was prepared with 5 mL of water by shaking vigorously for 5-10 s. After standing for 2 min for equilibration, a total of 2.5 g Levovist (6 mL 400 mg/mL concentration) was injected manually through a 20-gauge canula placed in an antecubital vein at a speed of 1 mL/s and flushed by an additional 5 mL of normal saline.
Contrast-enhanced C-cube gray scale US was performed with a commercially available US system, Technos DU6 US system (Esaote Biomedica, Genoa, Italy), equipped with C3-ModeTM technology. A convex-arrayed wide band transducer CA 421 was used at a frequency of 2-5 MHz. There were two states in contrast examination: LOW state and C3-Mode state. At the LOW state, the system transmitted pulses at a low acoustic power with a mechanical index of 0.2-0.4 that theoretically would not destroy microbubbles. At C3-Mode state, the system transmitted pulses at a high acoustic power with a mechanical index of 1.0-1.4 to destroy microbubbles in the scanning plane. We could transfer from the LOW state to the C3-Mode state by simply pressing a keyboard button to obtain signals of microbubble collapse, and the system automatically returned to the LOW state then.
Conventional US was performed on all lesions before contrast-enhanced study started. An ideal plane was selected for clearly showing the lesion and the surrounding liver parenchyma. Following administration of Levovist, we monitored the same scanning plane using LOW state and obtained a series of C3-Mode images by pressing the key button manually for 7-8 minutes. During 15-120 s after injection of Levovist, we obtained C3-Mode images of high mechanical index every 5-10 s. After that (121-480 s after injection of Levovist), we obtained C3-Mode images every 20-30 s. During the entire scanning procedure, we held the transducer and unfroze it during the same stage of the patient’s respiration to maintain the same scanning plane. The time delay between the initiation of contrast injection and the time at which the C3-Mode image obtained was automatically recorded on the US system.
All US images were recorded on digital videotapes and still images were stored digitally on a hard disk in the US system. Videotapes and still images were reviewed by two authors who were unaware of the findings on other imaging modalities and the final diagnosis of the lesions.
The time of occurrence of microbubble signals in the lesion and in surrounding liver parenchyma after administration of Levovist (injection-enhancement delay time), and subsequent time of microbubble signals decreased in the lesion on C3-Mode images (injection-decrease delay time) were carefully recorded. Enhancement decrease in a lesion was defined as the echogenicity lower in the lesion than that in the same-depth of surrounding liver parenchyma. In this way, we calculated enhancement duration of various lesions.
The entire procedure of contrast-enhanced C-cube gray scale US was classified into three phases. As vascular transit time of blood through the liver was associated with liver diseases, such as hepatic cirrhosis[7-9], we modified the scheme that Kim et al[10] used on dynamic CT. Arterial phase prior to the enhancement appeared in the liver parenchyma, approximately 15-50 s after administration of Levovist, and portal venous phase appeared 51-120 s after administration of Levovist, and delayed phase appeared 121 s after administration of Levovist.
Based on the enhancement of microbubble signals in the lesion relative to the surrounding liver parenchyma, vascular findings in lesions on C3-Mode images in the arterial phase were classified as peripheral enhancement (continuous or discontinuous ring enhancement occurred in the periphery of the lesion), homogenous enhancement (enhancement occurred in the whole lesion), mosaic enhancement (enhancement occurred in some area of the lesion), and no enhancement (no microbubble signal occurred in the lesion while enhancement in the liver parenchyma occurred). All the enhancement patterns including peripheral, homogenous and mosaic enhancements were defined as positive enhancement, namely positive detection of intranodular vascularity.
All the data were normal distribution and homogeneity of variance after tested. The data were analyzed using SAS (SAS 6.04 for windows). Continuous variables were compared by means of independent t-test. Categorical data were analyzed with chi square test. A P value < 0.05 was considered to be significant.
The detection rate of intranodular vascularity was 96.6% (56/58) on C-cube gray scale US with administration of Levovist. All PL Cs, metasta ses, h eman g io mas, FNH s, an d angiomyolipomas presented positive enhancement. No microbubble signals appeared in the lesions of IPT, resulting in a vascular defect when liver parenchymal perfusion was observed on C-cube gray scale US.
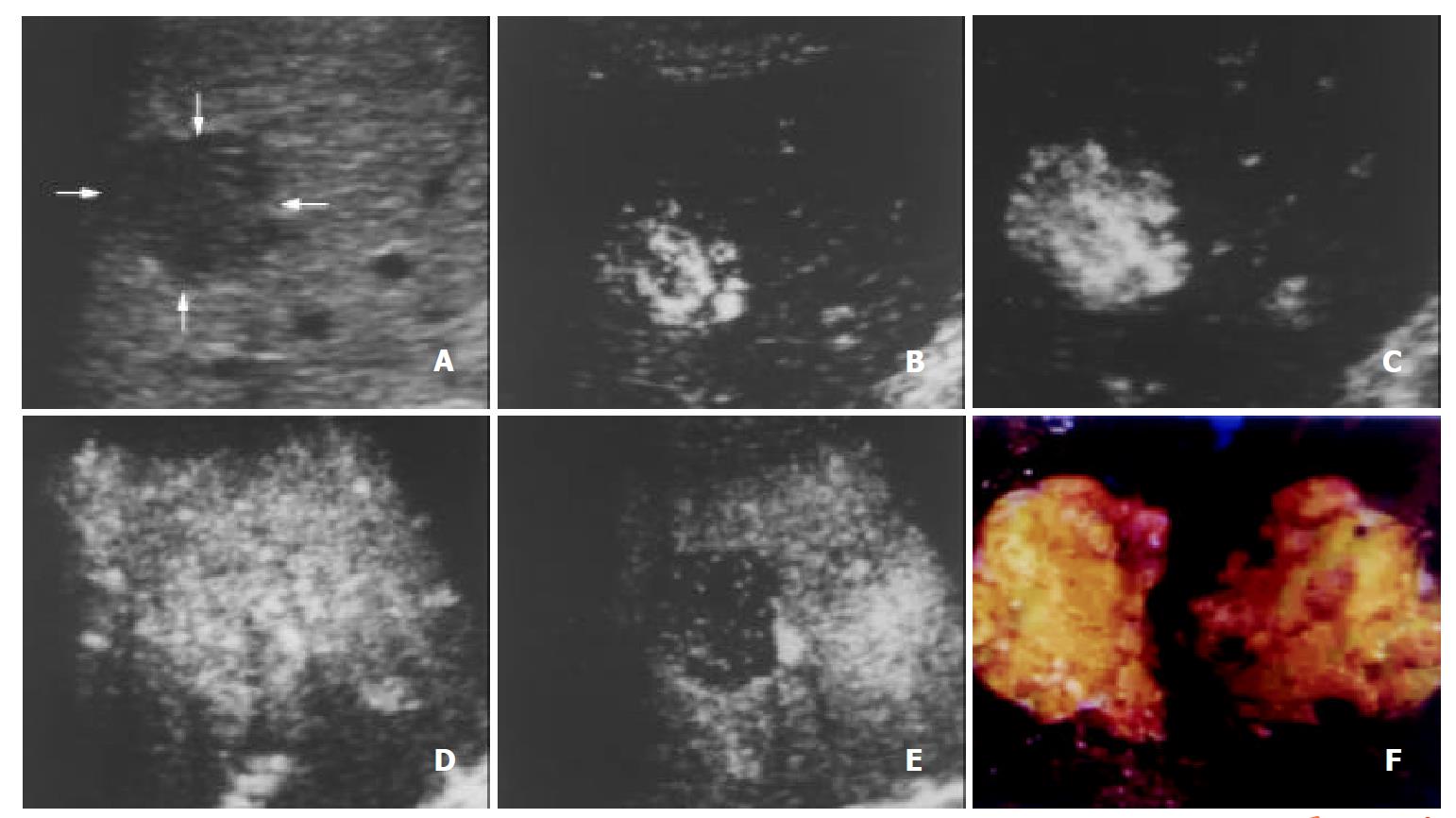
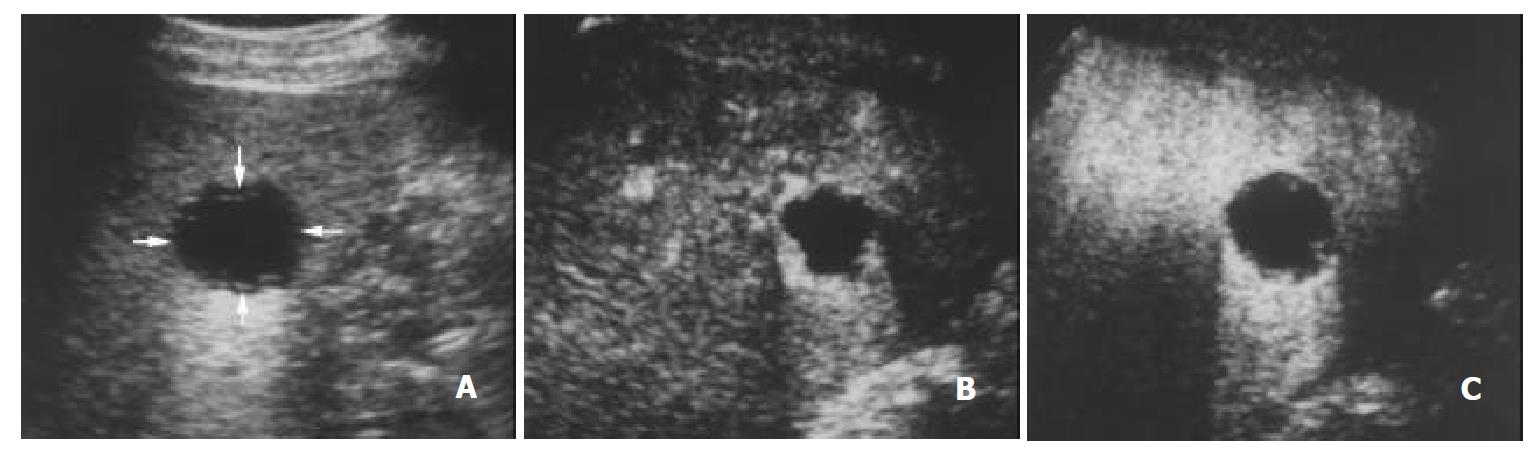
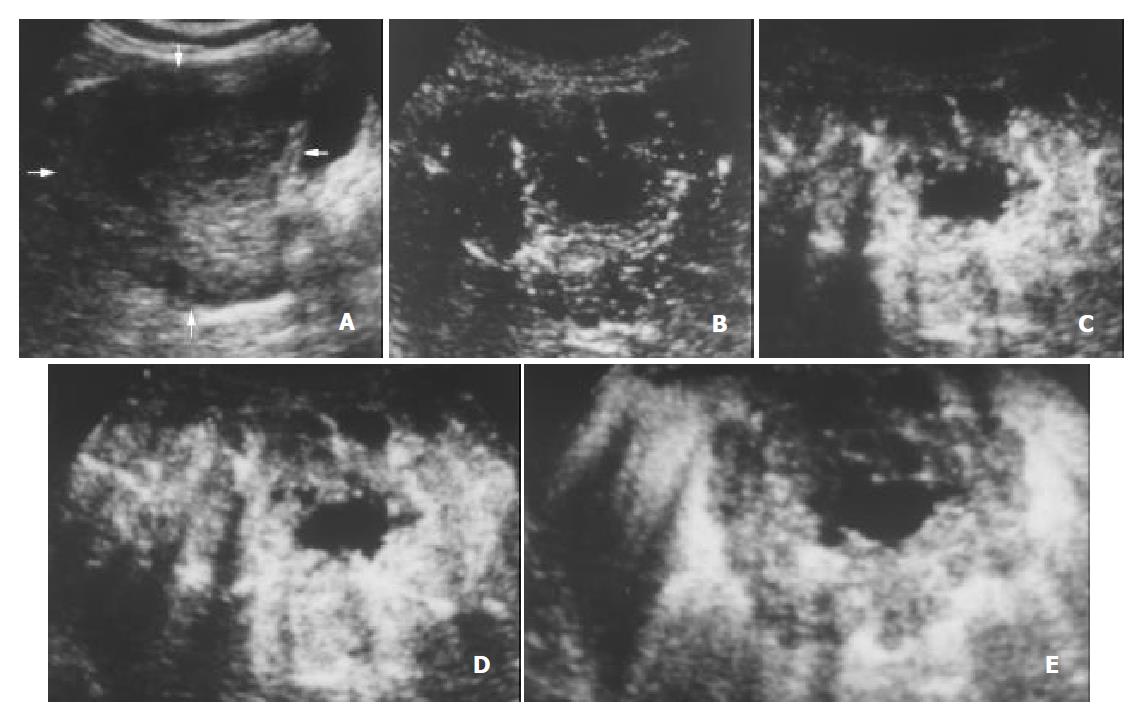
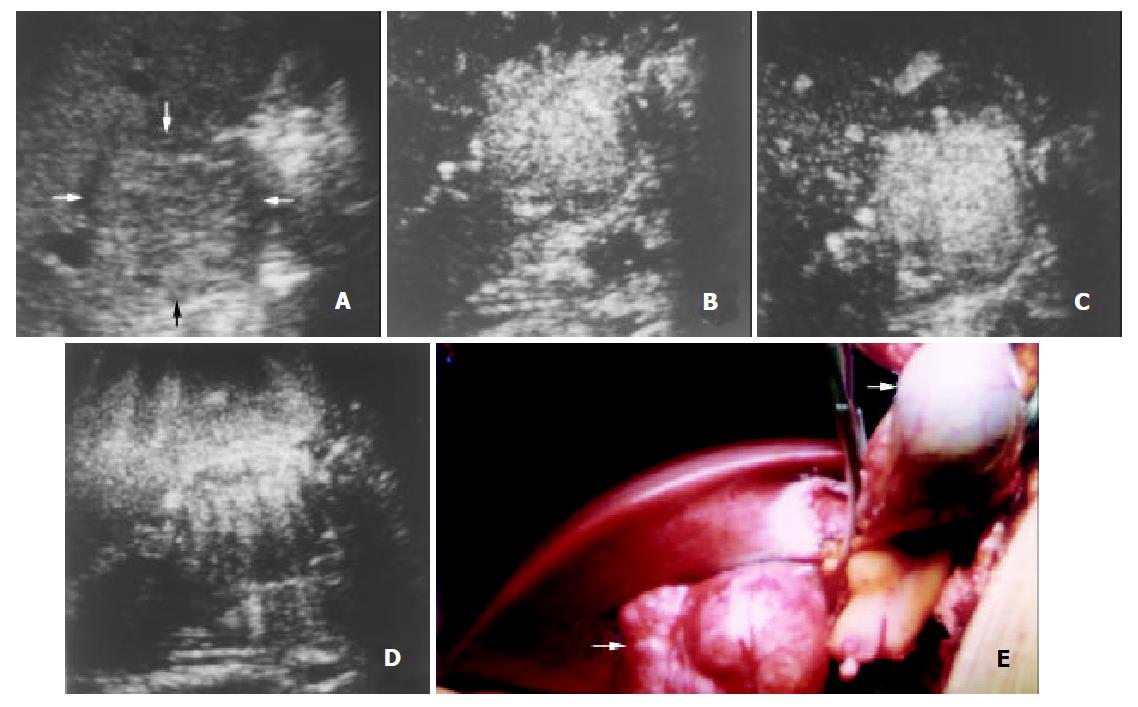
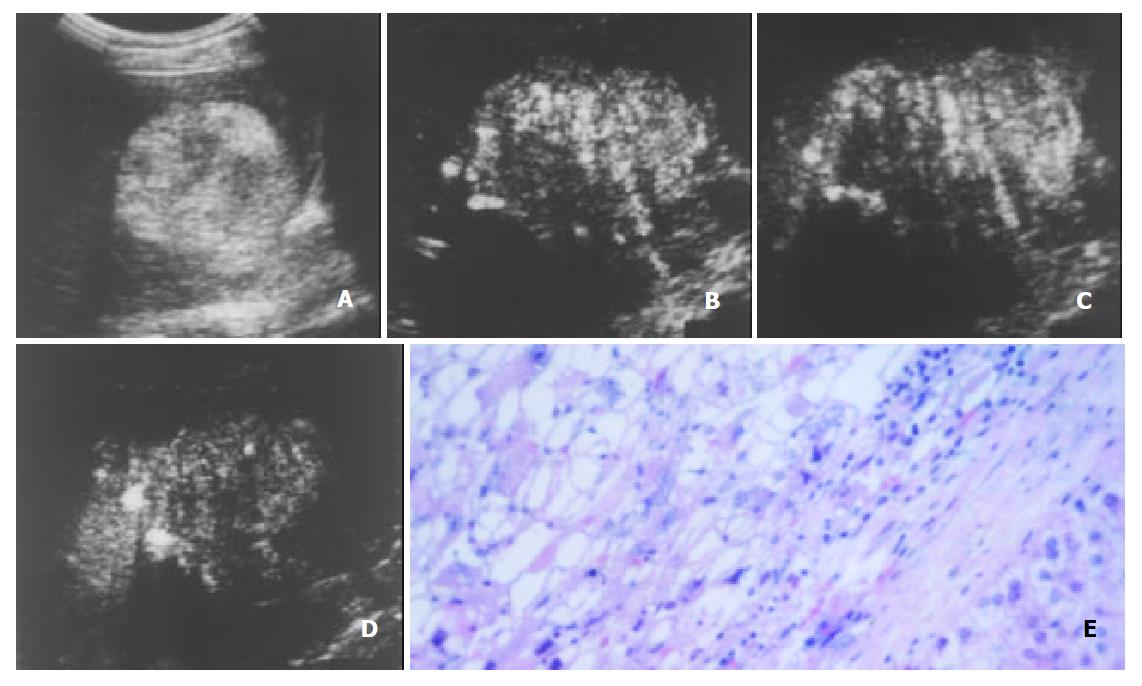
The initiation time of microbubble enhancement in various lesions and in the surrounding liver parenchyma after administration of Levovist, as well as enhancement duration of various lesions are listed in Table 1. Except hemangioma, all other lesions enhanced earlier than the liver parenchyma. The initiation time of enhancement in hemangioma (48 ± 12 s after administration of Levovist) was significantly later when compared to other lesions (P < 0.05). The enhancement in HCC (Figure 1), cholangiocarcinoma (Figure 2), and metastasis (Figure 3) decreased more rapidly when compared to benign liver lesions. The enhancement duration of malignancies (69 ± 33 s in PLC, 59 ± 22 s in metastasis, respectively) was significantly shorter when compared to benign liver lesions (P < 0.05).
| Types of lesion | No. of lesions | Injection-enhancement delaytime (mean ± SD) (s) | Enhancement duration (mean ± SD) (s) | |||
| Liver | Lesion | P Value | Lesion | P Value | ||
| Primary liver cancer | 29 | 33 ± 10 | 23 ± 6 | < 0.05 | 69 ± 33b | — |
| Metastasis | 4 | 26 ± 7 | 26 ± 11 | < 0.05 | 59 ± 22c | — |
| Hemangioma | 8 | 36 ± 8 | 48 ± 12a | — | 221 ± 47 | < 0.05 |
| Focal nodular hyperplasia | 12 | 29 ± 9 | 20 ± 6 | < 0.05 | 196 ± 96 | < 0.05 |
| Angiomyolipoma | 3 | 37 ± 12 | 26 ± 6 | < 0.05 | 177 ± 90 | < 0.05 |
If the intranodular enhancement appeared at arterial phase and enhancement decreased at portal venous phase in a lesion was considered characteristic for malignancy, the sensitivity, specificity and positive predictive values for contrast-enhanced C-cube gray scale US to differentiate malignancies from benign liver lesions were 90.9% (30/33), 92.0% (23/25), and 93.8% (30/32), respectively.
Vascular findings in all lesions at the arterial phase on contrast-enhanced C-cube gray scale US are shown in Table 2. Peripheral enhancement was detected in 50.0% (2/4) of metastases and 87.5% (7/8) of hemangiomas, homogenous enhancement in 55.2% (16/29) of PLCs, 100% (12/12) of FNHs, and mosaic enhancement in 41.4% (12/29) of PLCs, and 66.7% (2/3) of angiomyolipomas.
| Enhancement patterns | Peripheral | Homogenous | Mosaic | Negative | Total |
| Primary liver cancer | 1 | 16 | 12 | 0 | 29 |
| Metastasis | 2 | 0 | 2 | 0 | 4 |
| Hemangioma | 7 | 0 | 1 | 0 | 8 |
| Focal nodular hyperplasia | 0 | 12 | 0 | 0 | 12 |
| Angiomyolipoma | 0 | 1 | 2 | 0 | 3 |
| Inflammatory pseudotumor | 0 | 0 | 0 | 2 | 2 |
| Total | 10 | 29 | 17 | 2 | 58 |
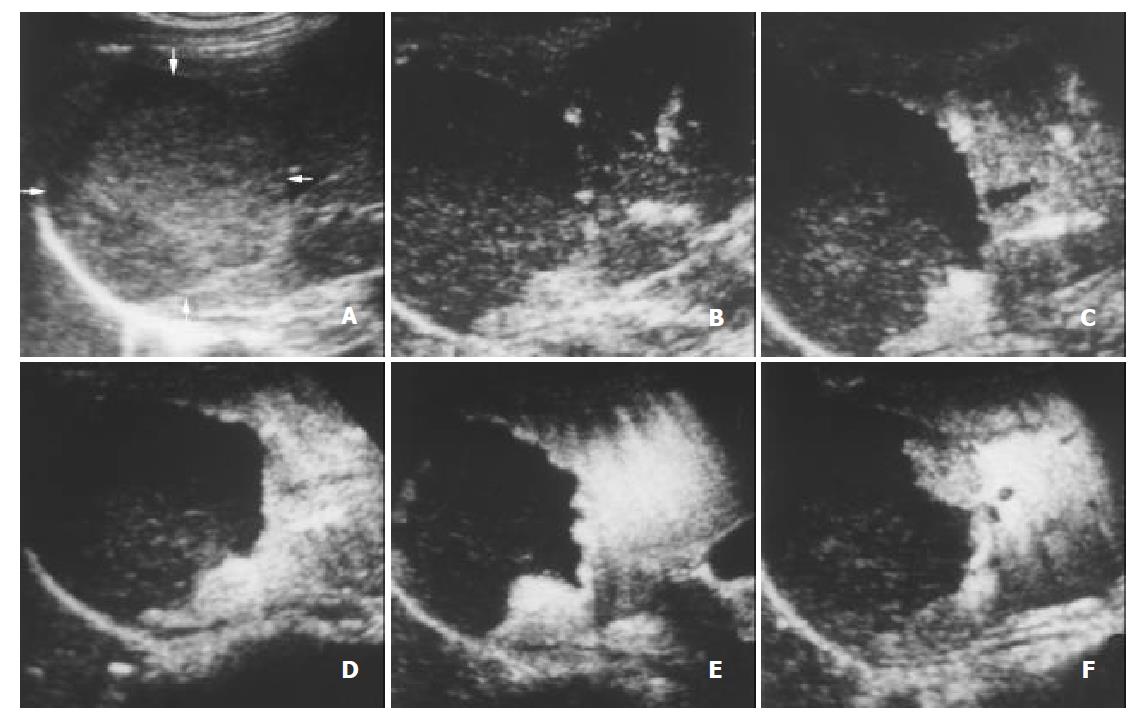
With respect to the enhancement patterns on contrast-enhanced C-cube gray scale US, no specific enhancement pattern was found among malignancies. For benign liver lesions, however, the enhancement patterns were specific. After administration of Levovist, hemangioma enhanced later than the liver parenchyma, and peripheral enhancement was obtained in most hemangiomas (Figure 4). All FNHs enhanced earlier than the liver parenchyma and presented homogenous enhancement (Figure 5). Most angiomyolipomas enhanced early and showed mosaic enhancement pattern (Figure 6).
If the intranodular enhancement did not appear earlier than the surrounding liver parenchyma after administration of Levovist, and the peripheral enhancement patterns were regarded as positive findings for hemangioma, the sensitivity, specificity and positive predictive values were 87.5% (7/8), 94.0% (47/50) and 70.0% (7/10), respectively.
If the intranodular enhancement appeared in the arterial phase, and the homogenous enhancement pattern sustained in the whole portal venous phase were regarded as positive findings for FNH, the sensitivity, specificity and positive predictive values were 100% (12/12), 95.7% (44/46) and 85.7% (12/14), respectively.
The characterization of focal liver lesions depends closely on their specific intranodular hemodynamics[11-14]. Various imaging modalities have been studied for demonstrating the typical vasculature of focal liver lesions. Contrast-enhanced US may be especially promising for depicting intranodular vascularity not only because of its easy performance, but also because of its particular advantages of providing dynamic flow information on a tomographic plane basis[4,15,16].
Levovist is the most widely used microbubble contrast agent for intravenous administration in the clinical setting. The backscatter signals caused by vibration or disruption of the microbubbles are received and used for image formation. Interval delay imaging or intermittent harmonic imaging[4,16-19], which transmits an ultrasound beam with a flexible interval, destroys most of the microbubbles in a region of interest with high acoustic power, and permits refreshment of microbubbles in the scanning plane during the low acoustic power period due to fresh blood inflow. C-cube gray scale US is a newly available technique using interval delay scanning to destroy microbubbles in the scanning plane. With the help of the low acoustic power image on LOW state, we could obtain intranodular capillary flow signals on the same sonographic scanning plane. Moreover, a series of C3-Mode images with microbubbles collapse provide dynamic information of enhancement patterns of focal liver lesions. Therefore, we could continuously observe the lesion of interest from the arterial phase to the delayed phase. The detailed enhancement process of each lesion could be obtained just as a single-level dynamic CT study[20]. In the present study, positive enhancement was detected in all focal liver lesions except for two IPT with complete necrosis. No false negative nodule was found on contrast-enhanced C-cube gray scale US.
The blood of normal liver is supplied both by the portal vein and by the hepatic artery, whereas the blood of most neoplastic tumors of the liver are supplied by the hepatic artery. The typical vascular patterns of PLC are high velocity signals on color Doppler US[21,22], and high attenuation at the early phase and low attenuation at the portal venous phase relative to the liver parenchyma on dynamic CT[23]. In this study, the injection-enhancement delay time of PLC was shorter than that of the liver parenchyma after administration of Levovist, presenting an early enhancement at the arterial phase. As the portal venous phase arrived, the concentration of microbubbles in the hepatic artery decreased. We recorded the injection-decrease delay time of various focal liver lesions and found that the injection-decrease delay time of PLC was relatively shorter compared to benign liver lesions. The difference of enhancement duration between PLC and benign liver lesions was statistically significant (P < 0.05). The hemodynamics of PLC was that, namely, it enhanced early in the arterial phase and washed out rapidly in the portal venous phase. These findings were highly corresponding to the appearance of PLC on contrast-enhanced dynamic CT[23-25].
As to intranodular enhancement patterns of PLC in the arterial phase, homogenous or mosaic enhancements were found in most lesions of PLC (96.7%, 28/29), which were also found in other focal liver lesions.
Similarly, 4 liver matestases enhanced early in the arterial phase and the enhancement decreased rapidly in the portal venous phase, resulting in a shorter enhancement duration compared to benign liver lesions (P < 0.05). This was probably due to their hemodynamics of mainly arterial blood supply. The enhancement patterns of metastases were peripheral or mosaic enhancements, which were also nonspecific and found in other liver lesions. Further study may be needed to clarify this results.
Hemangioma is usually rich in vessels with a sluggish blood circulation. After administration of a contrast agent, the enhancement of most hemangiomas on helical CT was a peripheral puddle pattern in the arterial phase and cotton wool sign in post vascular phase[25,26]. On contrast-enhanced C-cube gray scale US, similarly, the injection-enhancement delay time of hemangioma was significantly longer compared to other types of focal liver lesions (Table 1). As the arterial phase ended, the enhancement in hemangioma sustained and lasted over a long period. With respect to the enhancement patterns, peripheral enhancement was shown in most hemangiomas (87.5%, 7/8). Since microbubbles were destroyed when they were imaged by ultrasound beam, intratumoral enhancement could not be obtained with C-cube gray scale US using 5-10 s interval transmission in relatively large hemangioma. Based upon our preliminary experience, we supposed that at least one minute of interval transmission would be needed to image the microbubbles in the center of hemangioma. The specific characteristics of hemangioma after administration of Levovist was that, in short, it enhanced slowly and mainly at the periphery of the lesion with a long period of enhancement.
Except hemangioma, other benign liver lesions including 12 FNHs and 3 angiomyolipomas demonstrated positive enhancement on contrast-enhanced C-cube gray scale US, presenting their hypervascular characteristics. These lesions enhanced early in the arterial phase and positive enhancement lasted over a long period until the delayed phase. Their enhancement duration was relatively longer compared to hepatic malignancies (P < 0.05).
FNH is not a neoplasm but a hyperplastic response of liver parenchyma to the presence of a preexisting vascular malformation. FNH is a hypervascular lesion bypassing the portal venous system and exhibiting a varying degree of arteriovenous shunting[27]. On contrast-enhanced C-cube gray scale US, all FNH lesions showed early enhancement and appeared homogenously. In the portal venous and delayed phase, the intranodular enhancement was somewhat greater than that in the same-depth of surrounding liver parenchyma (Figure 5). The enhancement pattern of FNH seemed to be exclusively caused by arterial vascularization in the lesion, an absent capillary bed, and a vascular volume in excess of that of the normal liver[27-29].
Hepatic angiomyolipoma is a rare benign mesenchymal tumor, composed of a varying heterogenous mixture of three tissue components: blood vessels, smooth muscle and adipose cells. Most angiomyolipoma markedly enhanced with curved vessels in the arterial phase, and remained enhancement in the portal venous phase on spiral CT[30]. Its appearance on contrast-enhanced C-cube gray scale US was corresponding to that on helical CT. It enhanced inhomogenously because of the presence of the multiple ingredients within the lesion.
Contrast-enhanced C-cube gray scale US images microbubbles intermittently with a high mechanical index. This technique allows the detection of blood in the capillary bed, where the flow velocity is too low to be detected with Doppler US flow techniques. On the other hand, however, tumor vessel cannot be shown real-timely. It is because the microbubbles in small vessels (i.e. tumor vessels) cannot be detected with a low mechanical index on the LOW state, and real time C3-Mode state with a high mechanical index breaks microbubbles when they are imaged. This is one limitation of C-cube gray scale US.
In conclusion, C-cube gray scale US with administration of Levovist can demonstrate dynamic intranodular enhancement in various focal hepatic lesions. The information provided by this methodology may be useful in the differential diagnosis of hepatic lesions on the basis of demonstrating characteristic appearances of various hepatic lesions. Additional study with a greater number of cases is in progress to gather additional data to support and replenish these results.
Edited by Zhang JZ
| 1. | Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Eur J Radiol. 1998;27 Suppl 2:S157-S160. [PubMed] |
| 2. | Kono Y, Moriyasu F, Mine Y, Nada T, Kamiyama N, Suginoshita Y, Matsumura T, Kobayashi K, Chiba T. Gray-scale second harmonic imaging of the liver with galactose-based microbubbles. Invest Radiol. 1997;32:120-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Choi BI, Kim TK, Han JK, Kim AY, Seong CK, Park SJ. Vascularity of hepatocellular carcinoma: assessment with contrast-enhanced second-harmonic versus conventional power Doppler US. Radiology. 2000;214:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Wilson SR, Burns PN, Muradali D, Wilson JA, Lai X. Harmonic hepatic US with microbubble contrast agent: initial experience showing improved characterization of hemangioma, hepatocellular carcinoma, and metastasis. Radiology. 2000;215:153-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 213] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Maresca G, Summaria V, Colagrande C, Manfredi R, Calliada F. New prospects for ultrasound contrast agents. Eur J Radiol. 1998;27 Suppl 2:S171-S178. [PubMed] |
| 6. | Hosoki T, Mitomo M, Chor S, Miyahara N, Ohtani M, Morimoto K. Visualization of tumor vessels in hepatocellular carcinoma. Power Doppler compared with color Doppler and angiography. Acta Radiol. 1997;38:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Blomley MJ, Albrecht T, Cosgrove DO, Jayaram V, Eckersley RJ, Patel N, Taylor-Robinson S, Bauer A, Schlief R. Liver vascular transit time analyzed with dynamic hepatic venography with bolus injections of an US contrast agent: early experience in seven patients with metastases. Radiology. 1998;209:862-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Albrecht T, Blomley MJ, Cosgrove DO, Taylor-Robinson SD, Jayaram V, Eckersley R, Urbank A, Butler-Barnes J, Patel N. Non-invasive diagnosis of hepatic cirrhosis by transit-time analysis of an ultrasound contrast agent. Lancet. 1999;353:1579-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 163] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Bang N, Nielsen MB, Rasmussen AN, Osterhammel PA, Pedersen JF. Hepatic vein transit time of an ultrasound contrast agent: simplified procedure using pulse inversion imaging. Br J Radiol. 2001;74:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kim T, Murakami T, Takahashi S, Tsuda K, Tomoda K, Narumi Y, Oi H, Sakon M, Nakamura H. Optimal phases of dynamic CT for detecting hepatocellular carcinoma: evaluation of unenhanced and triple-phase images. Abdom Imaging. 1999;24:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Kudo M. Morphological diagnosis of hepatocellular carcinoma: special emphasis on intranodular hemodynamic imaging. Hepatogastroenterology. 1998;45 Suppl 3:1226-1231. [PubMed] |
| 12. | Koito K, Namieno T, Morita K. Differential diagnosis of small hepatocellular carcinoma and adenomatous hyperplasia with power Doppler sonography. AJR Am J Roentgenol. 1998;170:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Brancatelli G, Federle MP, Grazioli L, Blachar A, Peterson MS, Thaete L. Focal nodular hyperplasia: CT findings with emphasis on multiphasic helical CT in 78 patients. Radiology. 2001;219:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Kudo M. Imaging diagnosis of hepatocellular carcinoma and premalignant/borderline lesions. Semin Liver Dis. 1999;19:297-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 68] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kim TK, Choi BI, Han JK, Hong HS, Park SH, Moon SG. Hepatic tumors: contrast agent-enhancement patterns with pulse-inversion harmonic US. Radiology. 2000;216:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 169] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 16. | Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Hepatocellular carcinoma: depiction of tumor parenchymal flow with intermittent harmonic power Doppler US during the early arterial phase in dual-display mode. Radiology. 2001;220:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Heckemann RA, Cosgrove DO, Blomley MJ, Eckersley RJ, Harvey CJ, Mine Y. Liver lesions: intermittent second-harmonic gray-scale US can increase conspicuity with microbubble contrast material-early experience. Radiology. 2000;216:592-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Ding H, Kudo M, Onda H, Suetomi Y, Minami Y, Maekawa K. Contrast-enhanced subtraction harmonic sonography for evaluating treatment response in patients with hepatocellular carcinoma. AJR Am J Roentgenol. 2001;176:661-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Hancock J, Dittrich H, Jewitt DE, Monaghan MJ. Evaluation of myocardial, hepatic, and renal perfusion in a variety of clinical conditions using an intravenous ultrasound contrast agent (Optison) and second harmonic imaging. Heart. 1999;81:636-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Ueda K, Matsui O, Kawamori Y, Nakanuma Y, Kadoya M, Yoshikawa J, Gabata T, Nonomura A, Takashima T. Hypervascular hepatocellular carcinoma: evaluation of hemodynamics with dynamic CT during hepatic arteriography. Radiology. 1998;206:161-166. [PubMed] |
| 21. | Gaiani S, Casali A, Serra C, Piscaglia F, Gramantieri L, Volpe L, Siringo S, Bolondi L. Assessment of vascular patterns of small liver mass lesions: value and limitation of the different Doppler ultrasound modalities. Am J Gastroenterol. 2000;95:3537-3546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Taylor KJ, Ramos I, Carter D, Morse SS, Snower D, Fortune K. Correlation of Doppler US tumor signals with neovascular morphologic features. Radiology. 1988;166:57-62. [PubMed] |
| 23. | Lee HM, Lu DS, Krasny RM, Busuttil R, Kadell B, Lucas J. Hepatic lesion characterization in cirrhosis: significance of arterial hypervascularity on dual-phase helical CT. AJR Am J Roentgenol. 1997;169:125-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Van Hoe L, Baert AL, Gryspeerdt S, Vandenbosh G, Nevens F, Van Steenbergen W, Marchal G. Dual-phase helical CT of the liver: value of an early-phase acquisition in the differential diagnosis of noncystic focal lesions. AJR Am J Roentgenol. 1997;168:1185-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Nino-Murcia M, Olcott EW, Jeffrey RB, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology. 2000;215:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 124] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Gryspeerdt S, Van Hoe L, Marchal G, Baert AL. Evaluation of hepatic perfusion disorders with double-phase spiral CT.. Radiographics. 1997;17:337-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Uggowitzer M, Kugler C, Gröll R, Mischinger HJ, Stacher R, Fickert P, Weiglein A. Sonographic evaluation of focal nodular hyperplasias (FNH) of the liver with a transpulmonary galactose-based contrast agent (Levovist). Br J Radiol. 1998;71:1026-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Ruppert-Kohlmayr AJ, Uggowitzer MM, Kugler C, Zebedin D, Schaffler G, Ruppert GS. Focal nodular hyperplasia and hepatocellular adenoma of the liver: differentiation with multiphasic helical CT. AJR Am J Roentgenol. 2001;176:1493-1498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Miyayama S, Matsui O, Ueda K, Kifune K, Yamashiro M, Yamamoto T, Komatsu T, Kumano T. Hemodynamics of small hepatic focal nodular hyperplasia: evaluation with single-level dynamic CT during hepatic arteriography. AJR Am J Roentgenol. 2000;174:1567-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Yan F, Zeng M, Zhou K, Shi W, Zheng W, Da R, Fan J, Ji Y. Hepatic angiomyolipoma: various appearances on two-phase contrast scanning of spiral CT. Eur J Radiol. 2002;41:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 1.8] [Reference Citation Analysis (0)] |