Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1554
Revised: March 1, 2003
Accepted: March 4, 2003
Published online: July 15, 2003
AIM: Rodent testes are generally more susceptible to cadmium (Cd)-induced toxicity than liver. To clarify the molecular mechanism of Cd-induced toxicity in testes, we compared metallothionein (MT) gene expression, MT protein accumulation, and Cd retention at different time in freshly isolated testicular interstitial cells and liver of rats treated with Cd.
METHODS: Adult male Sprague-Dawley rats weighing 250-280 g received a s.c injection of 4.0 μmol Cd/kg and were euthanized by CO2 asphyxiation 1 h, 3 h, 6 h, or 24 h later. Tissue was sampled and testicular interstitial cells were isolated. There were three replicates per treatment and 3 animals per replicate for RNA analyses, others, three replicates per treatment and one animal per replicate. MT1 and MT2 mRNA levels were determined by semi-quantitative RT-PCR analysis followed by densitometry scanning, and MT was estimated by the enzyme-linked immunosorbent assay (ELISA) method. Cadmium content was determined by atomic absorption spectrophotometry. The same parametersd were also analyzed in the liver, since this tissue unquestionably accumulate MT.
RESULTS: The rat testis expressed MT1 and MT2, the major isoforms. We also found that untreated animals contained relatively high basal levels of both isoform mRNA, which were increased after Cd treatment in liver and peaked at 3 h, followed by a decline. In contrast, the mRNA levels in interstitial cells peaked at 6 h. Interestingly, the induction of MT1 mRNA was lower than MT2 mRNA in liver of rat treated with Cd, but it was opposite to interstitial cells. Cd exposure substantially increased hepatic MT (3.9-fold increase), but did not increase MT translation in interstitial cells.
CONCLUSION: Cd-induced expression of MT isoforms is not only tissue dependent but also time-dependent. The inability to induce the metal-detoxicating MT-protein in response to Cd, may account for a higher susceptibility of testes to Cd toxicity and carcinogenesis compared to liver.
-
Citation: Ren XY, Zhou Y, Zhang JP, Feng WH, Jiao BH. Expression of
metallothionein gene at different time in testicular interstitial cells and liver of rats treated with cadmium. World J Gastroenterol 2003; 9(7): 1554-1558 - URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1554
Metallothionein (MT) gene expression appears to be not only tissue specific but also cell specific[1,2]. In rodents, the testes and ventral prostate have been shown to have a higher sensitivity to cadmium (Cd)-induced carcinogenesis than many other tissues[3]. These tissues have also been shown to have either no induction or a reduced expression of MT gene when animals were exposed to Cd or some other MT inducer agents[4,5]. It has been reported that testicular Cd is bound to a protein different from MT. The testicular Cd-binding protein contains less cysteine and more glutamate than MT[6]. However, some other studies have shown that MT is constitutively expressed in the whole testes or specific testicular cells at levels higher than that in some other organs, e.g. the liver, and that in vivo or in vitro Cd exposure increases MT gene expression in the testes[7-9].
Therefore, the published data are inconclusive as to whether MT exists in the testes under physiological conditions and whether this protein plays an important role in the detoxification of testicular Cd. Some of the controversies may derive from analyzing the whole testicular tissue instead of individual cell types, since MT gene expression appears to be not only tissue specific but also cell specific[1,2]. On the other hand, MT might affect testicular Cd accumulation toxicokinetically by sequestering the metal in the liver, thus diverting it from target tissues, such as the testes. It has been well established that Cd significantly enhances hepatic MT gene expression and MT synthesis. Such enhancement of liver MT might lead to lower testicular Cd uptake and toxicity.
In rodents testicular Cd appears to be localized in the interstitial tissue and a high incidence of interstitial cell (Leydig cell) tumors can occur following Cd exposure. However, testicular lesions as a result of Cd exposure have not been reported in men[10]. A single s.c. dose ≥ 5 μmol Cd/kg could result in a high incidence of testicular interstitial cell tumors. An elevated incidence of testicular tumors in rats was also found after chronic oral exposure to Cd. Within the interstitial tissue, Leydig cells appeared to be highly sensitive to Cd cytotoxicity[11]. However, most of the studies on MT gene expression and synthesis have been focused on the whole testes, which might mask the results on MT activity in these specific cells, since they only contributed to 11% of parenchymal volume. Some authors investigated Cd-induction of MT expression in cultured Leydig cell lines established from Leydig cell tumors[12], but their findings gave little insight into MT synthesis in normal testicular interstitial cells or purified Leydig cells isolated from animals exposured to Cd.
Furthermore, MT gene expression is time-dependent. It was reported that both MT isoform mRNA levels in rat livers were substantially increased 4-6 h after Cd treatment, followed by a reduction and chromatography demonstrated a significant time-related increase in MT protein level. In vitro studies showed that MT induction was dose- and time-dependent in both Leydig and Sertoli cells[13]. Therefore some of the controversies may derive from the different study time.
In general, the studies on Cd-induced MT gene expression and MT synthesis in the testis, have been carried out under acute exposure to toxic Cd levels. It is also conceivable that the biochemical changes occurring in the testes under such conditions of Cd toxicity might mask the molecular processes of MT gene expression at a lower Cd exposure dose.
Although there is a wealth of published information regarding MT gene expression and MT synthesis following induction by Cd[14-16], little systematic information is available on MT gene expression at different time in testes, particularly in testicular interstitial cells. The aim of this study therefore was to clone MT gene and to investigate MT gene expression, MT accumulation, and Cd retention at different time in testicular interstitial cells isolated by collagenase dispersion and density gradient centrifugation from rats treated with a non-toxic Cd dose. The same parameters in the liver were also analyzed, since this tissue unquestionably accumulated MT.
Adult male Sprague-Dawley rats weighing 250-280 g were obtained from the Animal Center of Second Military Medical University. Rats received a single s.c. injection of 4 μmol Cd/kg and were euthanized by CO2 asphyxiation 1 h, 3 h, 6 h, or 24 h later for RNA, total Cd, and MT analyses in interstitial cells and liver. Untreated rats (0 h) were also treated as described above. There were three replicates per treatment and 3 animals per replicate for RNA analyses, others, three replicates per treatment and one animal per replicate. Testes and liver were weighed and processed as described below.
Immediately after the testes were removed, these cells were decapsulated, deveined and incubated in a solution of 2 mg/mL collagenase (334 μ/mg, Type IA, Worthington Biochemical Co.) with minimum essential medium (MEM) (6 mL/2 testes) at 37 °C in a shaking water bath at 90 oscillations/min for 15 min. DNase (Sigma Chemical Co.) was added as needed (5-10 drops of 0.1%) to reduce the clumping of cells to the DNA released from damaged cells. After collagenase dispersion, the digestion media were diluted with 12 mL prewarmed MEM and the tubules were allowed to settle. The resultant supernatant was centrifuged at 80 × g for 10 min at 4 °C. Crude interstitial cells from 2 testes were resuspended in ≤ 3 mL cold MEM and layered on the top of a linear (0%-50%) Percoll density gradient. The gradient was prepared in a LKB gradient mixer with 4 mL of a solution containing 50% Percoll, 0.15 M NaCl, and 0.07% bovine serum albumin (BSA) in the diluting chamber and 4 mL of an aqueous solution with 0.15 M NaCl, and 0.07% BSA in the mixing chamber. Crude interstitial cells were then separated from other cell types by centrifugation at 800 × g for 20 min in tubes (8 × g 1.5 cm, 3 tubes for one type of cells from one sample) at 4°C Purified interstitial cells formed a distinct band at 41-42 mm from the gradient bottom. Cell band was aspirated, diluted three times its volume with cold MEM to remove Percoll and centrifuged at 80 × g for 10 min. Testicular interstitial cells were then suspended in a minimal volume of MEM and processed for RNA, total Cd, or MT protein analysis after aliquots was taken for cell count. Morphological observations by light microscopy showed that the purity ranged from 80% to 85%.
Interstitial cells and liver tissue were wet-ashed in HNO3 (u.p.)-H2O2 and Cd was determined by atomic absorption spectrophotometry (detection limit: 0.001 ppm). Precautions were taken to avoid Cd contamination by acid-washing all glasswares and blanks were run along with the samples.
Total ribonucleic acids were extracted from freshly isolated interstitial cells and liver tissue using RNAzol (GibcoBRL). Total RNA was determined by UV absorbance at 260 nm and its purity was estimated by the absorbance ratio A260/A280 nm. In addition, RNA integrity was confirmed by ethidium bromide staining of ribosomal RNA following gel electrophoresis.
Oligonucleotide primers were synthesized using a DNA synthesizer (Worthington Biochemical Co.). The sequences of sense and antisense primers for rat MT-1 were 5'-ACTGCCTTCTTGTCGCTTA-3' and 5'-TGGAGGTGTA-CGGCAAGACT-3' respectively. They spanned a 310 bp fragment. The sequences of sense and antisense primers for rat MT-2 were 5'-CCAACTGCCGCCTCCATTCG-3' and 5'-GAAAAAAGTGTGGAGAACCG-3' respectively, spanning a 300bp fragment. The sequences of sense and antisense primers for β-actin were 5'-CCCATTGAACACGG-CATTG-3' and 5'-GGTACGACCAGAGGCATACA-3' respectively, spanning a 236bp fragment.
First-strand cDNA was synthesized with 1 μg total RNA, 50 pmol oligo (dT)18, 10U avian myloblastosis virus (AMV) reverse transciptase and 20U RNase inhibitor in a final 20 μL reaction mixture containing 1 × reverse transcriptase buffer, 8 mM MgCl2, 0.5 mM of each dNTP. The reaction mixture was incubated at 42 °C for 60 min. The cDNA products were stored at -20 °C until use.
PCR was carried out in a 25 μL reaction mixture containing 0.2 mM of each dNTP, 1 pmol of each sense and antisense primer and 0.625 unit of Taq DNA polymerase (TaKaRa), including 4 μL of cDNA products. After an initial denaturation at 95°Cfor 5 min, amplification was carried out for approximately 27 cycles comprising 1 min at 95 °C for denaturation, 1 min at 55 °C for annealing, 1 min at 72 °C for extension with a final entension step at 72 °C for 5 min. The PCR products were applied to electrophoresis using a 2.0% (w/v) agarose gel, which was stained with ethidium bromide and visualized under UV light. In order to confirm that there was no significant contamination in the total RNA preparation, we synthesized the first-strand DNA and performed control reactions in the absence of reverse transcriptase, and did not find any band on further PCR. In order to guarantee amplification in phase of exponential increase, we minimize the cycles of PCR in condition that the strap of gel electrophoresis could be detected. MT1 and MT2 mRNA levels were determined by RT-PCR analysis followed by densitometry scanning. All MT1 and MT2 RT-PCR products were normalized to the corresponding β-actin RT-PCR results that served as an internal control to ensure an approximate ratio of MTs mRNA.
PCR products were cloned using pUCm-T vector, The constructed plasmids were transfected into JM109, positive colonies were selected and the DNA sequence was analysed using a DNA sequencer (Worthington Biochemical Co.).
Testicular interstitial cells were suspended in 500 μL of 10 mM Tris-HCL, pH7.4, and lysed by sonication (3 × 10 s) on ice. Cytosol was then obtained by centrifugation at 18000 × g for 20 min at 4 °C. Liver tissue was homogenated in 10 mM Tris-HCL, pH7.4, with a glass homogenizer and a Teflon pestle. MT content analysis was performed by the enzyme-linked immunosorbent assay (ELISA) method. The test procedure was similar to that described by Ruitenberg et al[17] and affinity-purified sheep anti-(rat MT) IgG was provided by Environmental Health Sciences Division, National Institute for Environmental Studies, Japan.
Data were represented as means ± S.E. of three replicates per Cd treatment or untreatment. Differences between Cd-treated (1 h, 3 h, 6 h, 24 h) and untreated rats were evaluated by Student's t-test with P < 0.05 as the limit of significance.
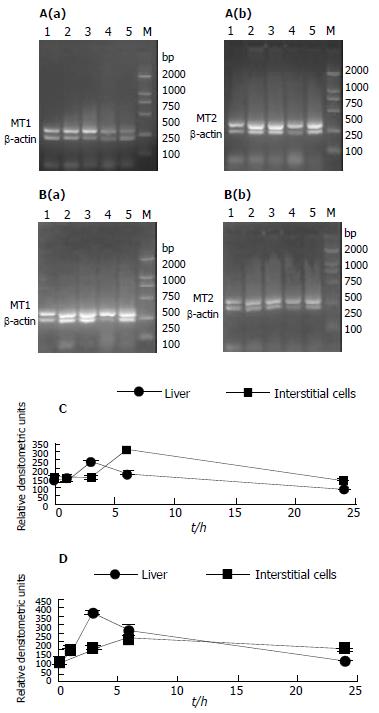
After cloning the RT-PCR products described above into a pUCm-T vector, we examined their DNA sequences (Figure 1) and compared with rat liver MT1 and MT2 cDNAs. These data clearly indicate that the MT1 and MT2 genes are constitutively expressed in the rat testis.
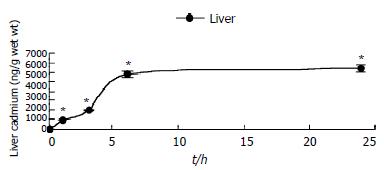
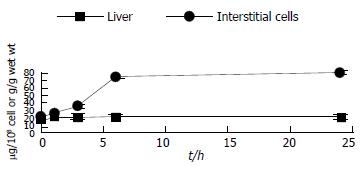
Cadmium accumulated in the liver rapidly after its administration, but then leveled off. 24 h after Cd content in the liver increased about 10000-fold whereas interstitial cell Cd was not detected (Figure 2). This indicated that the Cd content in interstitial cells might be below atomic absorption spectrophotometry detection limit (< 1 ng/107 cells).
To assess gene expression of both MT1 and MT2 genes, semi-quantitative RT-PCR was used. Results from the semi-quantitative RT-PCR (Figure 3) showed that untreated animals contained relatively high basal levels of both isoform mRNA, which were substantially increased after Cd treatment in the liver and peaked at 3 h, followed by a decline. In contrast, the mRNA levels in interstitial cells peaked at 6 h (Figure 3). Interestingly, the induction of MT1 mRNA was lower than MT2 mRNA in the liver of rats treated with Cd, but it was opposite to interstitial cells.
In parallel to the elevation of liver Cd, the level of hepatic MT increased significantly (3.9 hold increase) 24 h after Cd injection (Figure 4). In sharp contrast to the 3.9-fold elevation of hepatic MT, the MT in testicular interstitial cells of rats treated with Cd slightly declined as compared to untreated animals.
MTs exist not only in tissues from various animal species, but also in bacteria and plants, and are thought to play essential, but as yet unknown roles in cellular processes[18-22]. MTs are known to detoxify heavy metals. However, male genital organs, particularly testis, are extremely susceptible to Cd. Thus, it has been a long-standing issue as to whether MTs are present in male genital organs or not. We have previously summarized the major points of dispute arising from earlier studies that advocated either the presence or the absence of MTs in the testis, since these earlier studies utilized indirect experimental methods to characterize testicular Cd-binding proteins. Therefore, we considered it essential to analyse directly the cDNA sequence. It has been demonstrated by RT-PCR analysis and DNA sequence analysis of the cloned PCR products that MT1 and MT2 genes are transcripted as their respective mRNA in the rat testis. The present results have clearly shown that the rat testis contains MT1 and MT2, the major isoforms of MT, thus supporting the results of earlier studies, not only in turns of the amounts of MT(-like) proteins estimated by the Cd-binding method in the rat testis, but also in regard to the localization of MT in male genital tissues.
Our observations demonstrated that MT1 and MT2 mRNAs were expressed in interstitial cells under normal physiological conditions, which confirmed the results of others in the whole testes of rats and mice. Furthermore, our results showed that both MT1 and MT2 mRNA increased, but did not translate into any higher protein in interstitial cells in response to Cd treatment. Cd-inducibility of MT1 and MT2 mRNA that we observed in freshly isolated intertitial cells, corroborated the findings of others using cultured mouse interstitial tumor cell lines exposed to Cd in vitro. In contrast, other reports indicated decrease, no changes, or little increases of MTs mRNA levels in the whole testes of Cd-treated rats and mice[16,23]. The discrepancy in the results among the various studies might be due to analysis of whole testicular tissue rather than isolated interstitial cells, or species and strain differences in Cd-induced MT. MT expression in male reproductive tissue might also depend on doses, time course between Cd administration and tissue sampling, age and reproductive states of the animals[24]. The present data showed that untreated animals contained relatively high basal levels of both isoforms of mRNA, which were substantially increased after Cd treatment in the liver and peaked at 3 h, followed by a decline. In contrast, the mRNA levels in interstitial cells peaked at 6 h (Figure 3). Interestingly, the induction of MT1 mRNA was lower than MT2 mRNA in the liver of rats treated with Cd, but it was opposite to interstitial cells. This indicates that Cd-induced transcription of MT gene is not only tissue-dependent, but also time-dependent. Therefore, the time selected to analyse mRNA levels might be another source of discrepancy. Furthermore, both isoforms were different from Cd-induction in different tissues or cells. The cause is unclear, and might be related to methylation status of the MT gene, since DNA methylation controls MT gene expression in murine lymphoid cells.
We also found that interstitial cells isolated from unteated animals contained relatively high basal levels of MT protein, thus supporting the results of earlier studies that MT were constitutively present in the testes at levels higher than that in many other tissues, such as the liver and kidney[25].
MT might not have any significant role in the detoxification of Cd in the testes, since 20 μmol Cd/kg destroyed the testicular endothelium of both normal mice and mice with inactivated MT1 and MT2 genes. Furthermore, mouse strain differences in Cd-induced testicular toxicity have not been reported to be correlated with testicular MT levels. In fact, resistance to Cd-induced testicular necrosis is linked to the cdm gene, which is located in a different chromosome from the genes for MT1 and MT2.
One hypothesis, which may explain why Cd-induced MT mRNA in interstitial cells was not accompanied by an increase of MT synthesis, is that MT mRNA increases were not translated into the corresponding protein. The existence of nonfunctional MT translations in interstitial cells could explain the higher susceptibility of testes than the liver to Cd toxicity. On the other hand, the absence of an increase in the MT gene translation product in interstitial cells accompanied by a significant increase in the liver of Cd-treated animals, could also be due to kinetic differences between interstitial cells and liver on the rate at which MT mRNA molecules were translated into MT or the rate of MT degradation[1]. The discrepancy between MT mRNA and MT protein in interstitial cells suggested that MT synthesis was regulated at the level of post-transcription[26], since an increase of mRNA should not result in decreased protein if regulation was only through transcription. While it is well known that MT gene expression is specifically induced by metals through metal response elements and the heavy metal induction of MT is mediated through the transcription factor MTF-1[27-29]. We not determine whether the effect of these metals on MT mRNA translation and/or protein degradation was specific to MT. Therefore it is possible that the metals have a more widespread effect on translation of genes[30-32]. However, it is clear that metals do not have a widespread effect on gene transcription through stress or nonspecific events. For example, housekeeping genes, such as β-actin or dihydrofolate reductase, in the liver and kidney of CD-1 mice were unaffected when treated with 0.6 mg cadmium/kg.
In summary, our observations clearly demonstrate that both MT mRNA and MT are constitutively expressed in isolated interstitial cells and Cd-induced expression of MT isoforms are not only tissue dependent but also time-dependent. Our findings of Cd-induced MT mRNA without increases in MT protein in interstitial cells deserve further investigation. The inability to induce metal-detoxicating MT-protein, in response to Cd, might account for the higher susceptibility of testes to Cd toxicity and carcinogenesis compared to the liver.
We thank for Prof. Chiharu Tohyama for kindly providing affinity-purified sheep anti-(rat MT) IgG and Dr. Xu Qin-Hua for invaluable assistance with analysis of cadmium content.
Edited by Zhang JZ and Wang XL
| 1. | Vasconcelos MH, Tam SC, Hesketh JE, Reid M, Beattie JH. Metal- and tissue-dependent relationship between metallothionein mRNA and protein. Toxicol Appl Pharmacol. 2002;182:91-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 2. | Ren XY, Zhou Y, Zhang JP, Feng WH, Jiao BH. Metallothionein gene expression under different time in testicular Sertoli and spermatogenic cells of rats treated with cadmium. Reprod Toxicol. 2003;17:219-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Abe T, Yamamoto O, Gotoh S, Yan Y, Todaka N, Higashi K. Cadmium-induced mRNA expression of Hsp32 is augmented in metallothionein-I and -II knock-out mice. Arch Biochem Biophys. 2000;382:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Xu G, Zhou G, Jin T, Zhou T, Hammarström S, Bergh A, Nordberg G. Apoptosis and p53 gene expression in male reproductive tissues of cadmium exposed rats. Biometals. 1999;12:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Coogan TP, Shiraishi N, Waalkes MP. Minimal basal activity and lack of metal-induced activation of the metallothionein gene correlates with lobe-specific sensitivity to the carcinogenic effects of cadmium in the rat prostate. Toxicol Appl Pharmacol. 1995;132:164-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Mullins JE, Fredrickson RA, Fuentealba IC, Markham RJ. Purification and partial characterization of a cadmium-binding protein from the liver of rainbow trout (Onchorynchus mykiss). Can J Vet Res. 1999;63:225-229. [PubMed] |
| 7. | Suzuki JS, Kodama N, Molotkov A, Aoki E, Tohyama C. Isolation and identification of metallothionein isoforms (MT-1 and MT-2) in the rat testis. Biochem J. 1998;334:695-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Lee KF, Lau KM, Ho SM. Effects of cadmium on metallothionein-I and metallothionein-II mRNA expression in rat ventral, lateral, and dorsal prostatic lobes: quantification by competitive RT-PCR. Toxicol Appl Pharmacol. 1999;154:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Katakura M, Sugawara N. [Preventive effect of selenium against the testicular injury by cadmium]. Nihon Eiseigaku Zasshi. 1999;54:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Cook JC, Klinefelter GR, Hardisty JF, Sharpe RM, Foster PM. Rodent Leydig cell tumorigenesis: a review of the physiology, pathology, mechanisms, and relevance to humans. Crit Rev Toxicol. 1999;29:169-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 115] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | McKenna IM, Bare RM, Waalkes MP. Metallothionein gene expression in testicular interstitial cells and liver of rats treated with cadmium. Toxicology. 1996;107:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Shiraishi N, Hochadel JF, Coogan TP, Koropatnick J, Waalkes MP. Sensitivity to cadmium-induced genotoxicity in rat testicular cells is associated with minimal expression of the metallothionein gene. Toxicol Appl Pharmacol. 1995;130:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Wang SH, Chen JH, Lin LY. Functional integrity of metallothionein genes in testicular cell lines. J Cell Biochem. 1994;55:486-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Liu J, Corton C, Dix DJ, Liu Y, Waalkes MP, Klaassen CD. Genetic background but not metallothionein phenotype dictates sensitivity to cadmium-induced testicular injury in mice. Toxicol Appl Pharmacol. 2001;176:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Valverde M, Fortoul TI, Díaz-Barriga F, Mejia J, del Castillo ER. Induction of genotoxicity by cadmium chloride inhalation in several organs of CD-1 mice. Mutagenesis. 2000;15:109-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Zhou T, Zhou G, Song W, Eguchi N, Lu W, Lundin E, Jin T, Nordberg G. Cadmium-induced apoptosis and changes in expression of p53, c-jun and MT-I genes in testes and ventral prostate of rats. Toxicology. 1999;142:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Ruitenberg EJ, Steerenberg PA, Brosi BJ, Buys J. Reliability of the enzyme-linked immunosorbent assay (ELISA) for the serodiagnosis of Trichinella spiralis infections in conventionally raised pigs. Tijdschr Diergeneeskd. 1976;101:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 79] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Cai L, Deng DX, Jiang J, Chen S, Zhong R, Cherian MG, Chakrabarti S. Induction of metallothionein synthesis with preservation of testicular function in rats following long term renal transplantation. Urol Res. 2000;28:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Waalkes MP, Rehm S, Cherian MG. Repeated cadmium exposures enhance the malignant progression of ensuing tumors in rats. Toxicol Sci. 2000;54:110-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 34] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Klaassen CD, Liu J. Metallothionein transgenic and knock-out mouse models in the study of cadmium toxicity. J Toxicol Sci. 1998;23 Suppl 2:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Eid H, Géczi L, Mágori A, Bodrogi I, Institoris E, Bak M. Drug resistance and sensitivity of germ cell testicular tumors: evaluation of clinical relevance of MDR1/Pgp, p53, and metallothionein (MT) proteins. Anticancer Res. 1998;18:3059-3064. [PubMed] |
| 22. | Satoh M, Kaji T, Tohyama C. [Low dose exposure to cadmium and its health effects. (3) Toxicity in laboratory animals and cultured cells]. Nihon Eiseigaku Zasshi. 2003;57:615-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Shiraishi N, Waalkes MP. Enhancement of metallothionein gene expression in male Wistar (WF/NCr) rats by treatment with calmodulin inhibitors: potential role of calcium regulatory pathways in metallothionein induction. Toxicol Appl Pharmacol. 1994;125:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Betka M, Callard GV. Stage-dependent accumulation of cadmium and induction of metallothionein-like binding activity in the testis of the Dogfish shark, Squalus acanthias. Biol Reprod. 1999;60:14-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Cyr DG, Dufresne J, Pillet S, Alfieri TJ, Hermo L. Expression and regulation of metallothioneins in the rat epididymis. J Androl. 2001;22:124-135. [PubMed] |
| 26. | Vasconcelos MH, Tam SC, Beattie JH, Hesketh JE. Evidence for differences in the post-transcriptional regulation of rat metallothionein isoforms. Biochem J. 1996;315:665-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 33] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Dufresne J, Cyr DG. Effects of short-term methylmercury exposure on metallothionein mRNA levels in the testis and epididymis of the rat. J Androl. 1999;20:769-778. [PubMed] |
| 28. | Radtke F, Heuchel R, Georgiev O, Hergersberg M, Gariglio M, Dembic Z, Schaffner W. Cloned transcription factor MTF-1 activates the mouse metallothionein I promoter. EMBO J. 1993;12:1355-1362. [PubMed] |
| 29. | Saydam N, Georgiev O, Nakano MY, Greber UF, Schaffner W. Nucleo-cytoplasmic trafficking of metal-regulatory transcription factor 1 is regulated by diverse stress signals. J Biol Chem. 2001;276:25487-25495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 108] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 30. | Min KS, Kim H, Fujii M, Tetsuchikawahara N, Onosaka S. Glucocorticoids suppress the inflammation-mediated tolerance to acute toxicity of cadmium in mice. Toxicol Appl Pharmacol. 2002;178:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Ozawa N, Goda N, Makino N, Yamaguchi T, Yoshimura Y, Suematsu M. Leydig cell-derived heme oxygenase-1 regulates apoptosis of premeiotic germ cells in response to stress. J Clin Invest. 2002;109:457-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Matsuura T, Kawasaki Y, Miwa K, Sutou S, Ohinata Y, Yoshida F, Mitsui Y. Germ cell-specific nucleocytoplasmic shuttling protein, tesmin, responsive to heavy metal stress in mouse testes. J Inorg Biochem. 2002;88:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |