Published online Jul 15, 2003. doi: 10.3748/wjg.v9.i7.1491
Revised: March 4, 2003
Accepted: March 16, 2003
Published online: July 15, 2003
AIM: To investigate the safety and efficacy of long-term combination therapy with alpha interferon and lamivudine in non-responsive patients with anti-HBe-positive chronic hepatitis B.
METHODS: 34 patients received combination treatment (1 month lamivudine, 12 month lamivudine+interferon, 6 month lamivudine), 24 received lamivudine (12 mo), 24 received interferon (12 mo). Interferon was administered at 6 MU tiw and lamivudine at 100 mg orally once daily. Patients were followed up for 6 mo after treatment.
RESULTS: At the end of treatment, HBV DNA negativity rates were 88% with lamivudine + interferon, 99% with lamivudine and 55% with interferon, (P = 0.004, combination therapy vs. interferon, and P = 0.001 lamivudine vs. interferon), and serum transaminase normalization rates were 84%, 91% and 53% (P = 0.01 combination therapy vs. interferon, and P = 0.012 lamivudine vs. interferon). Six months later, HBV DNA negativity rates were 44% with lamivudine+interferon, 33% with lamivudine and 25% with interferon, and serum transaminase normalization rates were 61%, 42% and 45%, respectively, without statistical significance. No YMDD variants were observed with lamivudine+interferon (vs. 12% with lamivudine). The combination therapy appeared to be safe.
CONCLUSION: Although viral clearance and transaminase normalization are slower with long-term lamivudine + interferon than that with lamivudine alone, the combination regimen seems to provide more lasting benefits and to protect against the appearance of YMDD variants. Studies with other regimens regarding sequence and duration are needed.
- Citation: Jaboli MF, Fabbri C, Liva S, Azzaroli F, Nigro G, Giovanelli S, Ferrara F, Miracolo A, Marchetto S, Montagnani M, Colecchia A, Festi D, Reggiani LB, Roda E, Mazzella G. Long-term alpha interferon and lamivudine combination therapy in non-responder patients with anti-HBe-positive chronic hepatitis B: Results of an open, controlled trial. World J Gastroenterol 2003; 9(7): 1491-1495
- URL: https://www.wjgnet.com/1007-9327/full/v9/i7/1491.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i7.1491
Chronic hepatitis B virus (HBV) infection remains a major worldwide public health problem. Over 300 million people are affected by the disease[1], which is associated with high mortality and morbidity due to the associated risk of cirrhosis and hepatocellular carcinoma[2]. In addition, HBV infection is becoming an increasingly relevant problem for transplanted and immunodeficient patients, who are prone to develop more severe, accelerated liver disease[3].
Until recently, interferon-α (IFN) was the only drug approved throughout the world for chronic HBV infection[4,5]. Unfortunately, relapse with return of viremia and hepatitis occurs in up to 50% of cases[6]. The hepatitis B s antigen (HBsAg) may become negative in months to years after the end of treatment, suggesting total viral clearance[7]. IFN is less effective in the presence of HBeAg negativity[8], or in patients who have already developed cirrhosis or have had recurrence of HBV after a hepatic allograft.
Lamivudine (3TC) is the first potentially non-cytotoxic[9,10] oral nucleoside analogue approved for the treatment of chronic hepatitis B. It suppresses viral DNA replication by means of chain termination, and competitively inhibits viral polymerase, but does not act on supercoiled DNA. For these reasons, lamivudine rapidly reduces serum HBV DNA, enhances transaminase normalization, and improves the histological picture[11-13]. But only 16% achieve full HBeAg seroconversion, and after suspension of therapy serum HBV DNA levels and transaminases generally return to pretreatment values[14]. Furthermore, after clearance of HBV DNA, mutations in the sequence of the YMDD locus of the HBV RNA-dependent DNA polymerase appear in 15% of cases after one year of therapy, leading to recurrence of viremia[15]. Onset of resistance generally occurs after six months to several years of lamivudine therapy[16].
Combination therapy with IFN and lamivudine is being investigated in order to take advantage of the drugs' different mechanisms of action[17-21]. However, the results of short-term treatment studies have been not altogether encouraging[18]. The aim of the present study was to investigate whether long-term combination therapy was safe and well tolerated, and whether it could provide additional therapeutic benefits with respect to either of the single drug regimens. In particular, we set out to evaluate efficacy in terms of primary and sustained viremia and transaminase responses. We also assessed histological response, incidence of YMDD-variant HBV, and safety.
This open trial concerned 82 adult Italian patients with chronic anti-HBe positive hepatitis B (68 males, 14 females) enrolled between February 1997 and February 1999 who had either not responded to or had relapsed after previous interferon treatment. The experimental protocol was carried out in accordance with the Helsinki Declaration, and was approved by the Ethics Review Committee, and all patients gave written informed consent to participate in a trial involving long-term treatment either with interferon plus lamivudine or with lamivudine or interferon alone.
Inclusion criteria for the study were: age between 18 and 70 years, detectable hepatitis B surface antigen (HBsAg) in serum at the time of screening and for at least six months before the start of the study, serum HBV DNA levels of at least 5 pg/mL at screening, raised alanine transaminase values within three months before the start of therapy. Exclusion criteria were: presence of co-infection with hepatitis C and D virus or HIV, signs of hepatic decompensation or hepatocellular carcinoma, other possible causes of chronic liver damage (i.e. alcohol abuse, hemochromatosis, Wilson's disease, a1-antitrypsin deficiency, autoimmune diseases, drug-induced hepatotoxicity and congestive heart failure), assumption of immunosuppressive or antiviral therapy within six months before the study. Needle biopsy performed in all cases within 2 years of the study, showed that 33 patients had a Scheuer score for fibrosis > 3.
The patients were divided into three groups: group A (n = 34) for combination treatment, group B (n = 24) for treatment with lamivudine alone, group C (n = 24) for treatment with interferon alone.
Patients in group A received 100 mg/d of lamivudine (Glaxo-Wellcome Inc, Research Triangle Park, NC, USA) orally for one month, followed by lamivudine (100 mg/d) in association with six million units three times per week of natural interferon (Wellferon, Glaxo-Wellcome Inc, Research Triangle Park, NC, USA) for twelve months, and then lamivudine (100 mg/d) alone for six months. Patients in group B received lamivudine (100 mg/d) orally for twelve months. Patients in group C received six million units three times per week of natural interferon for twelve months. Interferon was administered subcutaneously by the patients after adequate training. Patients were followed up after the end treatment for twenty-four weeks. All patients had a liver biopsy within 2 years of entering the study, and were requested to have another at the end of treatment. Hepatitis flare up was defined as an increase in serum ALT during therapy and classified as mild, moderate or severe (> 1.5, 5, 10 times respectively) according to the preceding ALT value.
The main end point of the study was to evaluate the end-of-treatment and sustained response, defined as HBV DNA loss and serum ALT normalization at the end of therapy and follow-up, respectively. Secondary variables included histological response, incidence of YMDD-variant HBV, and safety.
Blood samples were obtained immediately before therapy, after 15 d, and then every month during treatment and follow-up. Routine biochemical tests and blood cell counts were performed. HBsAg, HBeAg, and anti-HBs and anti-HBe antibodies were determined by enzyme immunoassay. Serum HBV DNA was measured quantitatively by a solution-hybridization assay (Abbott, Chicago, USA) with a lower limit of 3.0 pg/mL of serum.
Presence of the YMDD variant was evaluated by a restriction fragment length polymorphism assay in all ends of treatment samples and in cases where initial loss of HBV DNA was followed by a return to positivity, regardless of transaminase values. The biopsy specimens were scored according to the Scheuer histological activity index[22].
Baseline characteristics of the patients were presented as mean values ± standard error (SE). Statistical evaluations were conducted according to the intention-to-treat procedure. The χ2 test or the Fisher's exact test was used to compare differences in proportion between treatment groups. Continuous data were analyzed using Kruskall-Wallis test and Mann-Whitney test. A two-tailed P value of less than 0.05 was considered to be statistically significant.
All treatment arms were well matched with regard to baseline characteristics (Table 1). Of the 82 patients, 79 completed the treatment schedule, while the remaining 3 dropped out of the study: one group A patient withdrew, one group B patient and one group C patient experienced depression.
| Characteristics | Lamivudine+ interferon(n = 34) | Lamivudine alone(n = 24) | Interferon alone(n = 24) |
| Age (year) | 44.0 ± 2.0 | 48.7 ± 2.4 | 44.4 ± 2.5 |
| Sex (m/f) | 30/4 | 18/6 | 20/4 |
| ALT (U/l) | 151 ± 21 | 160 ± 39 | 130 ± 17 |
| AST (U/l) | 81 ± 10 | 111 ± 37 | 66 ± 10 |
| GT (U/l) | 48 ± 3 | 57 ± 6 | 47 ± 3 |
| Albumin (gr/dl) | 3.94 ± 0.04 | 3.97 ± 0.04 | 4.01 ± 0.03 |
| HBV DNA (pg/ml) | 60 ± 25 | 85 ± 43 | 73 ± 36 |
| Histology: | |||
| Cirrhosis | 13 (38%) | 10 (42%) | 10 (42%) |
| Severe activity | 10 (29%) | 7 (29%) | 9 (37%) |
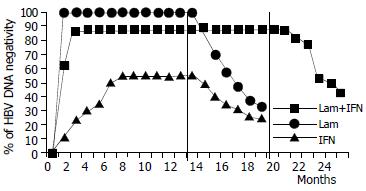
End-of-treatment response HBV DNA negativity was observed at the end of treatment in 30 (88%) patients who received combination therapy (i.e. group A), 23 (99%) who had lamivudine alone (i.e. group B), and 13 (55%) who had interferon alone (i.e. group C). HBV DNA negativity was achieved in week 12, 4 and 32, respectively, in the three groups. Two differences in primary response among the three groups were highly significant [combination vs. interferon: OR 6.34 (95%CI: 1.7%-23%), P = 0.004; lamivudine vs. interferon: OR 19.46 (95%CI: 3.3%-113%), P = 0.001].
Sustained response A total of 15 (44%) patients in group A, 8 (33%) in group B and 6 (25%) in group C were HBV DNA negative at the end of the follow-up. None of the differences in sustained virological response among the three groups reached statistical significance.
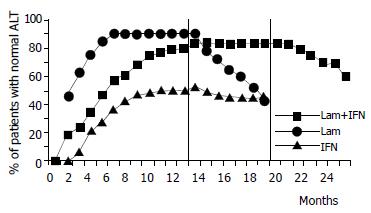
End-of-treatment response Serum transaminases became normal at the end of treatment in 29 (84%) patients in group A, 21 (91%) in group B and 13 (53%) in group C [combination vs. interferon: OR 4.9 (95%CI: 1.4%-17%), P = 0.01; lamivudine vs. interferon: OR 5.92 (95%CI: 1.28%-25.3%), P = 0.012]. Normalization was achieved in week 20, 8 and 38, respectively, in the three groups. A mild flare-up without bilirubin alteration was observed only in 8 group A patients. In all cases, serum transaminase normalization was preceded by a rise of HBV DNA at 2-8 mo of therapy. The flare-up was always followed 1-2 mo later by serum HBV DNA negativization and transaminase normalization. None of these patients had a YMDD variant.
Sustained response Sustained serum transaminase normalization was observed in 21 (61%) patients in group A, 10 (42%) in group B and 11 (45%) in group C. None of the differences in sustained transaminase response among the three groups had statistical significance. Only 4 of the 8 patients who experienced a flare-up during combined therapy mantained their primary response.
Histological findings Paired pre- and post-treatment liver biopsies were available for 22 (65%), 15 (62%) and 14 (58%) patients in groups A, B and C, respectively. None of the post-treatment biopsies showed a worsening or unchanged histological picture. Significant improvements in necro-inflammatory activity in parenchyma (P < 0.01) and portal space (P < 0.01) were observed in each group. Significant improvements in fibrosis were found in groups A and B (P < 0.05), but not in group C.
Incidence of YMDD variant HBV YMDD variants of HBV were not detected in any of the patients who received combination or IFN therapy. 3 of the 24 (12%) patients treated with lamivudine alone had a YMDD variant after 6-9 mo of therapy. In particular, two had a YVDD variant and the other had a YIDD variant. They continued lamivudine therapy and were monitored weekly. After a progressive rise of serum HBV DNA, two of the patients showed 5 times of elevation of transaminases (with respect to the upper limit) and another showed 1.5 times of elevation. However, after the emergence of the variant, the HBV DNA and transaminase levels always remained lower than the baseline.
The whole treatment program was completed by 33 (98%), 23 (96%) and 23 (94%) patients in groups A, B and C, respectively. No patient received reduced dosage but we directly stopped treatment. The side effects of the combination regimen were generally similar to those of interferon treatment. In addition, among the 34 patients in group A, symptomatic and reversible rises in serum levels of amylase, lipase and creatinine phosphokinase were recorded in 9 (28%), 2 (5%) and 1 (3%) cases, respectively. Worsening of pre-existing hypertriglyceridemia was recorded in 2 (7%) cases. By comparison, among the 24 patients in group B, lamivudine therapy was associated with an asymptomatic and reversible rise in amylase, lipase or creatinine phosphokinase in 6 (25%), 1 (4%) and 1 (4%), respectively. No case of hepatocellular carcinoma, liver decompensation or death was recorded, and no patient requested orthotopic liver transplantation (Table 2).
| Adverse events | Lamivudine + interferon(n = 34), n(%) | Lamivudine alone(n = 24), n(%) | Interferon alone(n = 24), n(%) |
| Influenza-like symptoms | 24 (70%) | none | 19 (79%) |
| Hair loss | 8 (23%) | none | 9 (38%) |
| Weight loss | 5 (16%) | none | 4 (15%) |
| Depression | none | 1 (4%) | 1 (6%) |
| Low white-cell count | 19 (56%) | none | 14 (59%) |
| Low platelet count | 14 (42%) | none | 10 (44%) |
| Amylase rise | 9 (28%) | 6 (25%) | none |
| Lipase rise | 2 (5%) | 1 (4%) | none |
| CPK rise | 1 (3%) | 1 (4%) | none |
| Hypertriglyceridemia | 2 (7%) | none | none |
The development of effective treatment strategies for patients with HBV remains a major clinical challenge. We investigated the possible benefits of a long-term interferon/lamivudine combination treatment regimen in a cohort of patients who had already received unsuccessful treatment with interferon alone. The effects of this long-term combination regimen were compared with single-agent treatment with either interferon or lamivudine in three similar groups of patients. In regard to virological response, we found that at the end of therapy, HBV DNA had become undetectable in 88% of the patients receiving combination treatment, as compared with 99% and 55% of those who had single-line therapy with lamivudine or interferon, respectively. These differences in end-of-treatment response were statistically significant in comparison of the combination therapy and lamivudine monotherapy with interferon monotherapy. The analysis of sustained response showed that 44% patients in the combination therapy group maintained DNA clearance as compared with only 33% in the lamivudine group and 25% in the interferon group. Although these differences did not have statistical significance, there was a trend in favor of the combination regimen. Similarly, although the end-of-treatment transaminase normalization rate was significantly higher in the combination treatment (84%) group and in the lamivudine group of patients (91%) than that in interferon (53%) group, the sustained response showed a non-significant trend in favor of the combination regimen (61% in the combination group vs. 42% and 45% with lamivudine or interferon alone, respectively).
As has been reported for short-term treatment[23], during our long-term combination therapy regimen there was a progressive viral decline, whereas with single-line lamivudine therapy the HBV DNA levels leveled off. Our data are in keeping with the concept that lamivudine therapy is more rapid and effective on virological and biochemical response than interferon alone or even in combination with lamivudine. However, after discontinuation of therapy, virological and biochemical relapses were considerably more common with lamivudine alone than those with the combination regimen (even though this difference did not actually reach statistical significance). Moreover, there was a trend suggesting that combination therapy delays relapse. The non-significant status of these trends could be due to the relatively small cohort sizes.
Among the two monotherapy options, interferon appeared less effective than lamivudine. In our study, interferon provided less end-of-treatment responses than lamivudine, and no advantages in terms of sustained response. The relatively poor capacity of interferon monotherapy to promote viral clearance may be explained by an inability to induce an efficient immune response.
After about 5 mo of combination therapy, we observed a mild flare-up in 8 patients following a rise in serum HBV DNA. The flare-up was always followed, after a further one to two months of therapy by a return to serum HBV DNA negativity and normalization of transaminases. They may have been caused by enhanced immune responses prompted by increased mutant viral replication other than YMDD, which however were unable to permanently eradicate the virus.
As regards the secondary criteria of response, all the patients who underwent a post-treatment biopsy derived histological benefit in terms of necro-inflammatory activity in parenchyma and in portal space, regardless of their serological response. However, in keeping with other reports[24], interferon monotherapy provided no improvement in fibrosis.
YMDD HBV variants were not detected in any of the patients who received combination therapy or interferon alone. After 6-9 mo of lamivudine monotherapy, 3 (12%) patients had YMDD, a rate similar to that reported in Asian patients. Interferon therapy seemed to exert a protective effect against the emergence of variants, probably because it lowers viral replication and enhances immunological response. In any case, in the three affected patients, the serum HBV DNA levels did not return completely to pretreatment levels. Thus, the mutant strains of HBV DNA that break through during prolonged therapy with lamivudine may not be as replicative-efficient or as pathogenic as wild-type strains. As in other reports[25], we found that 3 to 4 mo after lamivudine treatment was stopped, there was disappearance of this HBV mutant and re-emergence of the wild type virus. Honkoop et al[26] observed acute exacerbation of chronic HBV infection after withdrawal of lamivudine therapy and concluded that the relationship between the development of "lamivudine-withdrawal hepatitis" and the lamivudine-resistant mutant virus was currently unclear, since both the emergence of a lamivudine resistant viral mutant and the resurgence of wild-type virus after withdrawal of therapy were able to induce severe hepatitis exacerbations. Studies of rechallenge with lamivudine have yet to be reported, and the significance of YMDD variant is being evaluated in the ongoing follow-up clinical trials.
As regards safety, the combination therapy was well tolerated and no serious side effects were observed. The side effects observed during the course of the long-term combination regimen were those that were commonly associated with the two drugs. As expected, in the two monotherapy groups, the incidence of drug-related adverse events was much lower with lamivudine than that with interferon.
In conclusion, these results suggest that for patients who have already been unsuccessfully treated with interferon, long-term combination therapy may have some advantages over single-line treatment with lamivudine or interferon[27]. Other studies with other regimens regarding dose, sequence and duration of interferon and lamivudine are needed. Finally, correct staging of patients according to the particular phases in the natural history of chronic hepatitis B appears to be important to personalise patients' management. It is possible that combinations of two[28,29] or more nucleoside analogs may reduce the development of viral resistance, increase overall response rates, decrease adverse events. In general, multi-drug therapies[30] that take advantage of different modes of action concurrently may provide a more appropriate approach for particular subgroups of patients.
We are grateful to Robin MT Cooke for editing.
Edited by Xu XQ
| 1. | Maynard JE. Hepatitis B: global importance and need for control. Vaccine. 1990;8 Suppl:S18-20; discussion S21-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 214] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | de Jongh FE, Janssen HL, de Man RA, Hop WC, Schalm SW, van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology. 1992;103:1630-1635. [PubMed] |
| 3. | Davies SE, Portmann BC, O'Grady JG, Aldis PM, Chaggar K, Alexander GJ, Williams R. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology. 1991;13:150-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 282] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 4. | Dusheiko GM. Treatment and prevention of chronic viral hepatitis. Pharmacol Ther. 1995;65:47-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Korenman J, Baker B, Waggoner J, Everhart JE, Di Bisceglie AM, Hoofnagle JH. Long-term remission of chronic hepatitis B after alpha-interferon therapy. Ann Intern Med. 1991;114:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 267] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 6. | Hoofnagle JH, Lau D. Chronic viral hepatitis--benefits of current therapies. N Engl J Med. 1996;334:1470-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Mazzella G, Saracco G, Festi D, Rosina F, Marchetto S, Jaboli F, Sostegni R, Pezzoli A, Azzaroli F, Cancellieri C. Long-term results with interferon therapy in chronic type B hepatitis: a prospective randomized trial. Am J Gastroenterol. 1999;94:2246-2250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Hadziyannis SJ. Hepatitis B e antigen negative chronic hepatitis B: from clinical recognition to pathogenesis and treatment. Viral Hepatitis Rev. 1995;1:7-15. |
| 9. | Cui L, Yoon S, Schinazi RF, Sommadossi JP. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-beta-D-arabinofuranosyl)-5-iodouracil in human liver cells. J Clin Invest. 1995;95:555-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 92] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Nicoll A, Locarnini S. Review: Present and future directions in the treatment of chronic hepatitis B infection. J Gastroenterol Hepatol. 1997;12:843-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 619] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 12. | Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, Wu PC, Dent JC, Barber J. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1381] [Cited by in RCA: 1346] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 13. | Kweon YO, Goodman ZD, Dienstang JL. Lamivudine decreases fibrogenesis in chronic hepatitis B: an immunohistochemical study of paired liver biopsies. Hepatology. 2000;32:870P. |
| 14. | Honkoop P, de Man RA, Heijtink RA, Schalm SW. Hepatitis B reactivation after lamivudine. Lancet. 1995;346:1156-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Dienstag JL, Schiff ER, Mitchell M, Casey DE, Gitlin N, Lissoos T, Gelb LD, Condreay L, Crowther L, Rubin M. Extended lamivudine retreatment for chronic hepatitis B: maintenance of viral suppression after discontinuation of therapy. Hepatology. 1999;30:1082-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 16. | Hoofnagle JH. Therapy of viral hepatitis. Digestion. 1998;59:563-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Schiff ER, Dienstag JL, Karayalcin S, Grimm IS, Perrillo RP, Husa P, de Man RA, Goodman Z, Condreay LD, Crowther LM. Lamivudine and 24 wk of lamivudine/interferon combination therapy for hepatitis B e antigen-positive chronic hepatitis B in interferon nonresponders. J Hepatol. 2003;38:818-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Mutimer D, Naoumov N, Honkoop P, Marinos G, Ahmed M, de Man R, McPhillips P, Johnson M, Williams R, Elias E. Combination alpha-interferon and lamivudine therapy for alpha-interferon-resistant chronic hepatitis B infection: results of a pilot study. J Hepatol. 1998;28:923-929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Heathcote J, Schalm SW, Cianciara J, G Farrell, V Feinmann, M Shermann, AP Dhillon, AE Moorat and DF Gray. Lamivudine and Intron A combination treatment in patients with chronic hepatitis B infection. EASL April, 1998, Lisbon. . |
| 20. | Schalm SW, Heathcote J, Cianciara J, Farrell G, Sherman M, Willems B, Dhillon A, Moorat A, Barber J, Gray DF. Lamivudine and alpha interferon combination treatment of patients with chronic hepatitis B infection: a randomised trial. Gut. 2000;46:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 398] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 21. | Farrell G. Hepatitis B e antigen seroconversion: effects of lamivudine alone or in combination with interferon alpha. J Med Virol. 2000;61:374-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 22. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1193] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 23. | van Nunen AB, Hansen BE, Mutimer DJ. Viral kinetics during 16 wk of interferon and lamivudine monotherapy versus interferon-lamivudine combination therapy in chronic hepatitis B patients. Hepatology. 2000;32:878. |
| 24. | Brook MG, Petrovic L, McDonald JA, Scheuer PJ, Thomas HC. Histological improvement after anti-viral treatment for chronic hepatitis B virus infection. J Hepatol. 1989;8:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Chayama K, Suzuki Y, Kobayashi M, Kobayashi M, Tsubota A, Hashimoto M, Miyano Y, Koike H, Kobayashi M, Koida I. Emergence and takeover of YMDD motif mutant hepatitis B virus during long-term lamivudine therapy and re-takeover by wild type after cessation of therapy. Hepatology. 1998;27:1711-1716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 321] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 26. | Honkoop P, de Man RA, Niesters HG, Zondervan PE, Schalm SW. Acute exacerbation of chronic hepatitis B virus infection after withdrawal of lamivudine therapy. Hepatology. 2000;32:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 170] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Janssen HL, Schalm SW, Berk L, de Man RA, Heijtink RA. Repeated courses of alpha-interferon for treatment of chronic hepatitis type B. J Hepatol. 1993;17 Suppl 3:S47-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 28. | Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 339] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 29. | Lau GK, Tsiang M, Hou J, Yuen S, Carman WF, Zhang L, Gibbs CS, Lam S. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology. 2000;32:394-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 116] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 30. | Marques AR, Lau DT, McKenzie R, Straus SE, Hoofnagle JH. Combination therapy with famciclovir and interferon-alpha for the treatment of chronic hepatitis B. J Infect Dis. 1998;178:1483-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |